Abstract
Transfer of exponential-phase cells of Saccharomyces cerevisiae, previously grown in 2% glucose, to a derepression medium resulted in a prompt increase in the level of delta-aminolevulinate dehydratase, the rate-limiting enzyme of heme biosynthesis under these conditions. This derepression exhibited a lag of 35 min at 23 degrees C and required the participation of both RNA and protein syntheses. Dissection of the molecular events during this lag period disclosed that RNA synthesis, rnal gene function (messenger RNA transport from nucleus to cytosol), and initiation of protein synthesis were completed within less than 10, 18, and 24 min, respectively. The potential regulation of derepression by mitochondrial gene products and mitochondrial function was probed by means of a series of isogenic, respiration-deficient (rho-, pet-, and mit-) mutants; no such regulation was found.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bossinger J., Cooper T. G. Sequence of molecular events involved in induction of allophanate hydrolase. J Bacteriol. 1976 Apr;126(1):198–204. doi: 10.1128/jb.126.1.198-204.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deken R. H. The Crabtree effect: a regulatory system in yeast. J Gen Microbiol. 1966 Aug;44(2):149–156. doi: 10.1099/00221287-44-2-149. [DOI] [PubMed] [Google Scholar]
- De Deken R. H. The Crabtree effects and its relation to the petite mutation. J Gen Microbiol. 1966 Aug;44(2):157–165. doi: 10.1099/00221287-44-2-157. [DOI] [PubMed] [Google Scholar]
