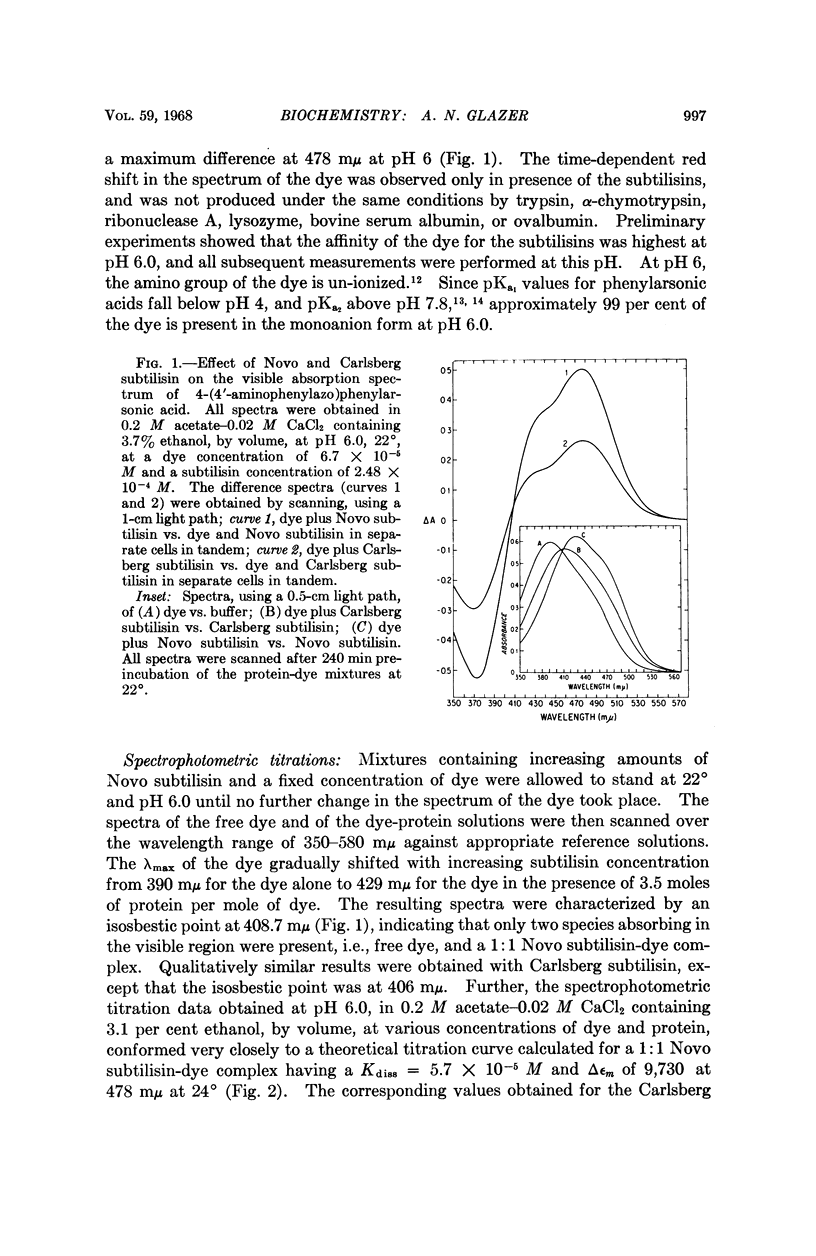
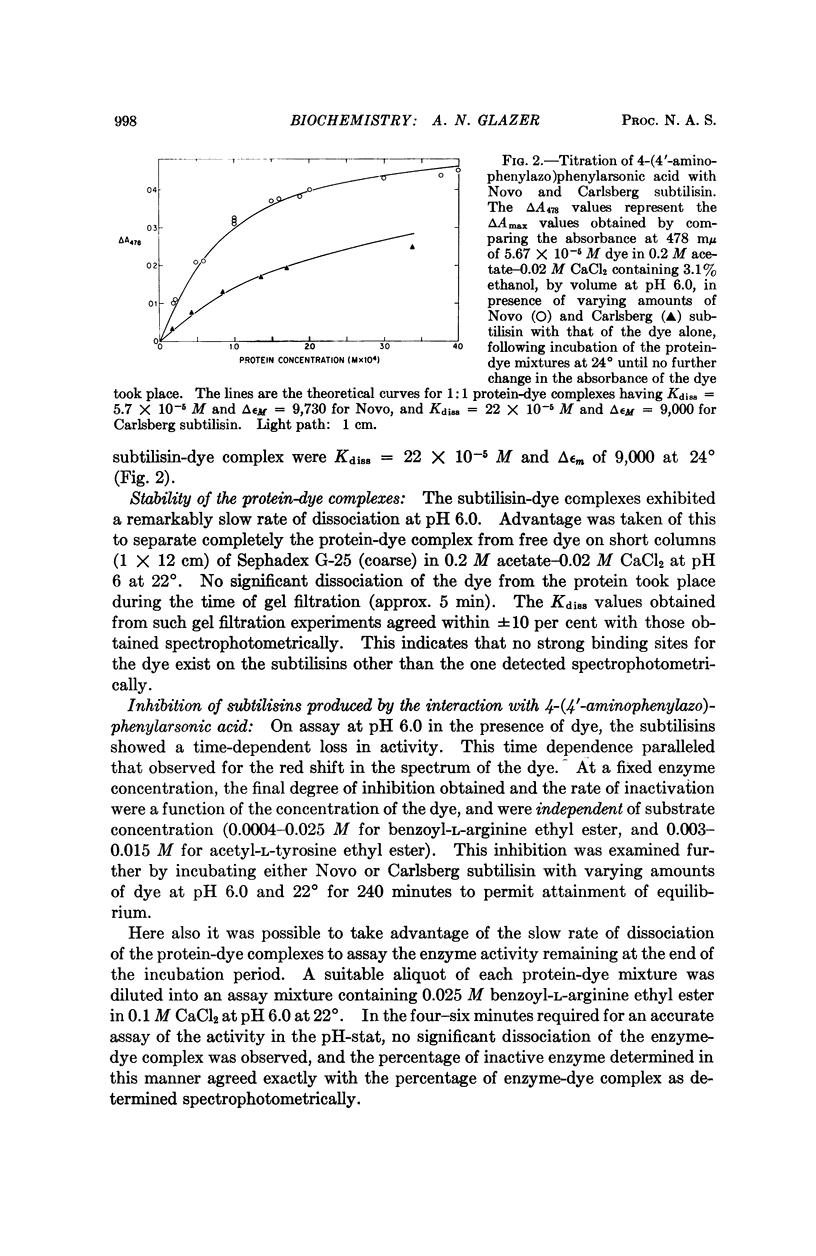
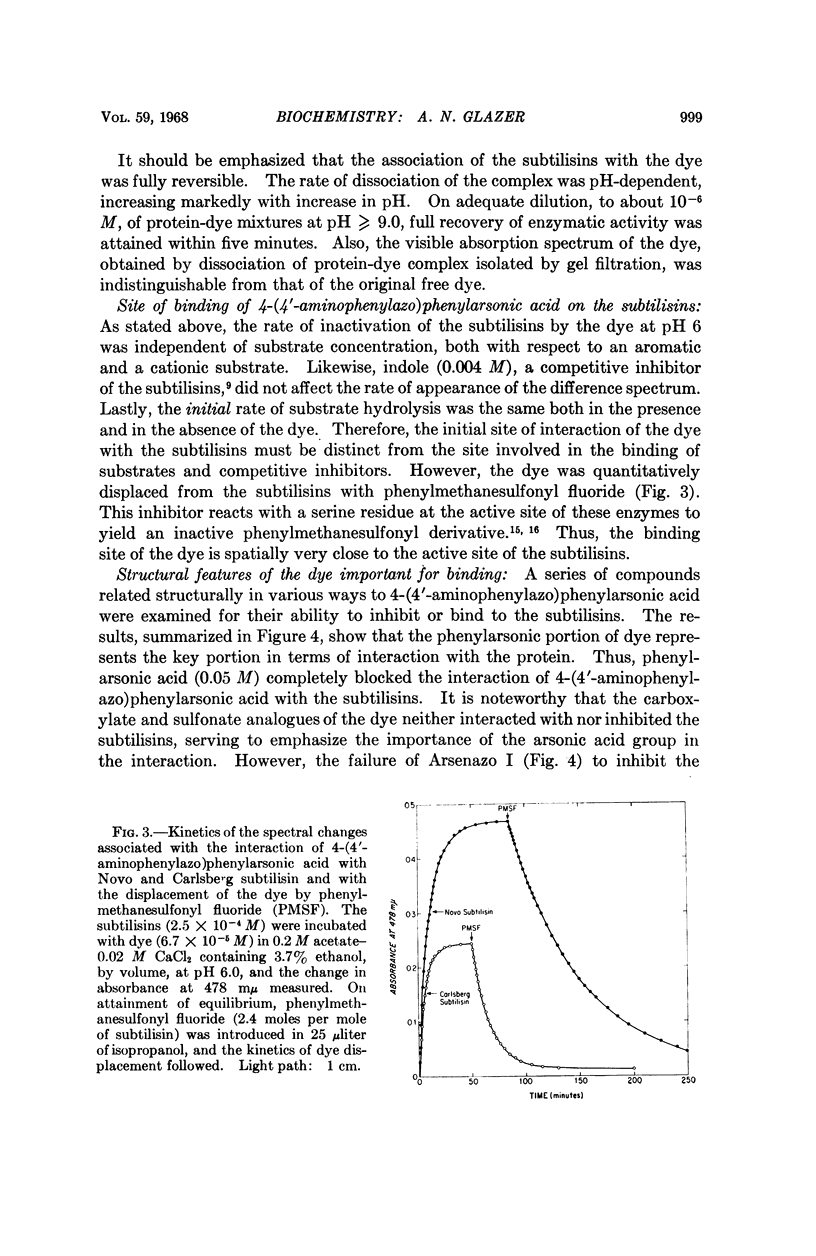
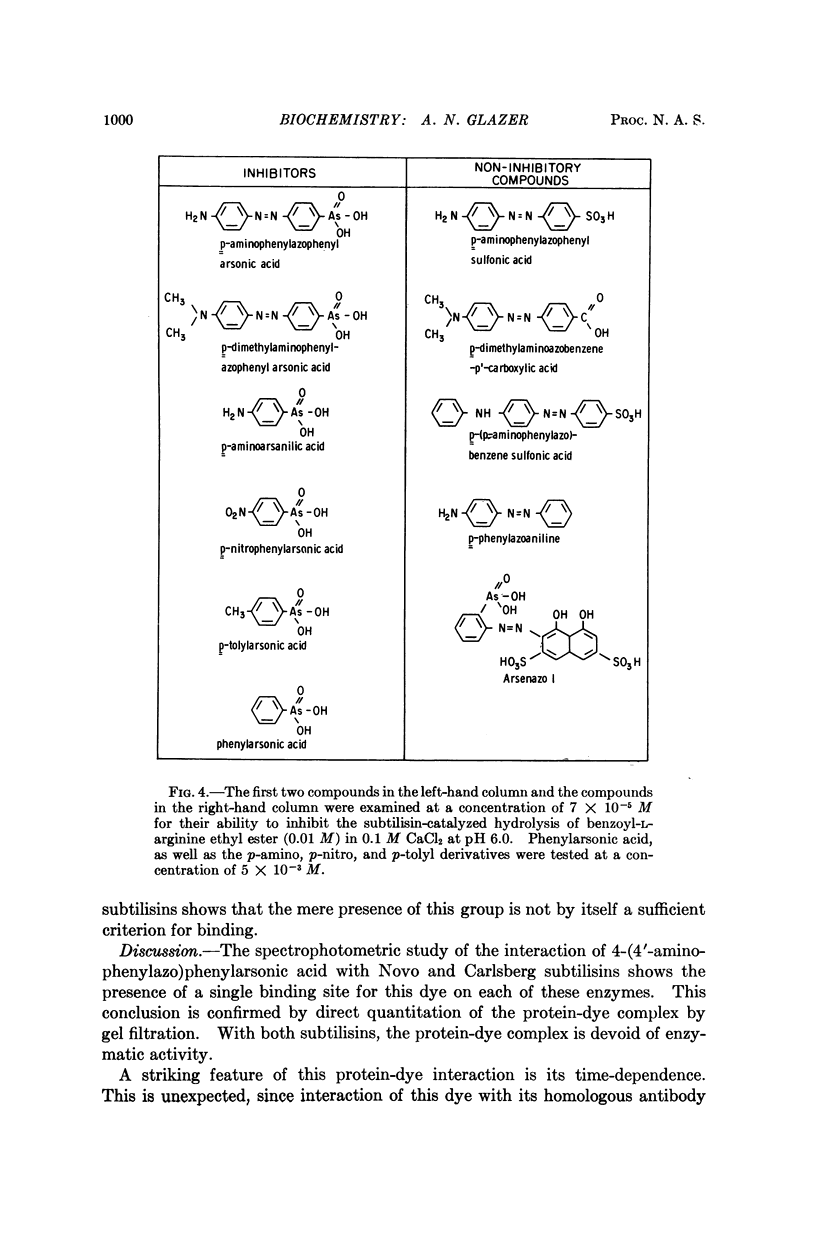
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernhard S. A., Gutfreund H. The optical detection of transients in trypsin- and chymotrypsin-catalyzed reactions. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1238–1243. doi: 10.1073/pnas.53.6.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard S. A., Lee B. F., Tashjian Z. H. On the interaction of the active side of alpha-chymotrypsin with chromophores: proflavin binding and enzyme conformation during catalysis. J Mol Biol. 1966 Jul;18(3):405–420. doi: 10.1016/s0022-2836(66)80033-5. [DOI] [PubMed] [Google Scholar]
