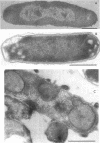
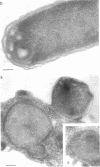
Abstract
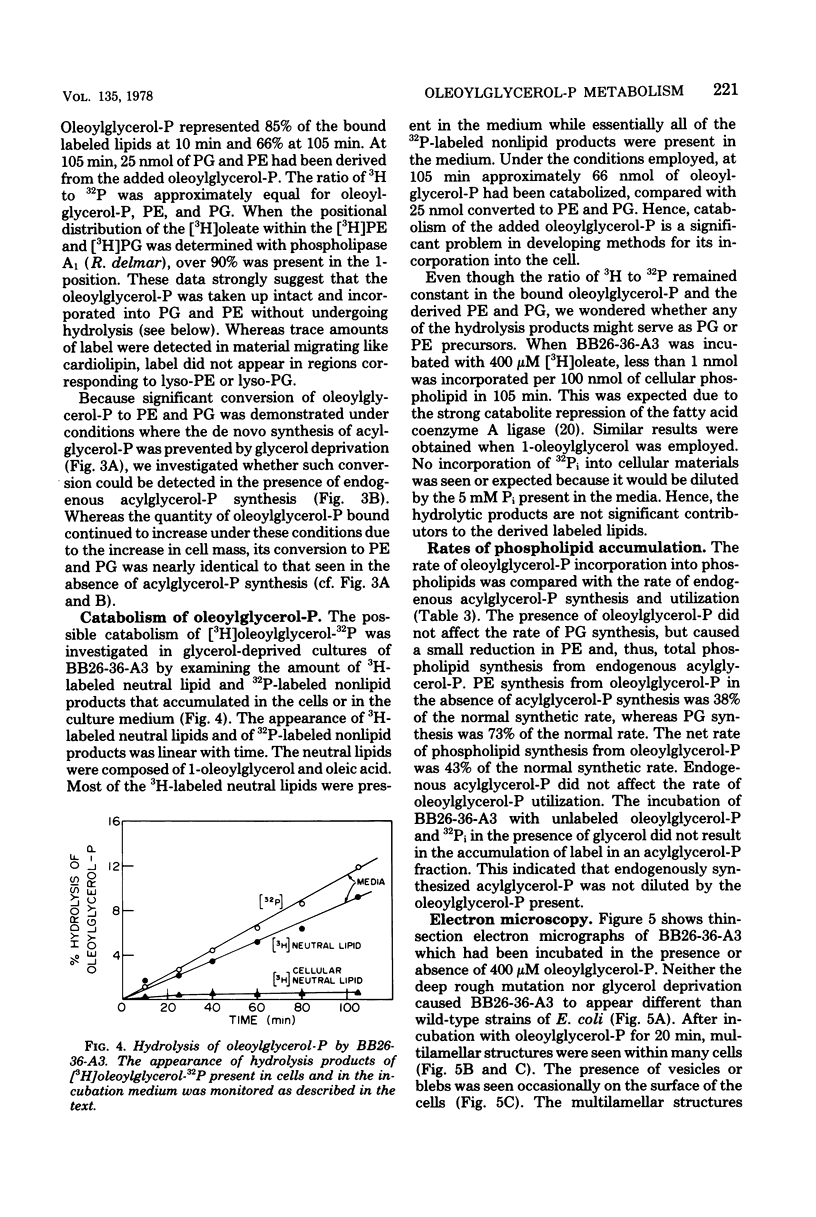
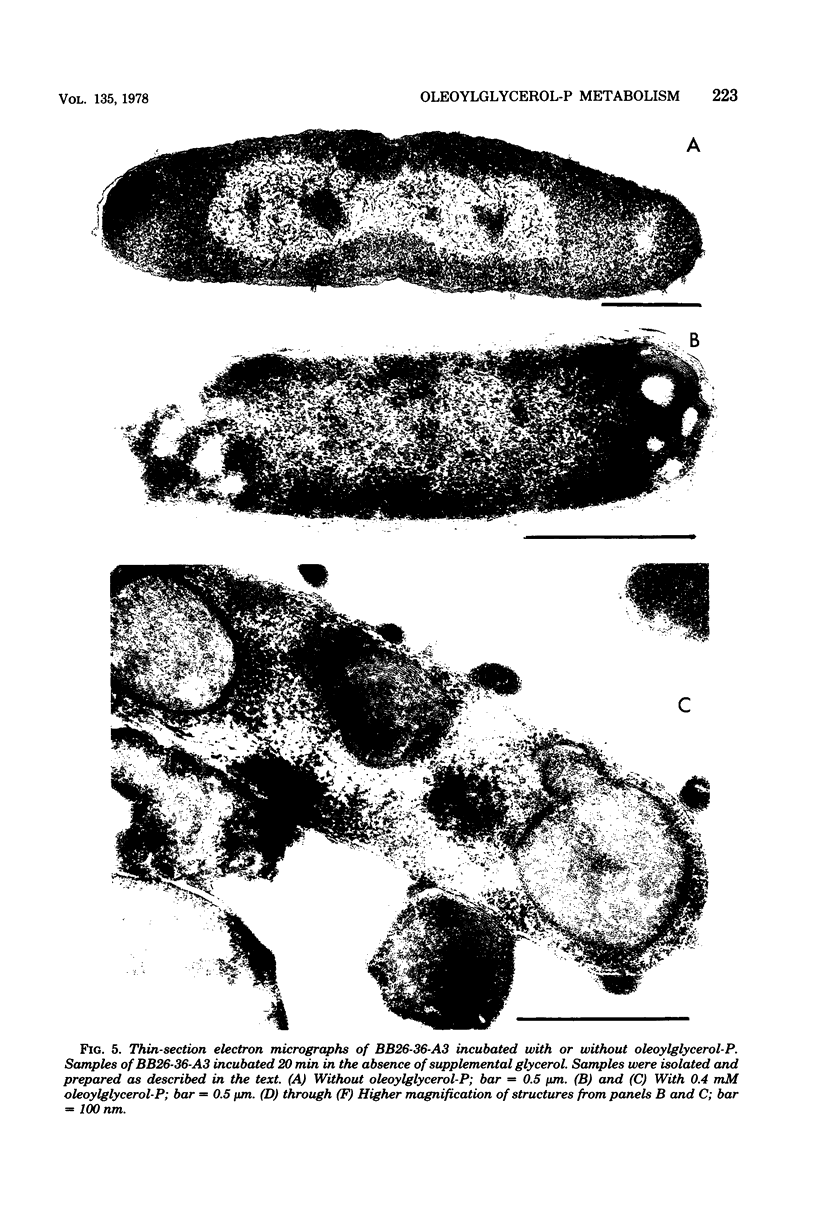
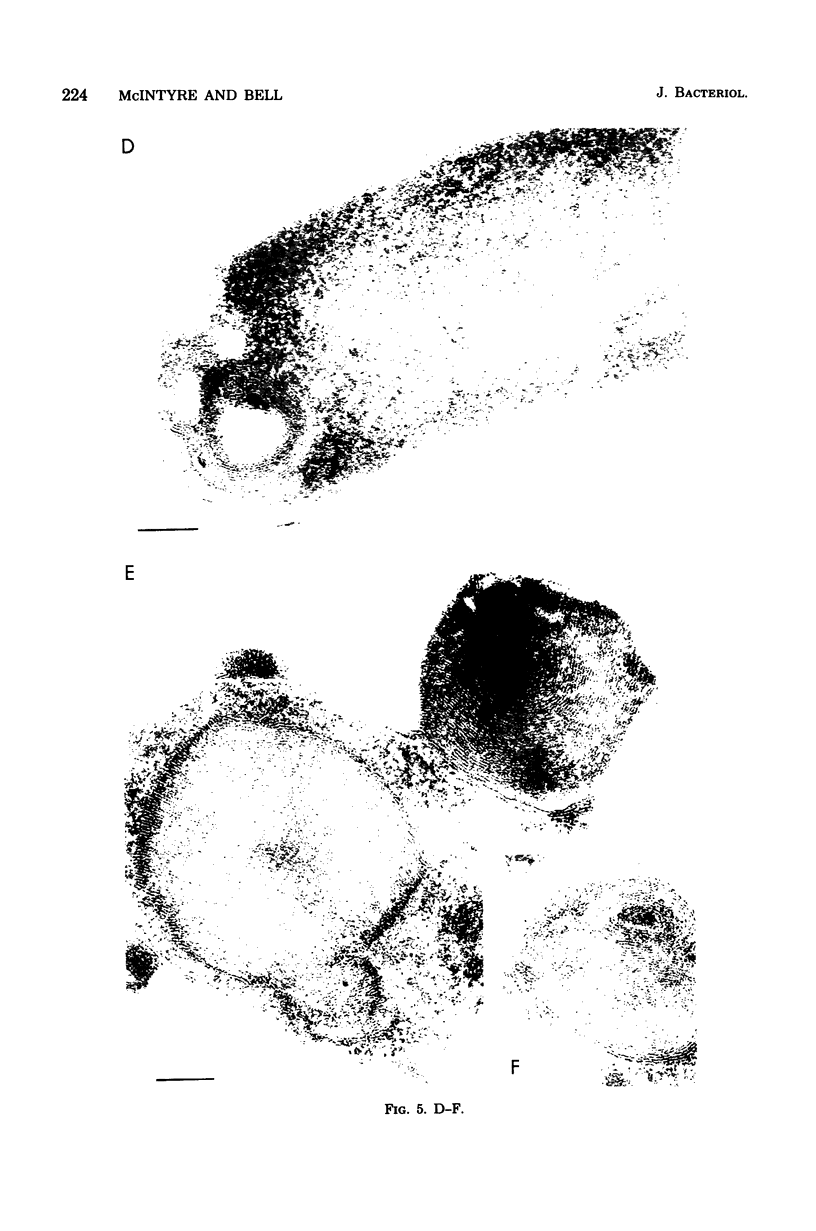
Mutants of Escherichia coli containing a defective sn-glycerol 3-phosphate acyltransferase are conditionally defective in the synthesis of acylglycerol phosphate (acylglycerol-P). Incubation of a deep rough derivative of one of these plsB strains with 1-[3H]oleoylglycerol-32P resulted in the binding of up to 70 nmol of oleoylglycerol-P per 100 nmol of cellular phospholipid. The binding was dependent on time, oleoylglycerol-P concentration, and the quantity of cells employed. The rate and extent of oleoylglycerol-P binding was affected by the deep rough mutation. The altered phospholipid composition due to oleoylglycerol-P binding was without consequence on cell growth and viability, but caused the appearance of intracellular multilamellar structures. Use of the double-labeled oleoylglycerol P demonstrated that the entire molecule was bound to the cell. Intact [3H]-oleoylglycerol-32P was converted to phosphatidylethanolamine and phosphotidyl-glycerol at a rate about 40% of that of de novo phospholipid synthesis. These data demonstrate the transmembrane movement of oleoylglycerol-P to the inner surface of the cytoplasmic membrane and suggest that it may become possible to supplement plsB strains of E. coli with acylglycerol-P's.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Ames B. N., Lee F. D., Durston W. E. An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc Natl Acad Sci U S A. 1973 Mar;70(3):782–786. doi: 10.1073/pnas.70.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANGHAM A. D., HORNE R. W. NEGATIVE STAINING OF PHOSPHOLIPIDS AND THEIR STRUCTURAL MODIFICATION BY SURFACE-ACTIVE AGENTS AS OBSERVED IN THE ELECTRON MICROSCOPE. J Mol Biol. 1964 May;8:660–668. doi: 10.1016/s0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barden R. E., Cleland W. W. 1-Acylglycerol 3-phosphate acyltransferase from rat liver. J Biol Chem. 1969 Jul 10;244(13):3677–3684. [PubMed] [Google Scholar]