- Feldman F., Mahler H. R. Mitochondrial biogenesis. Retention of terminal formylmethionine in membrane proteins and regulation of their synthesis. J Biol Chem. 1974 Jun 25;249(12):3702–3709. [PubMed] [Google Scholar]
- Gollub E. G., Liu K. P., Dayan J., Adlersberg M., Sprinson D. B. Yeast mutants deficient in heme biosynthesis and a heme mutant additionally blocked in cyclization of 2,3-oxidosqualene. J Biol Chem. 1977 May 10;252(9):2846–2854. [PubMed] [Google Scholar]
- Haarasilta S., Oura E. On the activity and regulation of anaplerotic and gluconeogenetic enzymes during the growth process of baker's yeast. The biphasic growth. Eur J Biochem. 1975 Mar 3;52(1):1–7. doi: 10.1111/j.1432-1033.1975.tb03966.x. [DOI] [PubMed] [Google Scholar]
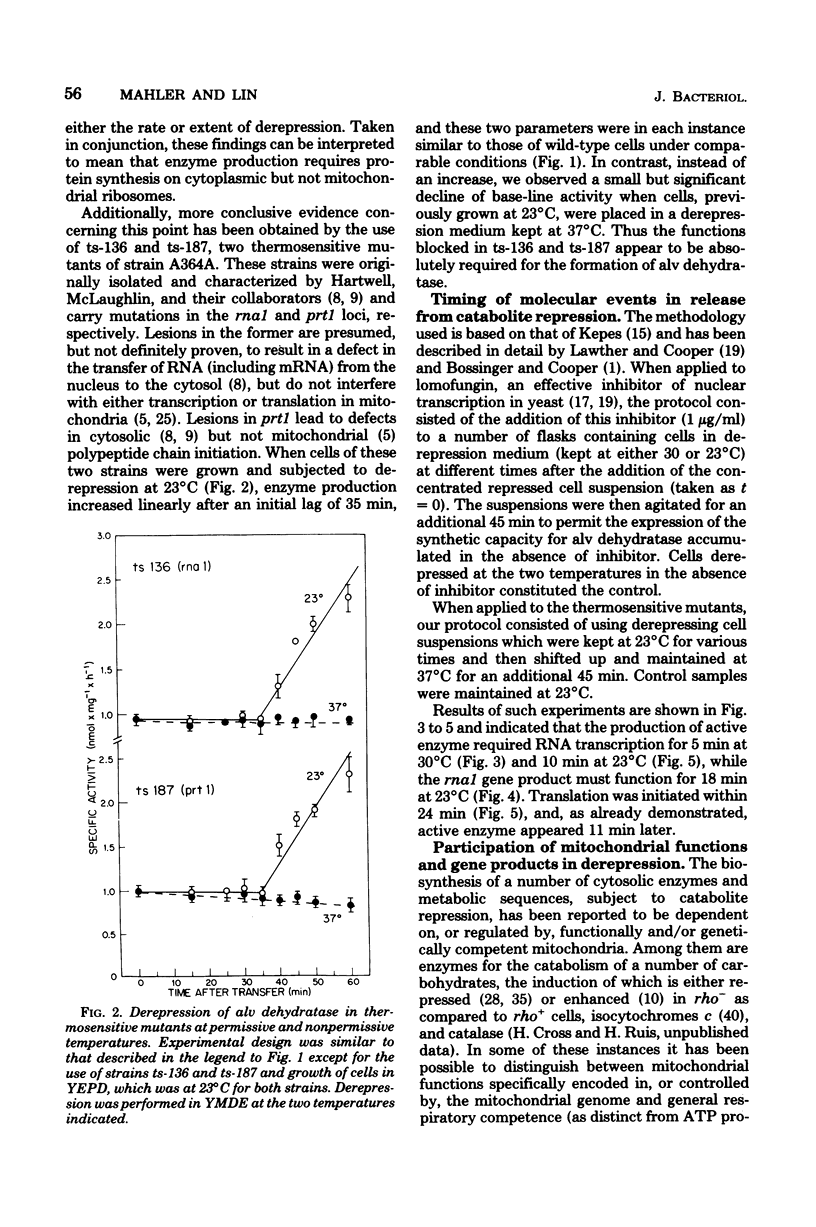
- Hartwell L. H., Hutchison H. T., Holland T. M., McLaughlin C. S. The effect of cycloheximide upon polyribosome stability in two yeast mutants defective respectively in the initiation of polypeptide chains and in messenger RNA synthesis. Mol Gen Genet. 1970;106(4):347–361. doi: 10.1007/BF00324052. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., McLaughlin C. S. A mutant of yeast apparently defective in the initiation of protein synthesis. Proc Natl Acad Sci U S A. 1969 Feb;62(2):468–474. doi: 10.1073/pnas.62.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann P., Zimmermann F. K. The role of mitochondria in carbon catabolite repression in yeast. Mol Gen Genet. 1976 Oct 18;148(2):205–211. doi: 10.1007/BF00268386. [DOI] [PubMed] [Google Scholar]
