Abstract
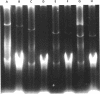
Large plasmids from Agrobacterium tumefaciens, Salmonella typhimurium, Escherichia coli, Pseudomonas putida, and Pseudomonas aeruginosa were routinely and consistently isolated using a procedure which does not require ultracentrifugation but includes steps designed to separate large-plasmid DNA from the bacterial folded chromosome. It also selectively removes fragments of broken chromosome. A variety of large plasmids was readily visualized with agarose gel electorphoresis, including five between 70 and 85 megadaltons (Mdal) in size, six between 90 and 143 Mdal, one that was larger than 200 Mdal, and one that was larger than 300 Mdal. This isolation procedure allowed initial estimation of the molecular sizes of the two IncP2 plasmids, pMG1 and pMG5, which were 312 and 280 Mdal, respectively. A standard curve for size determination by gel electrophoresis including plasmids between 23 and 143 Mdal in size did not extrapolate linearly for plasmids of the 300-Mdal size range. Unique response of different plasmids to the isolation procedure included sensitivity of IncP1 plasmids to high pH and the co-isolation of a 20-Mdal "cryptic" plasmid in conjunction.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Burgi A. W., Robinton J., Carlson C. L. Studies on the folded chromosome of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1974;38:43–51. doi: 10.1101/sqb.1974.038.01.007. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A. M. Plasmids in Pseudomonas. Annu Rev Genet. 1976;10:7–30. doi: 10.1146/annurev.ge.10.120176.000255. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Dunn N. W., Holloway B. W. Pleiotrophy of p-fluorophenylalanine-resistant and antibiotic hypersensitive mutants of Pseudomonas aeruginosa. Genet Res. 1971 Oct;18(2):185–197. doi: 10.1017/s0016672300012593. [DOI] [PubMed] [Google Scholar]
- Fennewald M., Prevatt W., Meyer R., Shapiro J. Isolation of inc P-2 plasmid DNA from Pseudomonas aeruginosa. Plasmid. 1978 Feb;1(2):164–173. doi: 10.1016/0147-619x(78)90036-7. [DOI] [PubMed] [Google Scholar]
- Grindley N. D., Humphreys G. O., Anderson E. S. Molecular studies of R factor compatibility groups. J Bacteriol. 1973 Jul;115(1):387–398. doi: 10.1128/jb.115.1.387-398.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsted J., Saunders J. R., Ingram L. C., Sykes R. B., Richmond M. H. Properties of a R factor which originated in Pseudomonas aeruginosa 1822. J Bacteriol. 1972 May;110(2):529–537. doi: 10.1128/jb.110.2.529-537.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helinski D. R., Clewell D. B. Circular DNA. Annu Rev Biochem. 1971;40:899–942. doi: 10.1146/annurev.bi.40.070171.004343. [DOI] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A. Properties of R plasmids determining gentamicin resistance by acetylation in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1974 Sep;6(3):239–252. doi: 10.1128/aac.6.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A. Properties of an R plasmid in Pseudomonas aeruginosa producing amikacin (BB-K8), butirosin, kanamycin, tobramycin, and sisomicin resistance. Antimicrob Agents Chemother. 1974 Dec;6(6):807–810. doi: 10.1128/aac.6.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J. B., Gunsalus I. C. Isolation of metabolic plasmid DNA from Pseudomonas putida. Biochem Biophys Res Commun. 1977 Mar 7;75(1):13–19. doi: 10.1016/0006-291x(77)91282-7. [DOI] [PubMed] [Google Scholar]
- Kline B. C., Miller J. R., Cress D. E., Wlodarczyk M., Manis J. J., Otten M. R. Nonintegrated plasmid-chromosome complexes in Escherichia coli. J Bacteriol. 1976 Aug;127(2):881–889. doi: 10.1128/jb.127.2.881-889.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline B. C., Miller J. R. Detection of nonintegrated plasmid deoxyribonucleic acid in the folded chromosome of Escherichia coli: physiochemical approach to studying the unit of segregation. J Bacteriol. 1975 Jan;121(1):165–172. doi: 10.1128/jb.121.1.165-172.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D. Molecular weights of coliphages and coliphage DNA. 3. Contour length and molecular weight of DNA from bacteriophages T4, T5 and T7, and from bovine papilloma virus. J Mol Biol. 1970 Dec 28;54(3):557–565. doi: 10.1016/0022-2836(70)90126-9. [DOI] [PubMed] [Google Scholar]
