Abstract
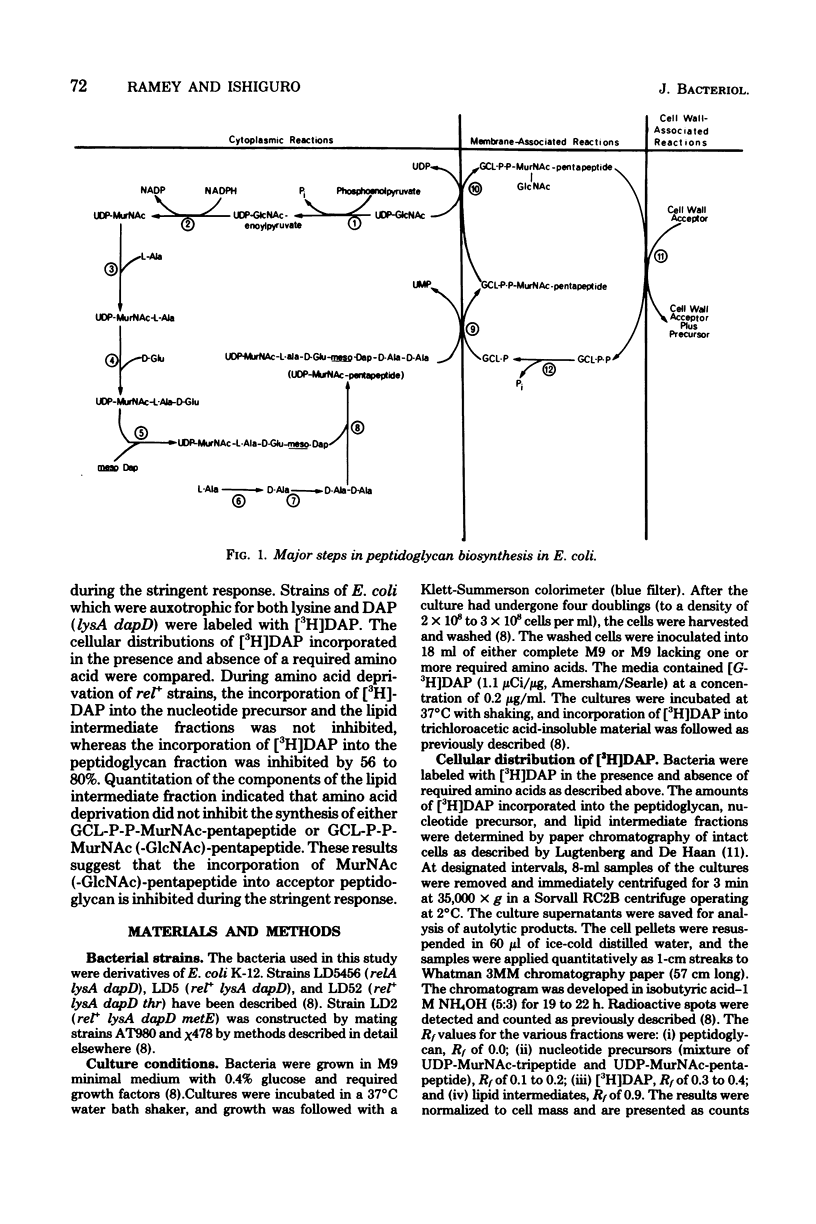
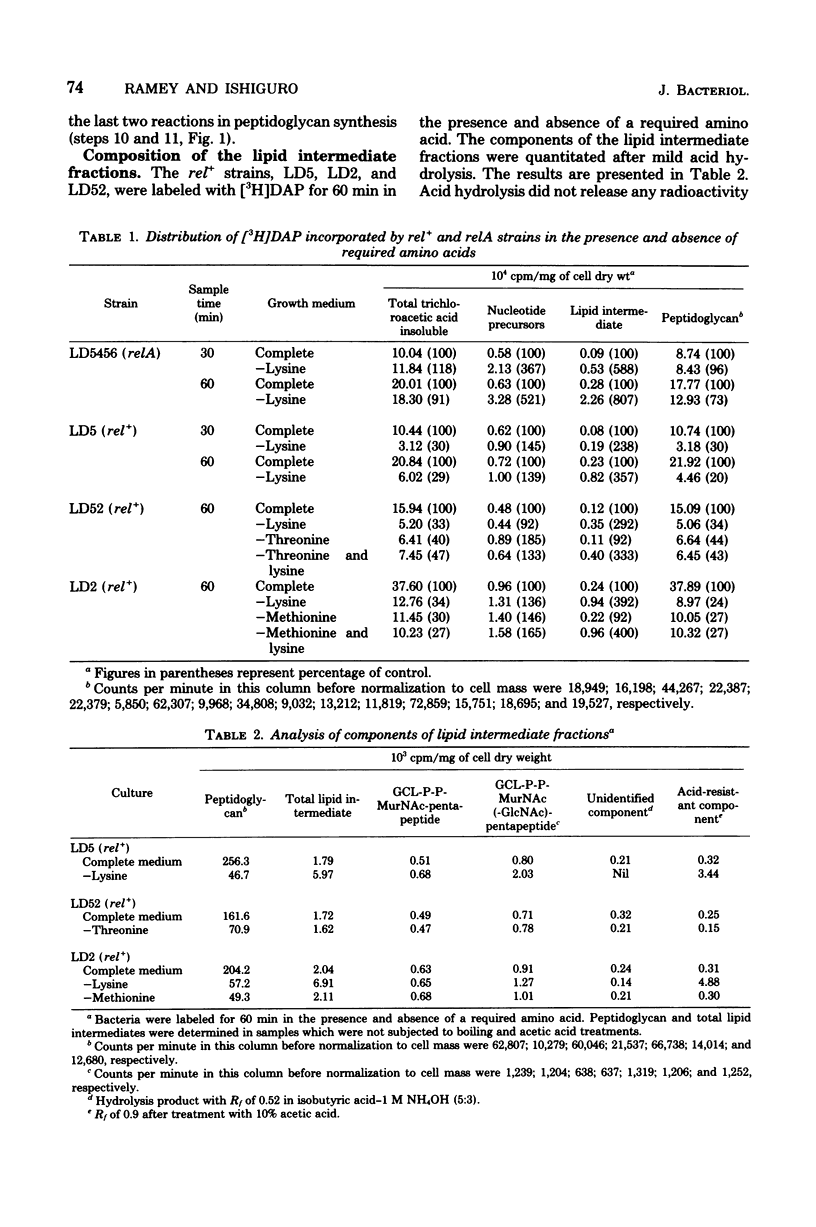
The site of inhibition of peptidoglycan synthesis during the stringent response in Escherichia coli was determined in strains which were auxotrophic for both lysine and diaminopimelic acid (DAP). Cells were labeled with [3H]DAP for 30 to 60 min in the presence and absence of required amino acids, and the cellular distribution of [3H]DAP was determined. In both stringent (rel+) and relaxed (relA) strains, amino acid deprivation did not inhibit the incorporation of [3H]DAP into the nucleotide precursor and lipid intermediate fractions. The amount of [3H]DAP incorporated into the peptidoglycan fraction by the amino acid-deprived relA strain was over 70% of the amount incorporated in the presence of required amino acids. In contrast, the amounts of labeled peptidoglycan in amino acid-deprived rel+ strains were only 20 to 44% of the amounts synthesized in the presence of amino acids. These results indicate that a late step in peptidoglycan synthesis is inhibited during the stringent response. The components of the lipid intermediate fraction synthesized by rel+ strains in the presence and absence of required amino acids were quantitated. Amino acid deprivation did not inhibit the synthesis of either the monosaccharide-pentapeptide or the disaccharide-pentapeptide derivatives of the lipid intermediate. Thus, the reaction which is most likely inhibited during the stringent response is the terminal one involving the incorporation of the disaccharide-pentapeptide into peptidoglycan.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. S., Matsuhashi M., Haskin M. A., Strominger J. L. Biosythesis of the peptidoglycan of bacterial cell walls. II. Phospholipid carriers in the reaction sequence. J Biol Chem. 1967 Jul 10;242(13):3180–3190. [PubMed] [Google Scholar]
- Braun V., Bosch V. In vivo biosynthesis of murein-lipoprotein of the outer membrane of E. coli. FEBS Lett. 1973 Aug 15;34(2):302–306. doi: 10.1016/0014-5793(73)80817-8. [DOI] [PubMed] [Google Scholar]
- Braun V., Wolff H. Attachment of lipoprotein to murein (peptidoglycan) of Escherichia coli in the presence and absence of penicillin FL 1060. J Bacteriol. 1975 Sep;123(3):888–897. doi: 10.1128/jb.123.3.888-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M. Regulation of bacterial ppGpp and pppGpp. Annu Rev Microbiol. 1975;29:301–318. doi: 10.1146/annurev.mi.29.100175.001505. [DOI] [PubMed] [Google Scholar]
