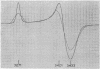
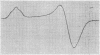
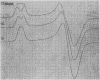
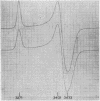
Full text
PDF


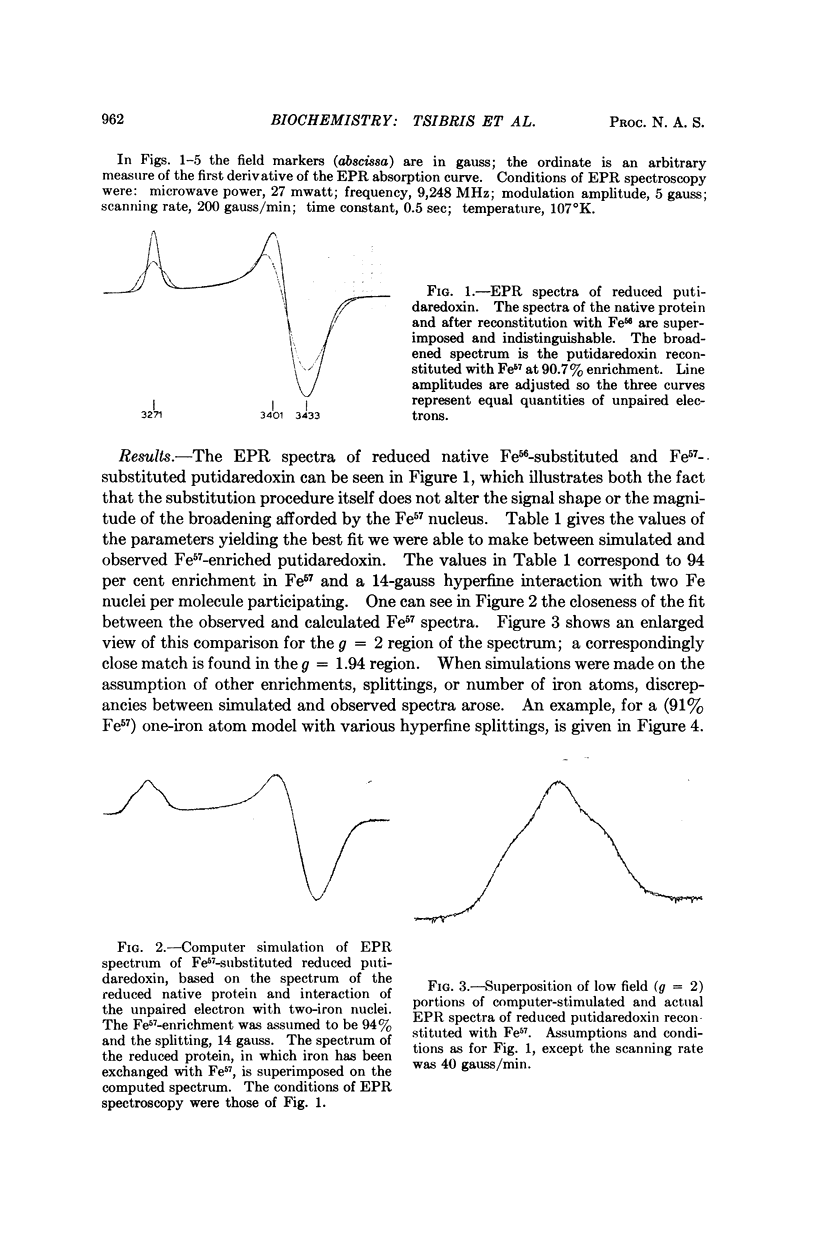
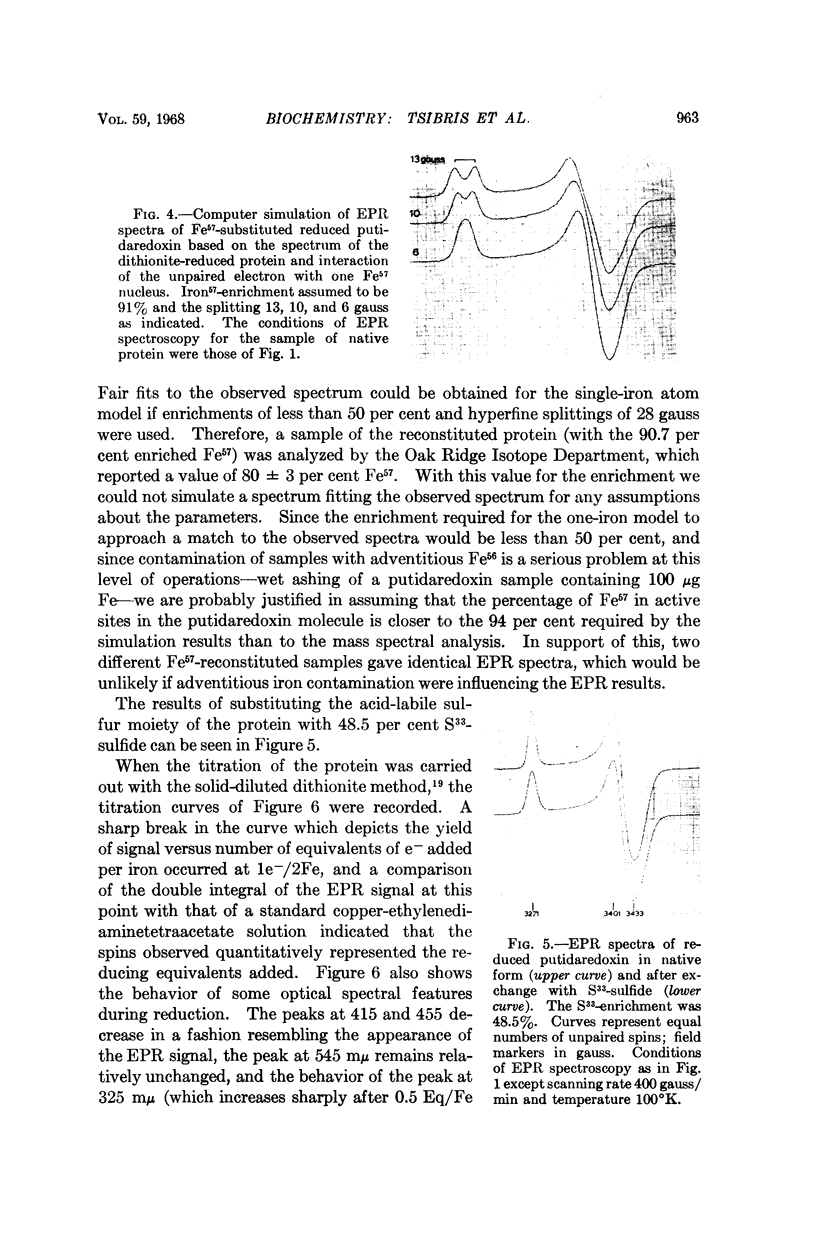
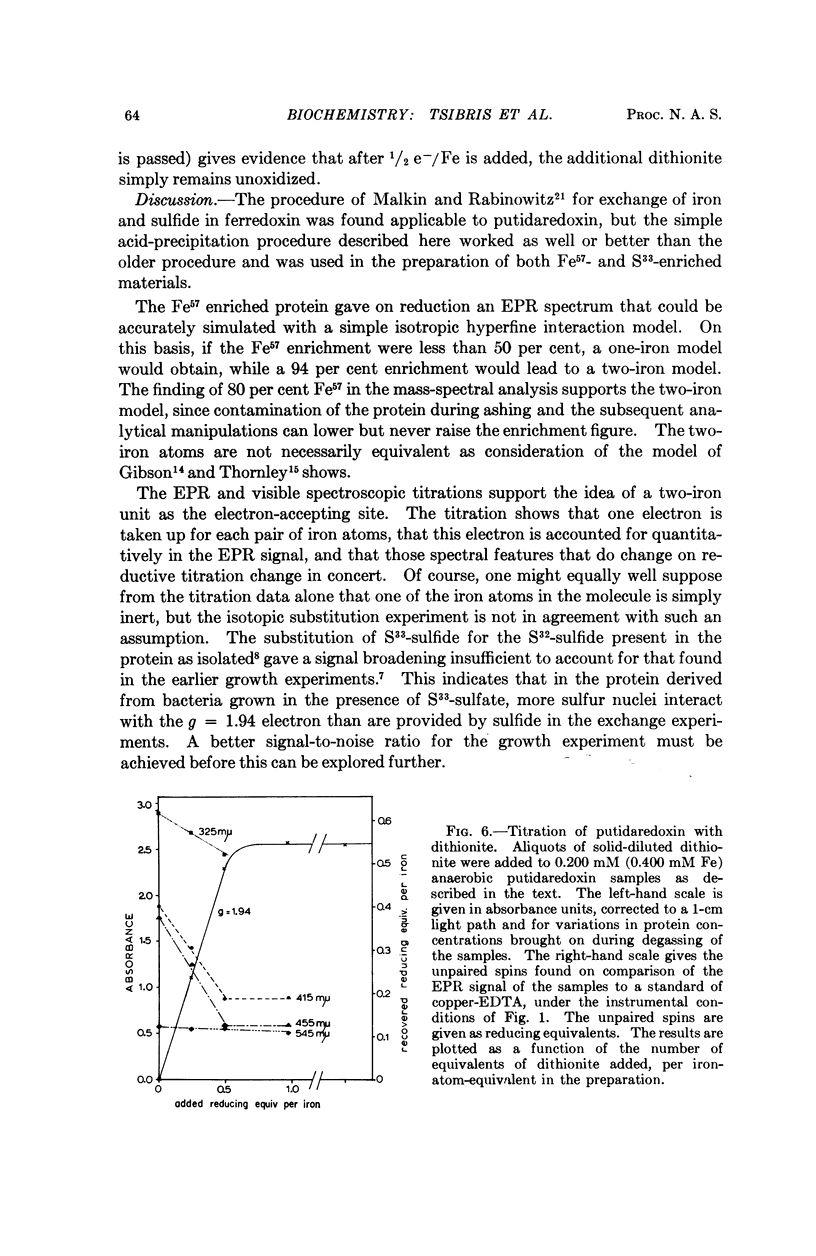

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEINERT H., DERVARTANIAN D. V., HEMMERICH P., VEEGER C., VAN VOORSTJ ON THE LIGAND FIELD OF REDOX ACTIVE NON-HEME IRON IN PROTEINS. Biochim Biophys Acta. 1965 Mar 22;96:530–533. doi: 10.1016/0005-2787(65)90573-3. [DOI] [PubMed] [Google Scholar]
- Beinert H., Palmer G. Contributions of EPR spectroscopy to our knowledge of oxidative enzymes. Adv Enzymol Relat Areas Mol Biol. 1965;27:105–198. doi: 10.1002/9780470122723.ch3. [DOI] [PubMed] [Google Scholar]
- Brintzinger H., Palmer G., Sands R. H. On the ligand field of iron in ferredoxin from spinach chloroplasts and related nonheme iron enzymes. Proc Natl Acad Sci U S A. 1966 Feb;55(2):397–404. doi: 10.1073/pnas.55.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman D. W., Tsai R. L., Gunsalus I. C. The ferroprotein component of a methylene hydroxylase. Biochem Biophys Res Commun. 1967 Mar 9;26(5):577–583. doi: 10.1016/0006-291x(67)90104-0. [DOI] [PubMed] [Google Scholar]