- Manis J. J., Miller J. R., Kline B. C. Partial characterization of nucleoids and nucleoid-plasmid complexes from Salmonella typhimurium. J Bacteriol. 1976 Jul;127(1):664–666. doi: 10.1128/jb.127.1.664-666.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis J. J., Whitfield H. J. Physical characterization of a plasmid cointegrate containing an F'his gnd element and the Salmonella typhimurium LT2 cryptic plasmid. J Bacteriol. 1977 Mar;129(3):1601–1606. doi: 10.1128/jb.129.3.1601-1606.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizusawa H., Tanaka S., Kobayashi M., Koike K. Alkali-labile colicinogenic factor E1 DNA molecules formed in the presence of N-methyl-N'-nitro-N-nitrosoguanidine. Biochem Biophys Res Commun. 1977 Jan 24;74(2):570–576. doi: 10.1016/0006-291x(77)90341-2. [DOI] [PubMed] [Google Scholar]
- Olsen R. H., Hansen J. Evolution and utility of a Pseudomonas aeruginosa drug resistance factor. J Bacteriol. 1976 Mar;125(3):837–844. doi: 10.1128/jb.125.3.837-844.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. H., Shipley P. L. RP1 properties and fertility inhibition among P, N, W, and X incompatibility group plasmids. J Bacteriol. 1975 Jul;123(1):28–35. doi: 10.1128/jb.123.1.28-35.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. H., Shipley P. Host range and properties of the Pseudomonas aeruginosa R factor R1822. J Bacteriol. 1973 Feb;113(2):772–780. doi: 10.1128/jb.113.2.772-780.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. H., Wright C. D. Interaction of Pseudomonas and Enterobacteriaceae plasmids in Aeromonas salmonicida. J Bacteriol. 1976 Oct;128(1):228–234. doi: 10.1128/jb.128.1.228-234.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchaudhuri S., Chakrabarty A. Isolation of plasmid deoxyribonucleic acid from Pseudomonas putida. J Bacteriol. 1976 Apr;126(1):410–416. doi: 10.1128/jb.126.1.410-416.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchaudhuri S. Molecular characterization of hydrocarbon degradative plasmids in Pseudomonas putida. Biochem Biophys Res Commun. 1977 Jul 25;77(2):518–525. doi: 10.1016/s0006-291x(77)80010-7. [DOI] [PubMed] [Google Scholar]
- Pemberton J. M., Clark A. J. Detection and characterization of plasmids in Pseudomonas aeruginosa strain PAO. J Bacteriol. 1973 Apr;114(1):424–433. doi: 10.1128/jb.114.1.424-433.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton J. M. Size of the chromosome of Pseudomonas aeruginosa PAO. J Bacteriol. 1974 Sep;119(3):748–752. doi: 10.1128/jb.119.3.748-752.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettijohn D. E., Hecht R. RNA molecules bound to the folded bacterial genome stabilize DNA folds and segregate domains of supercoiling. Cold Spring Harb Symp Quant Biol. 1974;38:31–41. doi: 10.1101/sqb.1974.038.01.006. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Shipley P. L., Olsen R. H. Isolation of a nontransmissible antibiotic resistance plasmid by transductional shortening of R factor RP1. J Bacteriol. 1975 Jul;123(1):20–27. doi: 10.1128/jb.123.1.20-27.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonington O. G., Pettijohn D. E. The folded genome of Escherichia coli isolated in a protein-DNA-RNA complex. Proc Natl Acad Sci U S A. 1971 Jan;68(1):6–9. doi: 10.1073/pnas.68.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Grant R. B. Incompatibility and bacteriophage inhibition properties of N-1, a plasmid belonging to the H2 incompatibility group. Mol Gen Genet. 1977 May 20;153(1):5–10. doi: 10.1007/BF01035990. [DOI] [PubMed] [Google Scholar]
- Watson B., Currier T. C., Gordon M. P., Chilton M. D., Nester E. W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975 Jul;123(1):255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]