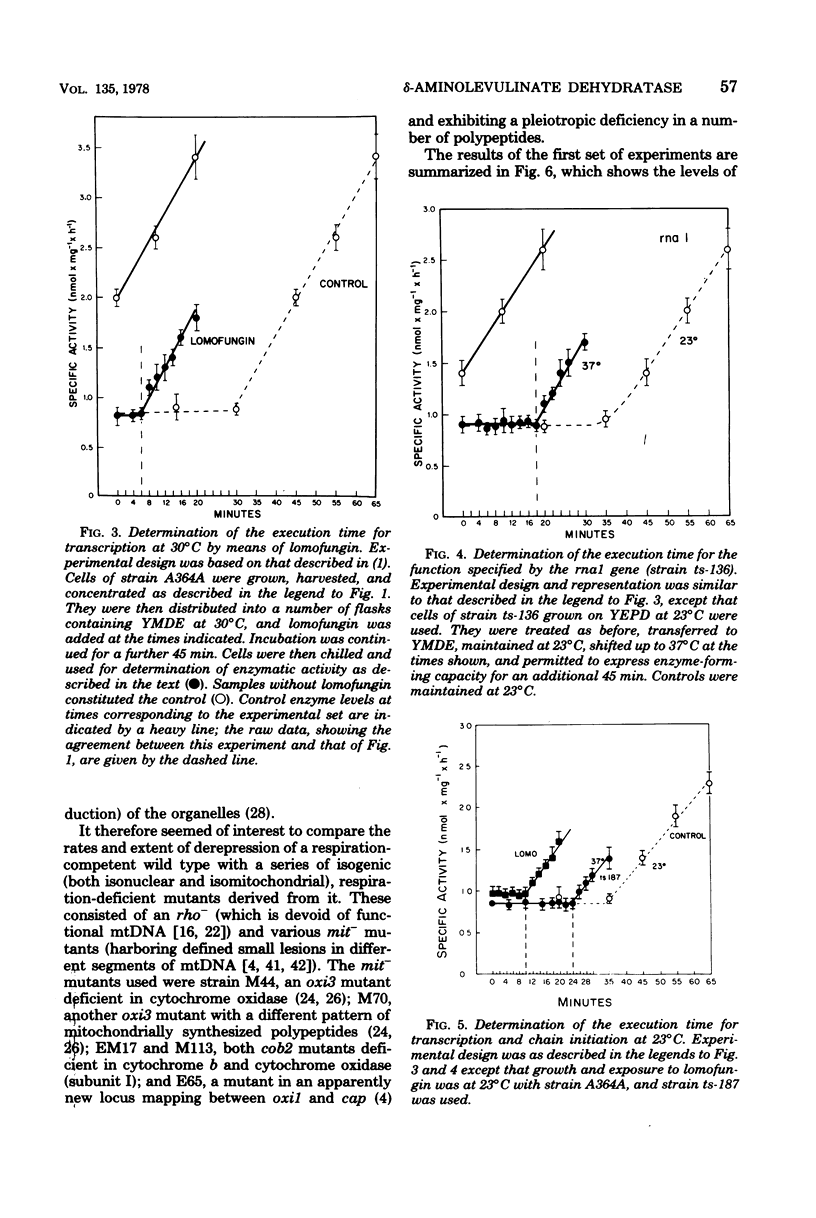
- Hutchison H. T., Hartwell L. H., McLaughlin C. S. Temperature-sensitive yeast mutant defective in ribonucleic acid production. J Bacteriol. 1969 Sep;99(3):807–814. doi: 10.1128/jb.99.3.807-814.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman J., Cotman C., Mahler H. R., Sharp C. W. Biochemical correlates of respiratory deficiency. VII. Glucose repression. Arch Biochem Biophys. 1966 Sep 26;116(1):224–251. doi: 10.1016/0003-9861(66)90029-4. [DOI] [PubMed] [Google Scholar]
- Jayaraman J., Padmanaban G., Malathi K., Sarma P. S. Haem synthesis during mitochondrogenesis in yeast. Biochem J. 1971 Feb;121(3):531–535. doi: 10.1042/bj1210531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEPES A. KINETICS OF INDUCED ENZYME SYNTHESIS. DETERMINATION OF THE MEAN LIFE OF GALACTOSIDASE-SPECIFIC MESSENGER RNA. Biochim Biophys Acta. 1963 Oct 15;76:293–309. [PubMed] [Google Scholar]
- Klo S. C., Cano F. R., Lampen J. O. Lomofungin, an inhibitor of ribonucleic acid synthesis in yeast protoplasts: its effect on enzyme formation. Antimicrob Agents Chemother. 1973 Jun;3(6):716–722. doi: 10.1128/aac.3.6.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovác L. Biochemical mutants: an approach to mitochondrial energy coupling. Biochim Biophys Acta. 1974 Oct 31;346(2):101–135. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labbe-Bois R., Volland C. Changes in the activities of the protoheme-synthesizing system during the growth of yeast under different conditions. Arch Biochem Biophys. 1977 Mar;179(2):565–577. doi: 10.1016/0003-9861(77)90145-x. [DOI] [PubMed] [Google Scholar]
