Abstract
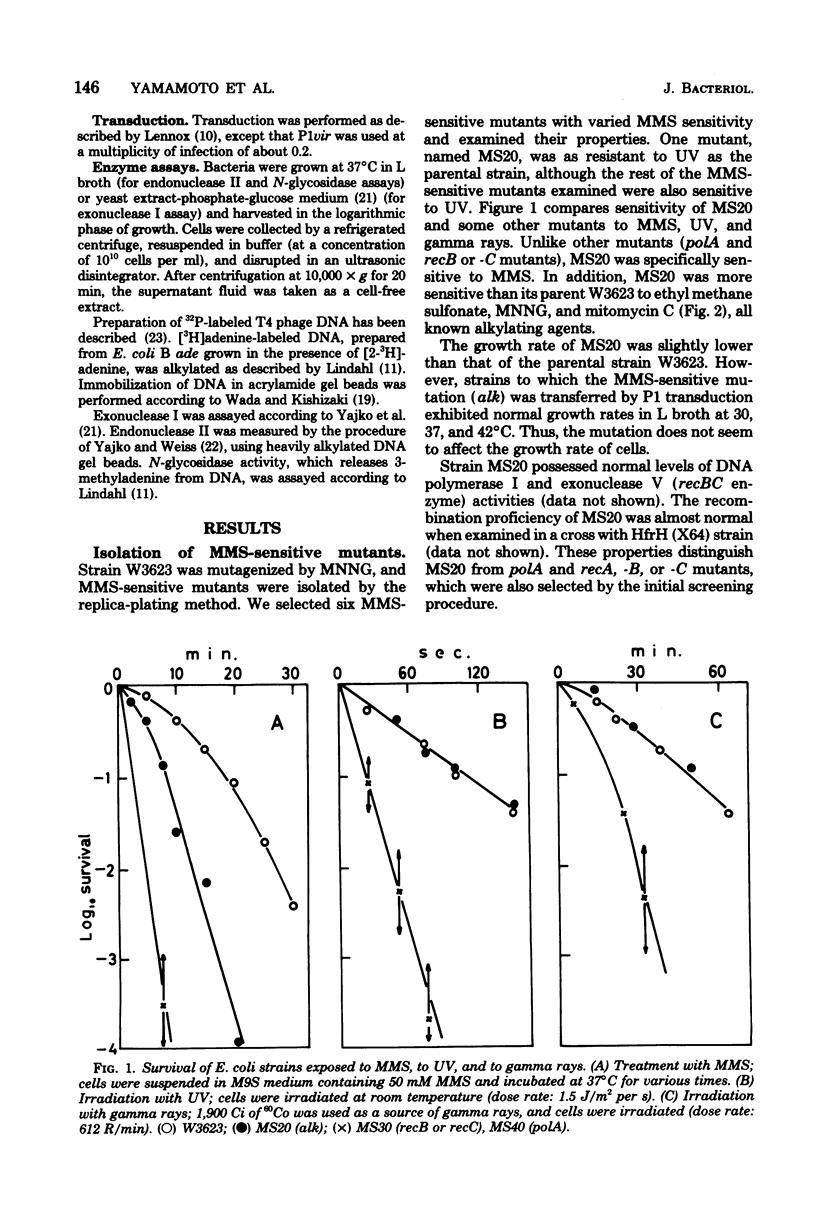
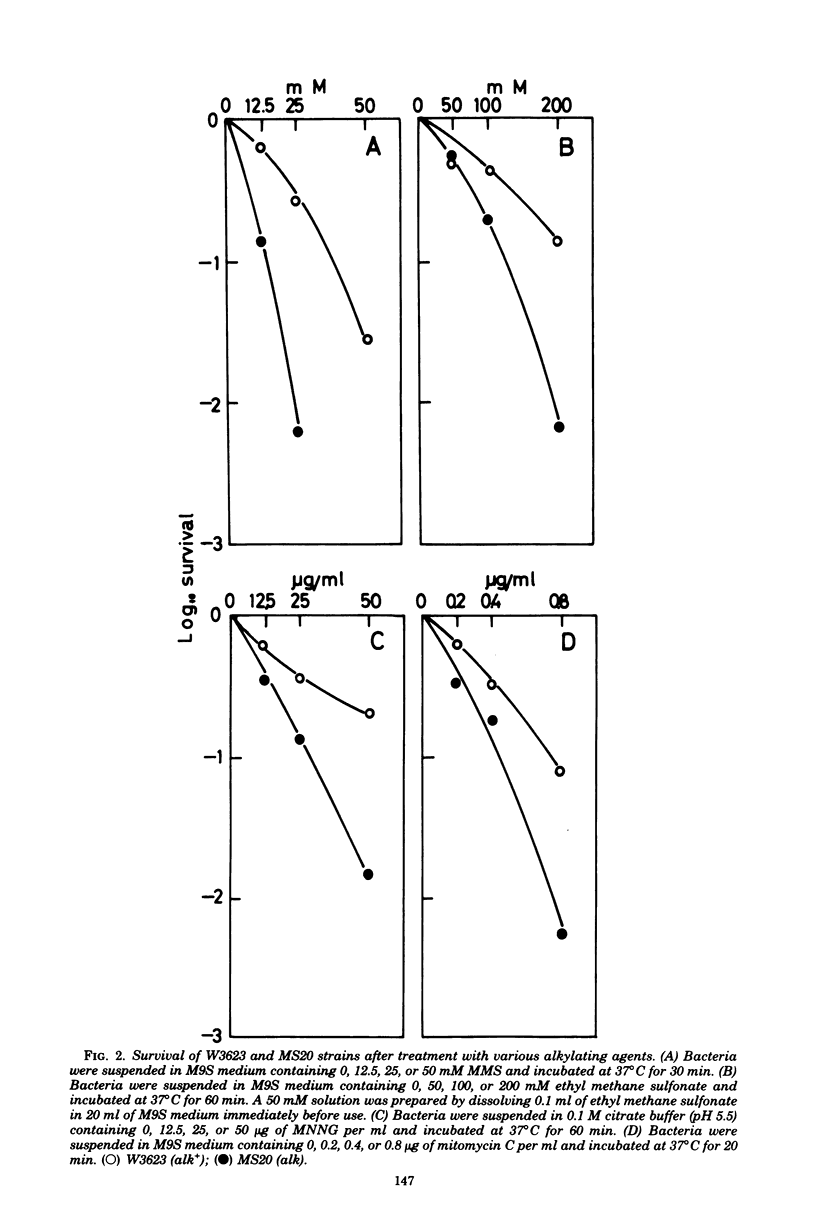
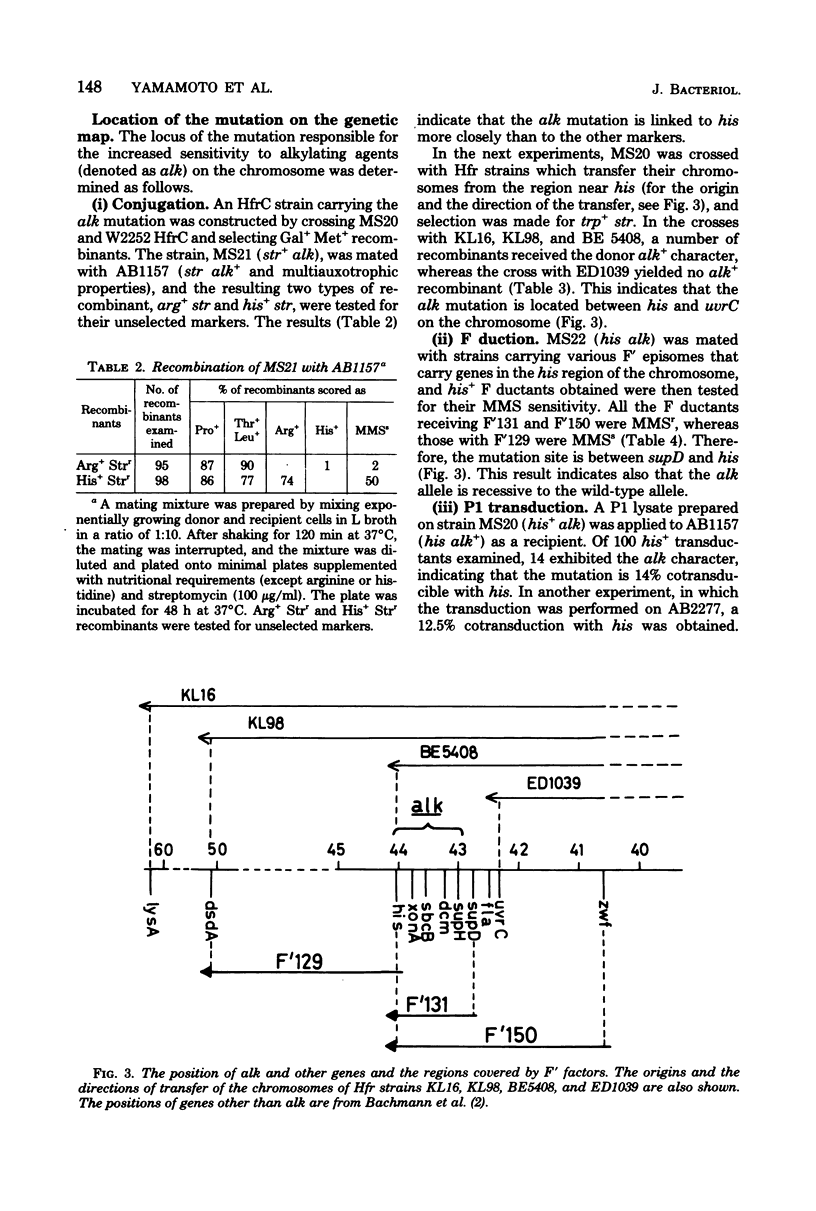
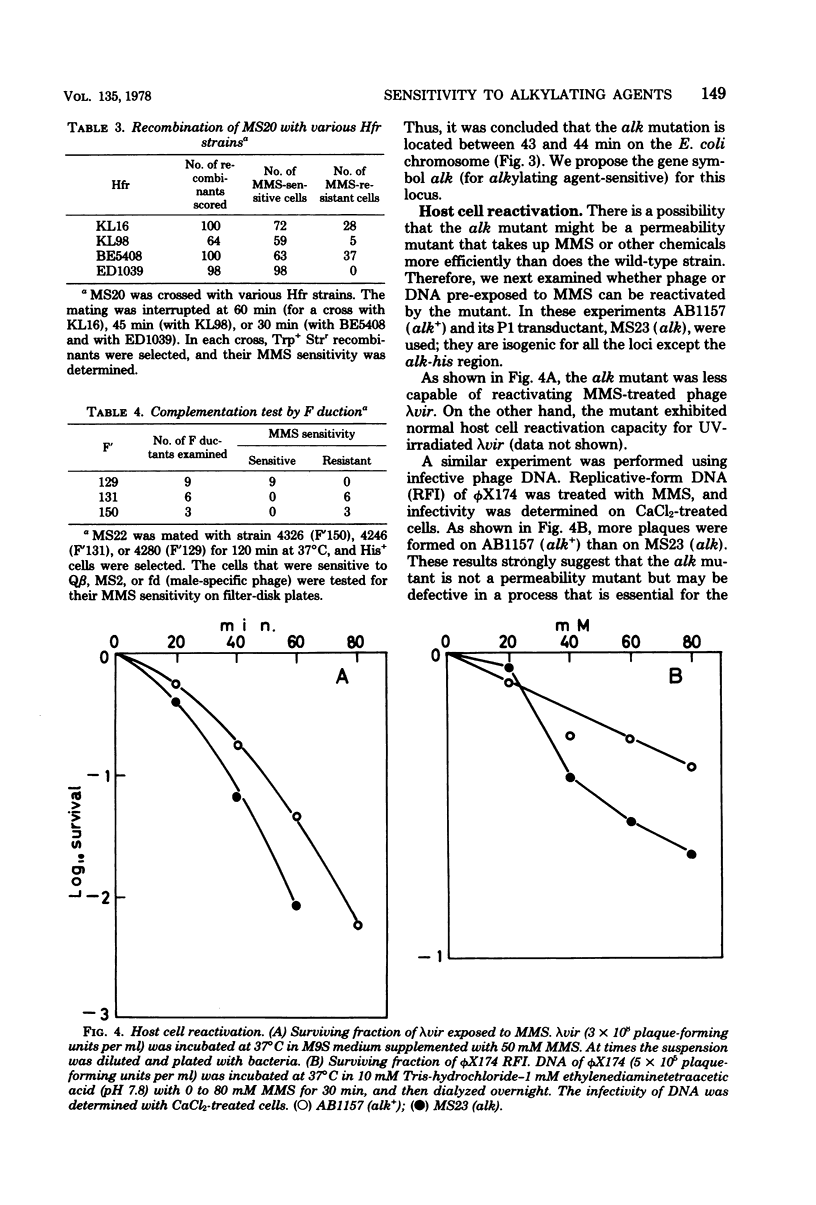
A new type of Escherichia coli mutant which shows increased sensitivity to methyl methane sulfonate but not to UV light or to gamma rays was isolated after mutagenesis with N-methyl-N'-nitro-N-nitrosoguanidine. The mutant is unable to reactivate phage lambdavir or double-stranded phiX174 DNA (replicative form) that had been treated with methyl methane sulfonate. The mutant is sensitive to other alkylating agents, such as ethyl methane sulfonate, mitomycin C, and N-methyl-N'-nitro-N-nitrosoguanidine, as well. It grows normally and exhibits almost normal recombination proficiency. The mutant possesses normal levels of DNA polymerase I, exonuclease I, exonuclease V, endonuclease specific for methyl methane sulfonate-treated DNA, and 3-methyladenine-DNA glycosidase activities. The genetic locus responsible has been named alk and is located near his on the chromosome.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Friedberg E. C., Goldthwait D. A. Endonuclease II of E. coli. I. Isolation and purification. Proc Natl Acad Sci U S A. 1969 Mar;62(3):934–940. doi: 10.1073/pnas.62.