- Dennis P. P., Bremer H. Macromolecular composition during steady-state growth of Escherichia coli B-r. J Bacteriol. 1974 Jul;119(1):270–281. doi: 10.1128/jb.119.1.270-281.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs-Cleveland E., Gilvarg C. Oligomeric intermediate in peptidoglycan biosynthesis in Bacillus megaterium. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4200–4204. doi: 10.1073/pnas.73.11.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro E. E., Ramey W. D. Stringent control of peptidoglycan biosynthesis in Escherichia coli K-12. J Bacteriol. 1976 Sep;127(3):1119–1126. doi: 10.1128/jb.127.3.1119-1126.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- Kelleher R. J., Jr, Heggeness M. Repression of diaminopimelic acid decarboxylase in Escherichia coli: gene dosage effects and escape synthesis. J Bacteriol. 1976 Jan;125(1):376–378. doi: 10.1128/jb.125.1.376-378.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., De Haas-Menger L., Ruyters W. H. Murein synthesis and identification of cell wall precursors of temperature-sensitive lysis mutants of Escherichia coli. J Bacteriol. 1972 Jan;109(1):326–335. doi: 10.1128/jb.109.1.326-335.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., de Haan P. G. A simple method for following the fate of alanine-containing components in murein synthesis in Escherichia coli. Antonie Van Leeuwenhoek. 1971;37(4):537–552. doi: 10.1007/BF02218524. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Yashouv-Gan Y., Schwarz U. Regulation of murein biosynthesis and septum formation in filamentous cells of Escherichia coli PAT 84. J Bacteriol. 1977 Mar;129(3):1593–1600. doi: 10.1128/jb.129.3.1593-1600.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis S. E., Guchhait R. B., Lane M. D. Stringent control of fatty acid synthesis in Escherichia coli. Possible regulation of acetyl coenzyme A carboxylase by ppGpp. J Biol Chem. 1973 Nov 25;248(22):7957–7966. [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- Torti S. V., Park J. T. Lipoprotein of gram-negative bacteria is essential for growth and division. Nature. 1976 Sep 23;263(5575):323–326. doi: 10.1038/263323a0. [DOI] [PubMed] [Google Scholar]
- WHITE P. J., KELLY B. PURIFICATION AND PROPERTIES OF DIAMINOPIMELATE DECARBOXYLASE FROM ESCHERICHIA COLI. Biochem J. 1965 Jul;96:75–84. doi: 10.1042/bj0960075. [DOI] [PMC free article] [PubMed] [Google Scholar]