- Lawther R. P., Cooper T. G. Kinetics of induced and repressed enzyme synthesis in Saccharomyces cerevisiae. J Bacteriol. 1975 Mar;121(3):1064–1073. doi: 10.1128/jb.121.3.1064-1073.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnane A. W., Haslam J. M., Lukins H. B., Nagley P. The biogenesis of mitochondria in microorganisms. Annu Rev Microbiol. 1972;26:163–198. doi: 10.1146/annurev.mi.26.100172.001115. [DOI] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
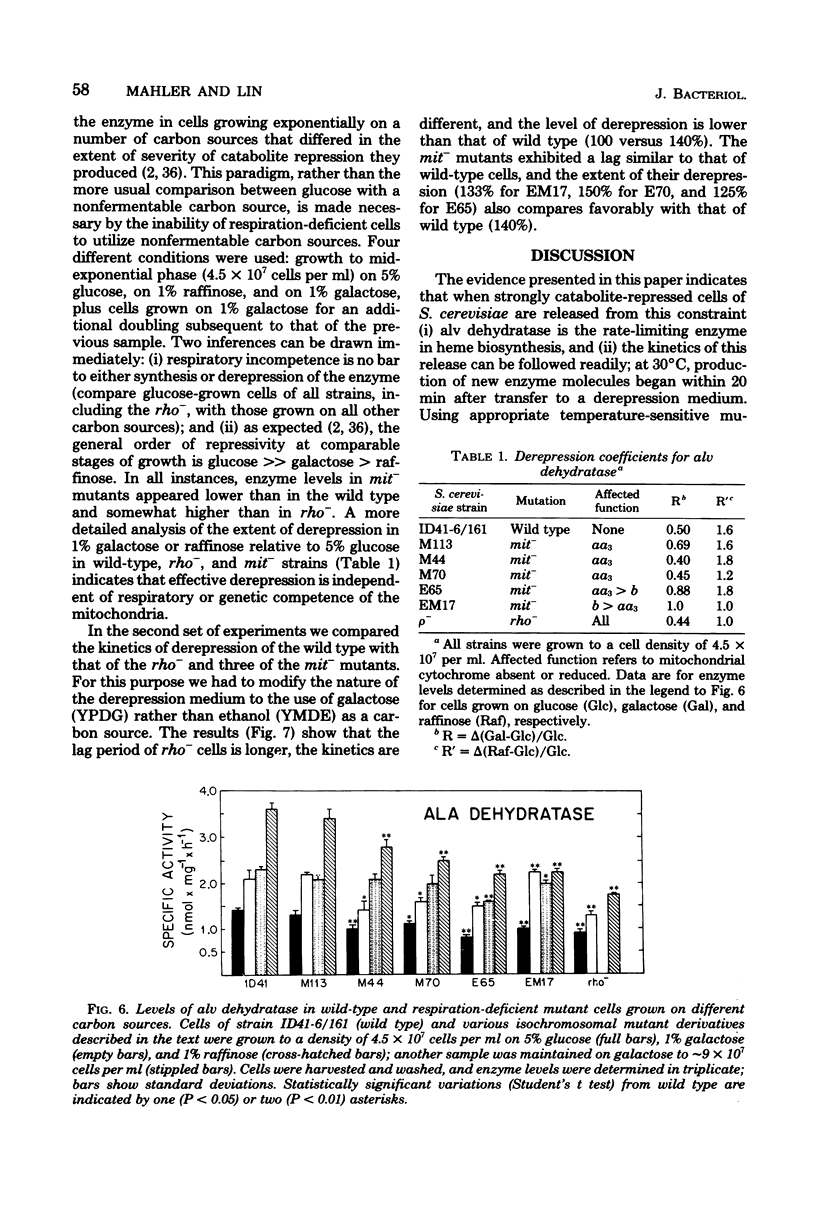
- Mahler H. R., Assimos K., Lin C. C. Derepression in Saccharomyces cerevisiae can be dissociated from cellular proliferation and deoxyribonucleic acid synthesis. J Bacteriol. 1975 Aug;123(2):637–641. doi: 10.1128/jb.123.2.637-641.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler H. R. Biogenetic autonomy of mitochondria. CRC Crit Rev Biochem. 1973 Aug;1(3):381–460. doi: 10.3109/10409237309105439. [DOI] [PubMed] [Google Scholar]
- Mahler H. R., Dawidowicz K. Autonomy of mitochondria in Saccharomyces cerevisiae in their production of messenger RNA. Proc Natl Acad Sci U S A. 1973 Jan;70(1):111–114. doi: 10.1073/pnas.70.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler H. R., Lin C. C. The derepression of delta-aminolevulinate synthetase in yeast. Biochem Biophys Res Commun. 1974 Dec 11;61(3):963–970. doi: 10.1016/0006-291x(74)90249-6. [DOI] [PubMed] [Google Scholar]