- Feinstein G., Feeney R. E. Binding of proflavine to alpha-chymotrypsin and trypsin and its displacement by avian ovomucoids.. Biochemistry. 1967 Mar;6(3):749–753. doi: 10.1021/bi00855a015. [DOI] [PubMed] [Google Scholar]
- Glazer A. N. Esteratic reactions catalyzed by subtilisins. J Biol Chem. 1967 Feb 10;242(3):433–436. [PubMed] [Google Scholar]
- Glazer A. N. Specific binding of thionine to the active site of trypsin. J Biol Chem. 1967 Jul 25;242(14):3326–3331. [PubMed] [Google Scholar]
- Glazer A. N. Spectral studies of the interaction of alpha-chymotrypsin and trypsin with proflavine. Proc Natl Acad Sci U S A. 1965 Jul;54(1):171–176. doi: 10.1073/pnas.54.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley B. S., Brown J. R., Kauffman D. L., Smillie L. B. Evolutionary similarities between pancreatic proteolytic enzymes. Nature. 1965 Sep 11;207(5002):1157–1159. doi: 10.1038/2071157a0. [DOI] [PubMed] [Google Scholar]
- Havsteen B. H. The kinetics of the two-step interaction of chymotrypsin with proflavin. J Biol Chem. 1967 Feb 25;242(4):769–771. [PubMed] [Google Scholar]
- KREITER V. P., PRESSMAN D. Antibodies formed in response to individual ionization states of benzenearsonic acid. Biochemistry. 1963 Jan-Feb;2:97–97. doi: 10.1021/bi00901a018. [DOI] [PubMed] [Google Scholar]
- MATSUBARA H., KASPER C. B., BROWN D. M., SMITH E. L. SUBTILISIN BPN'. I. PHYSICAL PROPERTIES AND AMINO ACID COMPOSITION. J Biol Chem. 1965 Mar;240:1125–1130. [PubMed] [Google Scholar]
- Neet K. E., Koshland D. E., Jr The conversion of serine at the active site of subtilisin to cysteine: a "chemical mutation". Proc Natl Acad Sci U S A. 1966 Nov;56(5):1606–1611. doi: 10.1073/pnas.56.5.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. L., Markland F. S., Kasper C. B., DeLange R. J., Landon M., Evans W. H. The complete amino acid sequence of two types of subtilisin, BPN' and Carlsberg. J Biol Chem. 1966 Dec 25;241(24):5974–5976. [PubMed] [Google Scholar]



