Abstract
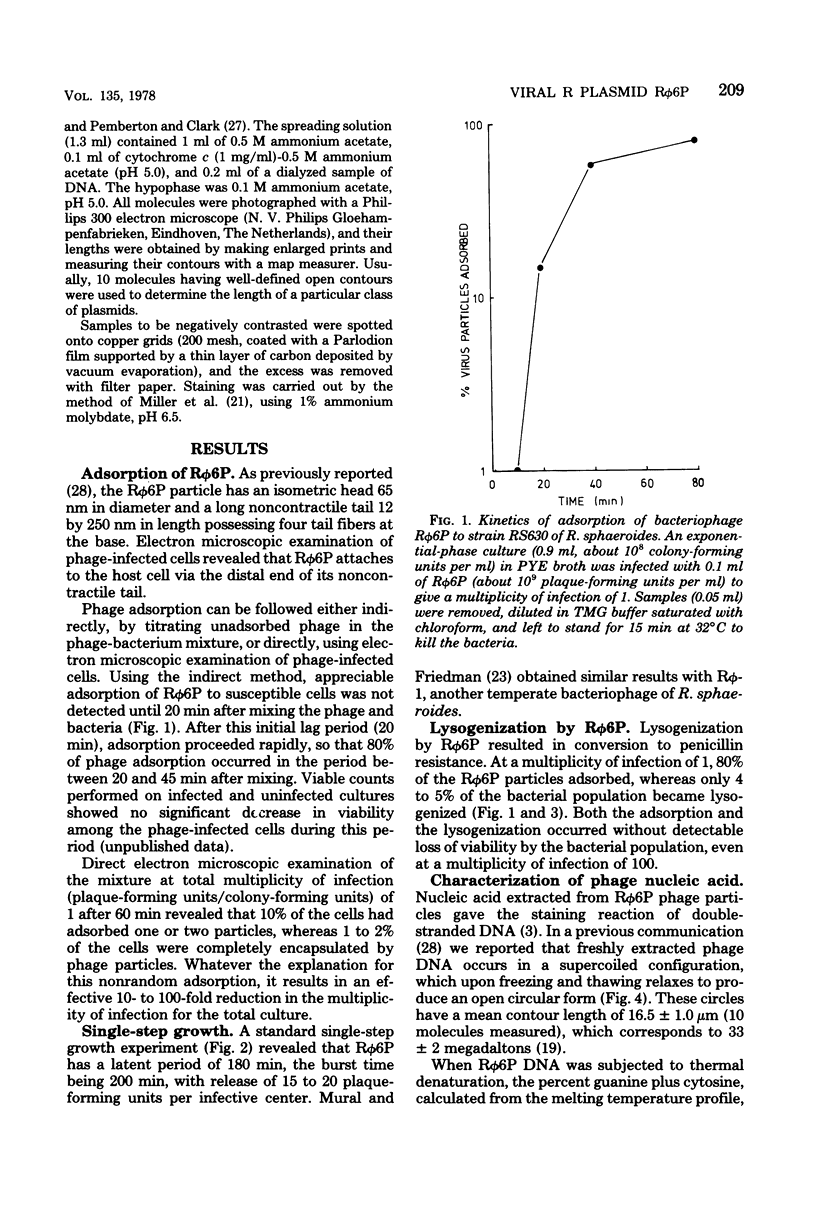
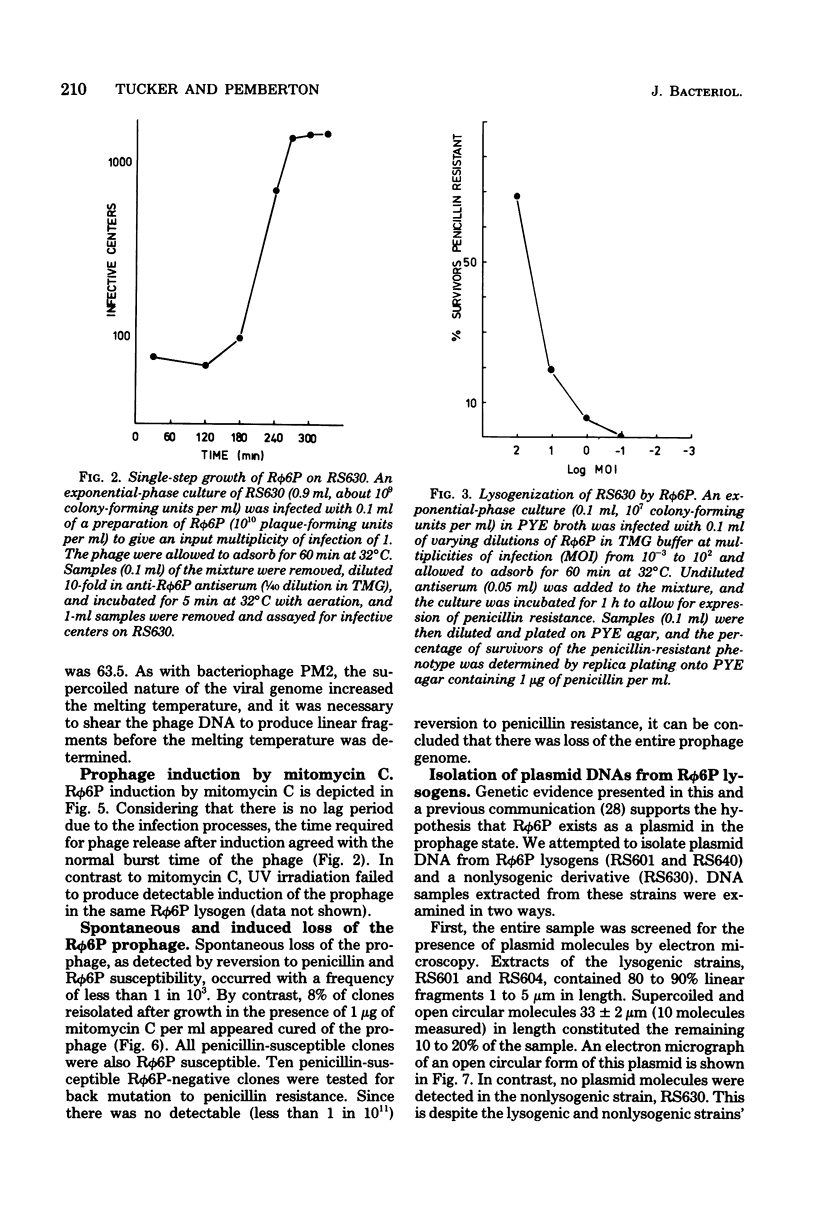
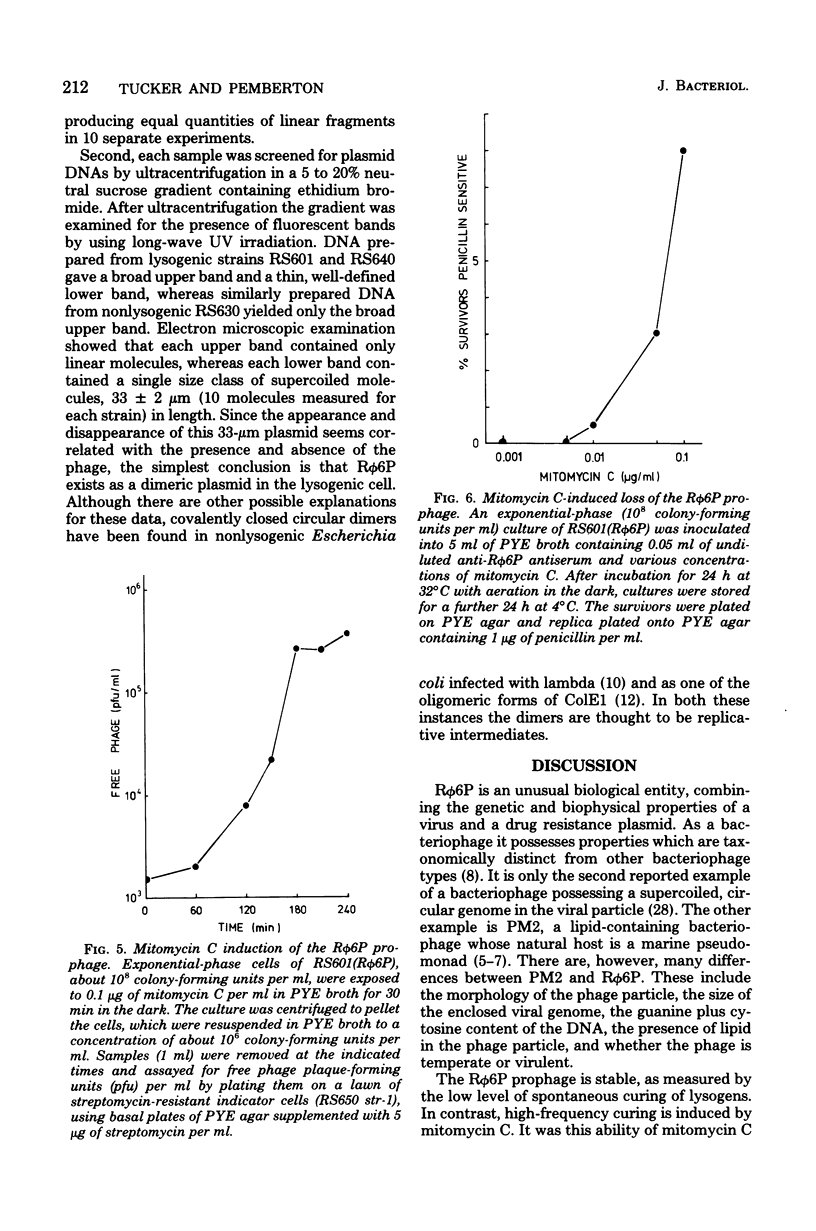
Properties of the viral R plasmid Rphi6P are described. As a temperate bacteriophage, it plaques on the facultative phototroph Rhodopseudomonas sphaeroides. Under aerobic conditions the phage had a latent period of 180 min, a burst time of 200 min, and a burst size of 15 to 20 particles per infective center. The encapsidated viral genome occurred as a supercoiled, circular DNA duplex with a mean contour length of 16.5 +/- 10 micron. Percent guanine plus cytosine, as calculated from thermal denaturation profiles, was 63.5. Mitomycin C-induced loss of the prophage suggested an extrachromosomal location in the host cell. Use of this curing agent enabled the isolation of a plasmid-free strain of R. sphaeroides. Biophysical analysis of the plasmid-free strain lysogenized with Rphi6P confirmed that the prophage occurred as a plasmid in the host cell.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Cleary P., Campbell A. A deletion analysis of prophage lambda and adjacent genetic regions. Proc Natl Acad Sci U S A. 1968 Nov;61(3):956–962. doi: 10.1073/pnas.61.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N. Transposable genetic elements and plasmid evolution. Nature. 1976 Oct 28;263(5580):731–738. doi: 10.1038/263731a0. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S. Properties and characterization of the host bacterium of bacteriophage PM2. J Bacteriol. 1968 May;95(5):1887–1891. doi: 10.1128/jb.95.5.1887-1891.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S. Properties of bacteriophage PM2: a lipid-containing bacterial virus. Virology. 1968 Apr;34(4):738–747. doi: 10.1016/0042-6822(68)90094-9. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. DNA of bacteriophage PM2: a closed circular double-stranded molecule. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1164–1168. doi: 10.1073/pnas.63.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner F. Classification and nomenclature of viruses. Second report of the International Committee on Taxonomy of Viruses. Intervirology. 1976;7(1-2):1–115. doi: 10.1159/000149938. [DOI] [PubMed] [Google Scholar]
- Freifelder D., Baran N., Folkmanis A., Freifelder D. L. Circular dimers of a lambda DNA in infected, nonlysogenic Escherichia coli. Virology. 1977 Sep;81(2):183–191. doi: 10.1016/0042-6822(77)90136-2. [DOI] [PubMed] [Google Scholar]
- Gibson K. D., Niederman R. A. Characterization of two circular satellite species of deoxyribonucleic acid in Rhodopseudomonas spheroides. Arch Biochem Biophys. 1970 Dec;141(2):694–704. doi: 10.1016/0003-9861(70)90190-6. [DOI] [PubMed] [Google Scholar]
- Goebel W., Helinski D. R. Generation of higher multiple circular DNA forms in bacteria. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1406–1413. doi: 10.1073/pnas.61.4.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. E., Yarmolinsky M. B. Integration-negative mutants of bacteriophage lambda. J Mol Biol. 1968 Feb 14;31(3):487–505. doi: 10.1016/0022-2836(68)90423-3. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Sublett R., Hedges R. W., Jacob A., Falkow S. Origin of the TEM-beta-lactamase gene found on plasmids. J Bacteriol. 1975 Apr;122(1):250–256. doi: 10.1128/jb.122.1.250-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. Prophage P1, and extrachromosomal replication unit. Cold Spring Harb Symp Quant Biol. 1968;33:791–798. doi: 10.1101/sqb.1968.033.01.091. [DOI] [PubMed] [Google Scholar]