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J., Gross M. Genetic analysis of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1166–1168. doi: 10.1038/2241166a0. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- Kirtikar D. M., Cathcart G. R., Goldthwait D. A. Endonuclease II, apurinic acid endonuclease, and exonuclease III. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4324–4328. doi: 10.1073/pnas.73.12.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtikar D. M., Goldthwait D. A. The enzymatic release of O6-methylguanine and 3-methyladenine from DNA reacted with the carcinogen N-methyl-N-nitrosourea. Proc Natl Acad Sci U S A. 1974 May;71(5):2022–2026. doi: 10.1073/pnas.71.5.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Templin A., Clark A. J. Genetic recombination in Escherichia coli: the role of exonuclease I. Proc Natl Acad Sci U S A. 1971 Apr;68(4):824–827. doi: 10.1073/pnas.68.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lawley P. D. Some chemical aspects of dose-response relationships in alkylation mutagenesis. Mutat Res. 1974 Jun;23(3):283–295. doi: 10.1016/0027-5107(74)90102-x. [DOI] [PubMed] [Google Scholar]
- Lindahl T. New class of enzymes acting on damaged DNA. Nature. 1976 Jan 1;259(5538):64–66. doi: 10.1038/259064a0. [DOI] [PubMed] [Google Scholar]
- Ljungquist S. A new endonuclease from Escherichia coli acting at apurinic sites in DNA. J Biol Chem. 1977 May 10;252(9):2808–2814. [PubMed] [Google Scholar]
- Ljungquist S., Lindahl T., Howard-Flanders P. Methyl methane sulfonate-sensitive mutant of Escherichia coli deficient in an endonuclease specific for apurinic sites in deoxyribonucleic acid. J Bacteriol. 1976 May;126(2):646–653. doi: 10.1128/jb.126.2.646-653.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Otsuji N., Iyehara H., Hideshima Y. Isolation and characterization of an Escherichia coli ruv mutant which forms nonseptate filaments after low doses of ultraviolet light irradiation. J Bacteriol. 1974 Feb;117(2):337–344. doi: 10.1128/jb.117.2.337-344.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Sekiguchi M. Studies on temperature-dependent ultraviolet light-sensitive mutants of bacteriophage T4: the structural gene for T4 endonuclease V. J Mol Biol. 1976 Mar 25;102(1):15–26. doi: 10.1016/0022-2836(76)90071-1. [DOI] [PubMed] [Google Scholar]
- Tanaka J. I., Sekiguchi M. Action of exonuclease V (the recBC enzyme) on ultraviolet-irradiated DNA. Biochim Biophys Acta. 1975 Mar 10;383(2):178–187. doi: 10.1016/0005-2787(75)90259-2. [DOI] [PubMed] [Google Scholar]