- DerVartanian D. V., Orme-Johnson W. H., Hansen R. E., Beinert H. Identification of sulfur as component of the EPR signal at g equals 1.94 by isotopic substitution. Biochem Biophys Res Commun. 1967 Mar 9;26(5):569–576. doi: 10.1016/0006-291x(67)90103-9. [DOI] [PubMed] [Google Scholar]
- Gibson J. F., Hall D. O., Thornley J. H., Whatley F. R. The iron complex in spinach ferredoxin. Proc Natl Acad Sci U S A. 1966 Sep;56(3):987–990. doi: 10.1073/pnas.56.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen R. E., Kalal T. T., Beinert H. A gas-flow system for control of the temperature of an electron paramagnetic resonance sample at temperatures between 80 and 273 degrees Kelvin. Anal Biochem. 1967 Jul;20(1):40–50. doi: 10.1016/0003-2697(67)90263-1. [DOI] [PubMed] [Google Scholar]
- Malkin R., Rabinowitz J. C. The reconstitution of clostridial ferredoxin. Biochem Biophys Res Commun. 1966 Jun 21;23(6):822–827. doi: 10.1016/0006-291x(66)90561-4. [DOI] [PubMed] [Google Scholar]
- Palmer G., Sands R. H. On the magnetic resonance of spinach ferredoxin. J Biol Chem. 1966 Jan 10;241(1):253–253. [PubMed] [Google Scholar]
- Röder A., Bayer E. ESR studies of model complexes for non-heme iron proteins. Angew Chem Int Ed Engl. 1967 Mar;6(3):263–264. doi: 10.1002/anie.196702631. [DOI] [PubMed] [Google Scholar]
- SHETHNA Y. I., WILSON P. W., HANSEN R. E., BEINERT H. IDENTIFICATION BY ISOTOPIC SUBSTITUTION OF THE EPR SIGNAL AT G = 1.94 IN A NON-HEME IRON PROTEIN FROM AZOTOBACTER. Proc Natl Acad Sci U S A. 1964 Nov;52:1263–1271. doi: 10.1073/pnas.52.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornley J. H., Gibson J. F., Whatley F. R., Hall D. O. Comment on a recent model of the iron complex in spinach ferredoxin. Biochem Biophys Res Commun. 1966 Sep 22;24(6):877–879. doi: 10.1016/0006-291x(66)90330-5. [DOI] [PubMed] [Google Scholar]
- Watari H., Kimura T. Study of the adrenal non-heme iron protein (adrenodoxin) by electron spin resonance. Biochem Biophys Res Commun. 1966 Jul 6;24(1):106–112. doi: 10.1016/0006-291x(66)90417-7. [DOI] [PubMed] [Google Scholar]