- KONDO E., MITSUHASHI S. DRUG RESISTANCE OF ENTERIC BACTERIA. IV. ACTIVE TRANSDUCING BACTERIOPHAGE P1 CM PRODUCED BY THE COMBINATION OF R FACTOR WITH BACTERIOPHAGE P1. J Bacteriol. 1964 Nov;88:1266–1276. doi: 10.1128/jb.88.5.1266-1276.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D. Molecular weights of coliphages and coliphage DNA. 3. Contour length and molecular weight of DNA from bacteriophages T4, T5 and T7, and from bovine papilloma virus. J Mol Biol. 1970 Dec 28;54(3):557–565. doi: 10.1016/0022-2836(70)90126-9. [DOI] [PubMed] [Google Scholar]
- Miller R. V., Pemberton J. M., Richards K. E. F116, D3 and G101: temperate bacteriophages of Pseudomonas aeruginosa. Virology. 1974 Jun;59(2):566–569. doi: 10.1016/0042-6822(74)90466-8. [DOI] [PubMed] [Google Scholar]
- Mise K., Arber W. Plaque-forming transducing bacteriophage P1 derivatives and their behaviour in lysogenic conditions. Virology. 1976 Jan;69(1):191–205. doi: 10.1016/0042-6822(76)90206-3. [DOI] [PubMed] [Google Scholar]
- Mural R. J., Friedman D. I. Isolation and characterization of a temperate bacteriophage specific for Rhodopseudomonas spheroides. J Virol. 1974 Nov;14(5):1288–1292. doi: 10.1128/jvi.14.5.1288-1292.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchaudhuri S., Chakrabarty A. Isolation of plasmid deoxyribonucleic acid from Pseudomonas putida. J Bacteriol. 1976 Apr;126(1):410–416. doi: 10.1128/jb.126.1.410-416.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton J. M., Clark A. J. Detection and characterization of plasmids in Pseudomonas aeruginosa strain PAO. J Bacteriol. 1973 Apr;114(1):424–433. doi: 10.1128/jb.114.1.424-433.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton J. M. Size of the chromosome of Pseudomonas aeruginosa PAO. J Bacteriol. 1974 Sep;119(3):748–752. doi: 10.1128/jb.119.3.748-752.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton J. M., Tucker W. T. Naturally occurring viral R plasmid with a circular supercoiled genome in the extracellular state. Nature. 1977 Mar 3;266(5597):50–51. doi: 10.1038/266050a0. [DOI] [PubMed] [Google Scholar]