- Mahler H. R., Wilkie D. Mitochondrial control of sugar utilization in Saccharomyces cerevisiae. Plasmid. 1978 Feb;1(2):125–133. doi: 10.1016/0147-619x(78)90033-1. [DOI] [PubMed] [Google Scholar]
- Matern H., Holzer H. Catabolite inactivation of the galactose uptake system in yeast. J Biol Chem. 1977 Sep 25;252(18):6399–6402. [PubMed] [Google Scholar]
- Muthukrishnan S., Malathi K., Padmanaban G. Delta-aminolaevulinate dehydratase, the regulatory enzyme of the haem-biosynthetic pathway in Neurospora crassa. Biochem J. 1972 Aug;129(1):31–37. doi: 10.1042/bj1290031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi D. L., Baker-Cohen K. F., Shemin D. Delta-aminolevulinic acid dehydratase of Rhodopseudomonas spheroides. J Biol Chem. 1968 Mar 25;243(6):1224–1230. [PubMed] [Google Scholar]
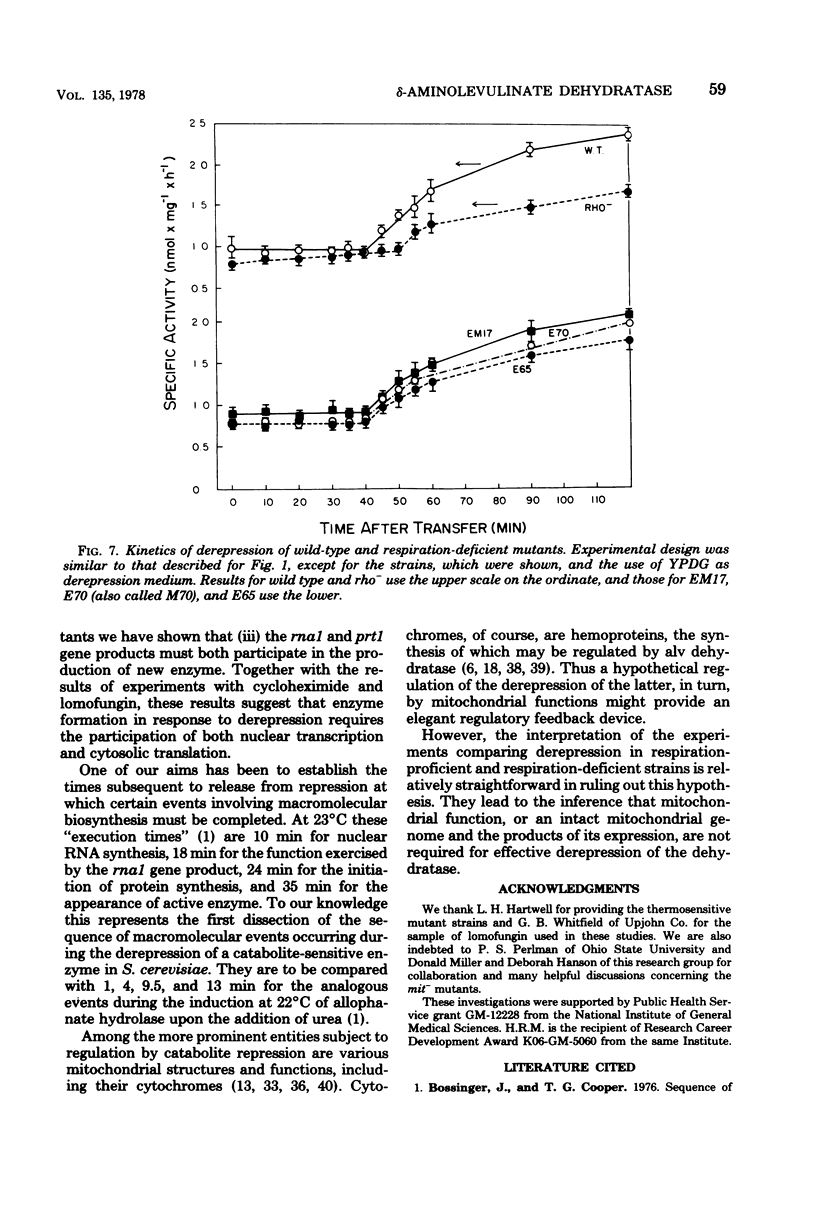
- Perlman P. S., Mahler H. R. Derepression of mitochondria and their enzymes in yeast: regulatory aspects. Arch Biochem Biophys. 1974 May;162(1):248–271. doi: 10.1016/0003-9861(74)90125-8. [DOI] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W. Changes in the enzyme activities of Saccharomyces cerevisiae during aerobic growth on different carbon sources. Biochem J. 1965 Oct;97(1):284–297. doi: 10.1042/bj0970284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi P. P., Algeri A. Role of the mitochondrion in the regulation of protein synthesis in the eucaryote Saccharomyces cerevisiae. Mol Gen Genet. 1971;110(2):110–117. doi: 10.1007/BF00332642. [DOI] [PubMed] [Google Scholar]