- Bell R. M., Mavis R. D., Osborn M. J., Vagelos P. R. Enzymes of phospholipid metabolism: localization in the cytoplasmic and outer membrane of the cell envelope of Escherichia coli and Salmonella typhimurium. Biochim Biophys Acta. 1971 Dec 3;249(2):628–635. doi: 10.1016/0005-2736(71)90144-1. [DOI] [PubMed] [Google Scholar]
- Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: macromolecular synthesis in an sn-glycerol 3-phosphate acyltransferase Km mutant. J Bacteriol. 1974 Mar;117(3):1065–1076. doi: 10.1128/jb.117.3.1065-1076.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H. G., Monner D. A. Characterization of lipopolysaccharides from Escherichia coli K-12 mutants. J Bacteriol. 1975 Feb;121(2):455–464. doi: 10.1128/jb.121.2.455-464.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: mapping of sn-glycerol 3-phosphate acyltransferase Km mutants. J Bacteriol. 1974 Oct;120(1):227–233. doi: 10.1128/jb.120.1.227-233.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: mapping of the structural gene for L-glycerol 3-phosphate dehydrogenase. J Bacteriol. 1974 May;118(2):598–605. doi: 10.1128/jb.118.2.598-605.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Ray T. K., Vagelos P. R. Selection and characterization of an E. coli mutant defective in membrane lipid biosynthesis. Proc Natl Acad Sci U S A. 1970 Mar;65(3):737–744. doi: 10.1073/pnas.65.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI S., KOCH J. P., LIN E. C. ACTIVE TRANSPORT OF L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3098–3105. [PubMed] [Google Scholar]
- Hawrot E., Kennedy E. P. Biogenesis of membrane lipids: mutants of Escherichia coli with temperature-sensitive phosphatidylserine decarboxylase. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1112–1116. doi: 10.1073/pnas.72.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Interaction of Salmonella typhimurium with phospholipid vesicles. Incorporation of exogenous lipids into intact cells. J Biol Chem. 1977 Oct 25;252(20):7398–7404. [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Translocation of phospholipids between the outer and inner membranes of Salmonella typhimurium. J Biol Chem. 1977 Oct 25;252(20):7405–7412. [PubMed] [Google Scholar]