- Richmond M. H. Extrachromosomal elements and the spread of antibiotic resistance in bacteria. Biochem J. 1969 Jun;113(2):225–234. doi: 10.1042/bj1130225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Saunders V. A., Saunders J. R., Bennett P. M. Extrachromosomal deoxyribonucleic acid in wild-type and photosynthetically incompetent strains of Rhodopseudomonas spheroides. J Bacteriol. 1976 Mar;125(3):1180–1187. doi: 10.1128/jb.125.3.1180-1187.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistrom W. R. Transfer of chromosomal genes mediated by plasmid r68.45 in Rhodopseudomonas sphaeroides. J Bacteriol. 1977 Aug;131(2):526–532. doi: 10.1128/jb.131.2.526-532.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Ikeda S. Phage P1 carrying kanamycin resistance gene of R factor. Virology. 1976 Mar;70(1):198–200. doi: 10.1016/0042-6822(76)90252-x. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Jr, Walker J. T. Genetic studies of coliphage P1. II. Relatedness to P7. J Virol. 1976 Jul;19(1):271–274. doi: 10.1128/jvi.19.1.271-274.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun T., Vapnek D. Electron microscopic analysis of bacteriophages P1, P1Cm, and P7. Determination of genome sizes, sequence homology, and location of antibiotic-resistance determinants. Virology. 1977 Mar;77(1):376–385. doi: 10.1016/0042-6822(77)90434-2. [DOI] [PubMed] [Google Scholar]
- Zabrovitz S., Segev N., Cohen G. Growth of bacteriophage P1 in recombination-deficient hosts of Escherichia coli. Virology. 1977 Jul 15;80(2):233–248. doi: 10.1016/s0042-6822(77)80001-9. [DOI] [PubMed] [Google Scholar]