- REILLY C., SHERMAN R. GLUCOSE REPRESSION OF CYTOCHROME A-SYNTHESIS IN CYTOCHROME-DEFICIENT MUTANTS OF YEAST. Biochim Biophys Acta. 1965 Apr 19;95:640–651. doi: 10.1016/0005-2787(65)90518-6. [DOI] [PubMed] [Google Scholar]
- Saltzgaber-Müller J., Schatz G. Heme is necessary for the accumulation and assembly of cytochrome c oxidase subunits in Saccharomyces cerevisiae. J Biol Chem. 1978 Jan 10;253(1):305–310. [PubMed] [Google Scholar]
- Sanders H. K., Mied P. A., Briquet M., Hernandez-Rodriguez J., Gottal R. F., Mattoon J. R. Regulation of mitochondrial biogenesis: yeast mutants deficient in synthesis of delta-aminolevulinic acid. J Mol Biol. 1973 Oct 15;80(1):17–39. doi: 10.1016/0022-2836(73)90231-3. [DOI] [PubMed] [Google Scholar]
- Slonimski P. P., Tzagoloff A. Localization in yeast mitochondrial DNA of mutations expressed in a deficiency of cytochrome oxidase and/or coenzyme QH2-cytochrome c reductase. Eur J Biochem. 1976 Jan 2;61(1):27–41. doi: 10.1111/j.1432-1033.1976.tb09994.x. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Akai A., Needleman R. B., Zulch G. Assembly of the mitochondrial membrane system. Cytoplasmic mutants of Saccharomyces cerevisiae with lesions in enzymes of the respiratory chain and in the mitochondrial ATPase. J Biol Chem. 1975 Oct 25;250(20):8236–8242. [PubMed] [Google Scholar]
- Woods R. A., Sanders H. K., Briquet M., Foury F., Drysdale B. E., Mattoon J. R. Regulation of mitochondrial biogenesis: enzymatic changes in cytochrome-deficient yeast mutants requiring delta-aminolevulinic acid. J Biol Chem. 1975 Dec 10;250(23):9090–9098. [PubMed] [Google Scholar]