- KARNOVSKY M. J. Simple methods for "staining with lead" at high pH in electron microscopy. J Biophys Biochem Cytol. 1961 Dec;11:729–732. doi: 10.1083/jcb.11.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein K., Steinberg R., Fiethen B., Overath P. Fatty acid degradation in Escherichia coli. An inducible system for the uptake of fatty acids and further characterization of old mutants. Eur J Biochem. 1971 Apr;19(3):442–450. doi: 10.1111/j.1432-1033.1971.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavis R. D., Bell R. M., Vagelos P. R. Effect of phospholipase C hydrolysis of membrane phospholipids on membranous enzymes. J Biol Chem. 1972 May 10;247(9):2835–2841. [PubMed] [Google Scholar]
- McIntyre T. M., Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis. Effect of cessation of net phospholipid synthesis on cytoplasmic and outer membranes. J Biol Chem. 1975 Dec 10;250(23):9053–9059. [PubMed] [Google Scholar]
- McIntyre T. M., Chamberlain B. K., Webster R. E., Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis. Effects of cessation and reinitiation of phospholipid synthesis on macromolecular synthesis and phospholipid turnover. J Biol Chem. 1977 Jul 10;252(13):4487–4493. [PubMed] [Google Scholar]
- NICHOLS B. W. SEPARATION OF THE LIPIDS OF PHOTOSYNTHETIC TISSUES: IMPROVEMENTS IN ANALYSIS BY THIN-LAYER CHROMATOGRAPHY. Biochim Biophys Acta. 1963 Aug 27;70:417–422. doi: 10.1016/0006-3002(63)90771-6. [DOI] [PubMed] [Google Scholar]
- Nakae T., Nikaido H. Outer membrane as a diffusion barrier in Salmonella typhimurium. Penetration of oligo- and polysaccharides into isolated outer membrane vesicles and cells with degraded peptidoglycan layer. J Biol Chem. 1975 Sep 25;250(18):7359–7365. [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Nishijima M., Akamatsu Y., Nojima S. Purification and properties of a membrane-bound phospholipase A1 from Mycobacterium phlei. J Biol Chem. 1974 Sep 10;249(17):5658–5667. [PubMed] [Google Scholar]