- Verly W. G., Rassart E. Purification of Escherichia coli endonuclease specific for apurinic sites in DNA. J Biol Chem. 1975 Oct 25;250(20):8214–8219. [PubMed] [Google Scholar]
- Wada A., Kishizaki A. Chromatographic studies with immobilized polynucleotide in acrylamide gel matrix. Biochim Biophys Acta. 1968 Aug 23;166(1):29–39. doi: 10.1016/0005-2787(68)90487-5. [DOI] [PubMed] [Google Scholar]
- White B. J., Hochhauser S. J., Cintron N. M., Weiss B. Genetic mapping of xthA, the structural gene for exonuclease III in Escherichia coli K-12. J Bacteriol. 1976 Jun;126(3):1082–1088. doi: 10.1128/jb.126.3.1082-1088.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajko D. M., Valentine M. C., Weiss B. Mutants of Escherichia coli with altered deoxyribonucleases. II. Isolation and characterization of mutants for exonuclease I. J Mol Biol. 1974 May 15;85(2):323–343. doi: 10.1016/0022-2836(74)90367-2. [DOI] [PubMed] [Google Scholar]
- Yajko D. M., Weiss B. Mutations simultaneously affecting endonuclease II and exonuclease III in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Feb;72(2):688–692. doi: 10.1073/pnas.72.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S., Sekiguchi M. T4 endonuclease involved in repair of DNA. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1839–1845. doi: 10.1073/pnas.67.4.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]