- Raetz C. R. Isolation of Escherichia coli mutants defective in enzymes of membrane lipid synthesis. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2274–2278. doi: 10.1073/pnas.72.6.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Kennedy E. P. The association of phosphatidylserine synthetase with ribosomes in extracts of Escherichia coli. J Biol Chem. 1972 Apr 10;247(7):2008–2014. [PubMed] [Google Scholar]
- Raetz C. R. Phosphatidylserine synthetase mutants of Escherichia coli. Genetic mapping and membrane phospholipid composition. J Biol Chem. 1976 Jun 10;251(11):3242–3249. [PubMed] [Google Scholar]
- Silbert D. F. Genetic modification of membrane lipid. Annu Rev Biochem. 1975;44:315–339. doi: 10.1146/annurev.bi.44.070175.001531. [DOI] [PubMed] [Google Scholar]
- Silbert D. F., Ladenson R. C., Honegger J. L. The unsaturated fatty acid requirement in Escherichia coli. Temperature dependence and total replacement by branched-chain fatty acids. Biochim Biophys Acta. 1973 Jul 6;311(3):349–361. doi: 10.1016/0005-2736(73)90315-5. [DOI] [PubMed] [Google Scholar]
- Snider M. D., Kennedy E. P. Partial purification of glycerophosphate acyltransferase from Escherichia coli. J Bacteriol. 1977 Jun;130(3):1072–1083. doi: 10.1128/jb.130.3.1072-1083.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Sato T., Matsuhashi M. Role of lipopolysaccharides in antibiotic resistance and bacteriophage adsorption of Escherichia coli K-12. J Bacteriol. 1971 Mar;105(3):968–975. doi: 10.1128/jb.105.3.968-975.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand R. A., Shively J. M., Greenawalt J. W. Formation and ultrastructure of extra membranes in Escherichia coli. J Bacteriol. 1970 Apr;102(1):240–249. doi: 10.1128/jb.102.1.240-249.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]