Abstract
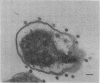
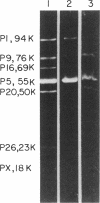
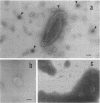
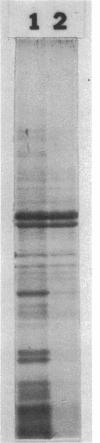
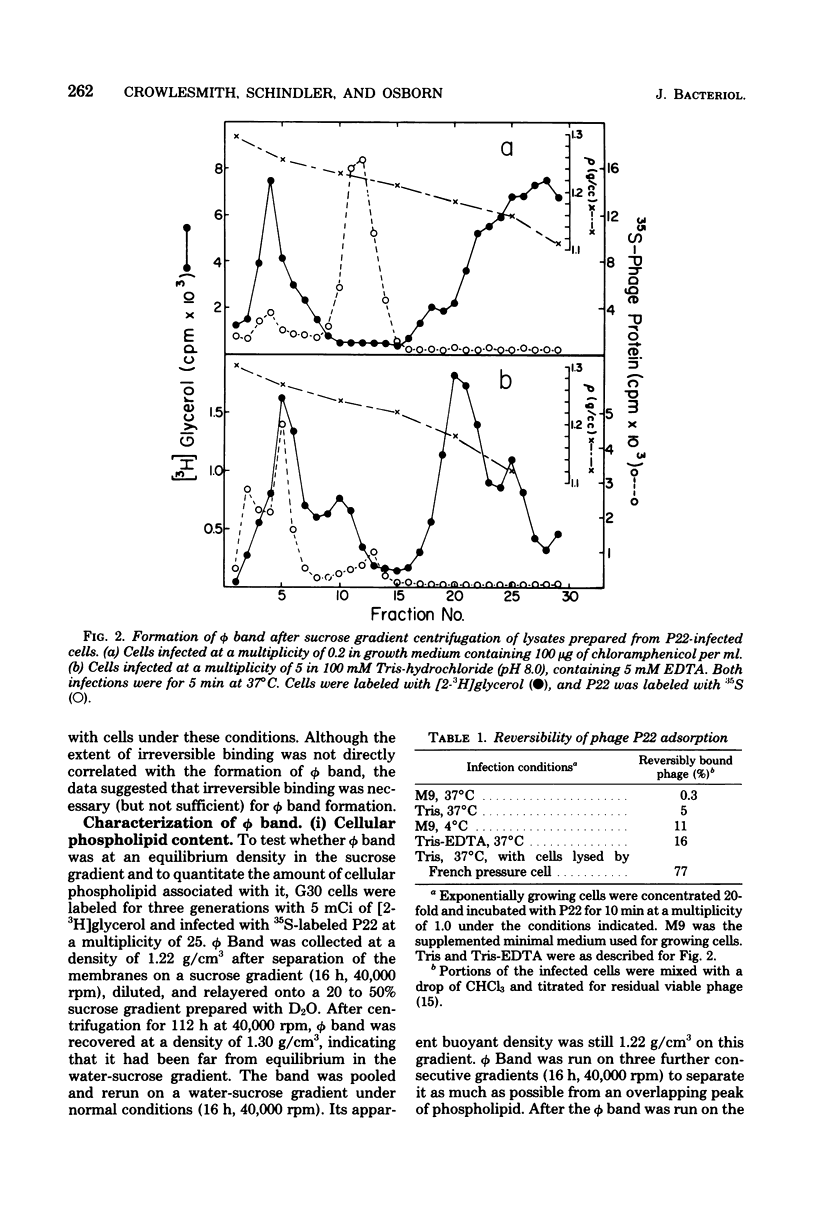
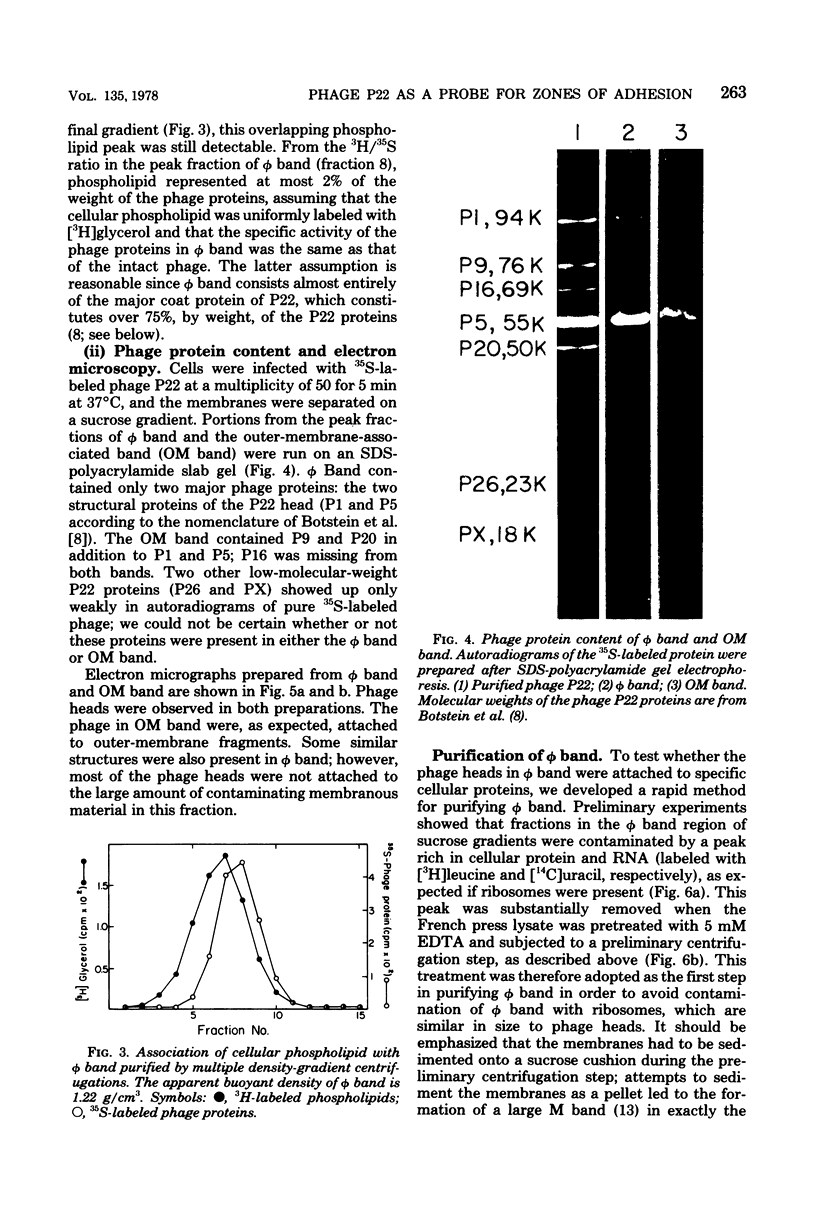
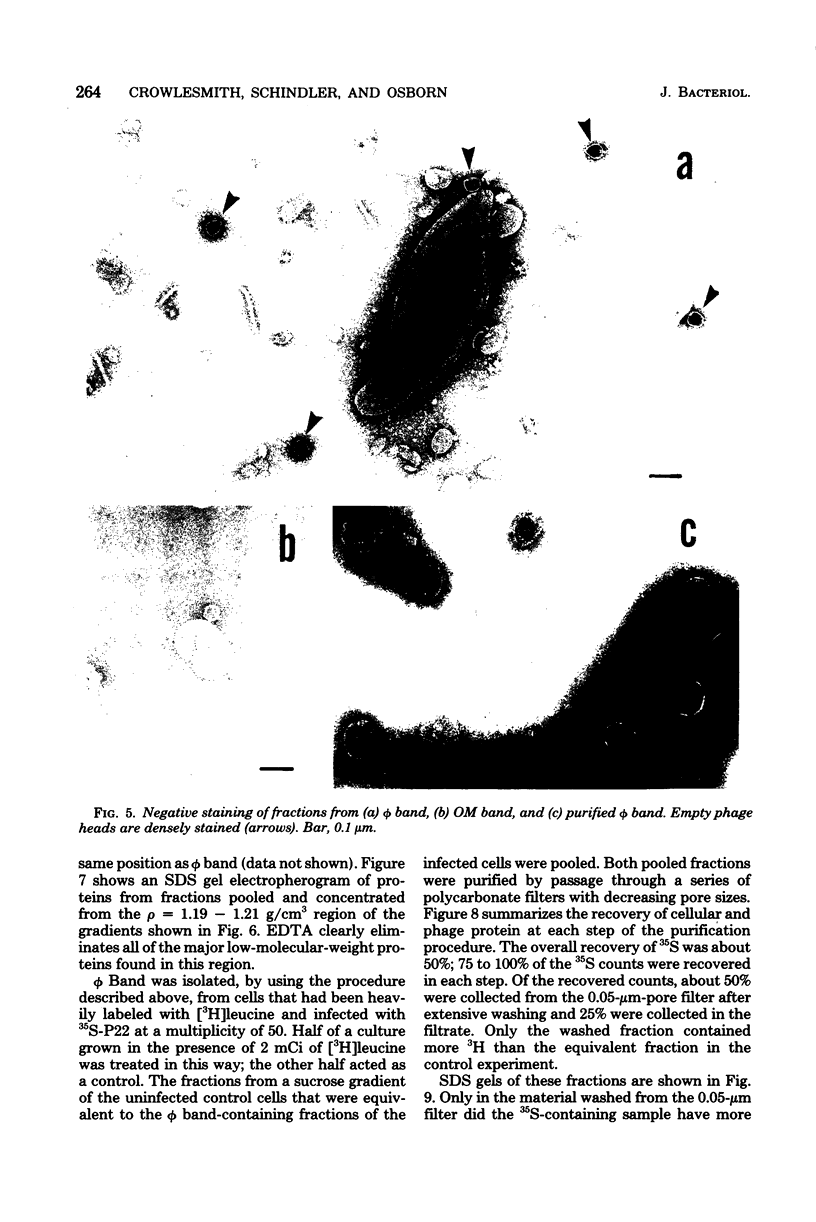
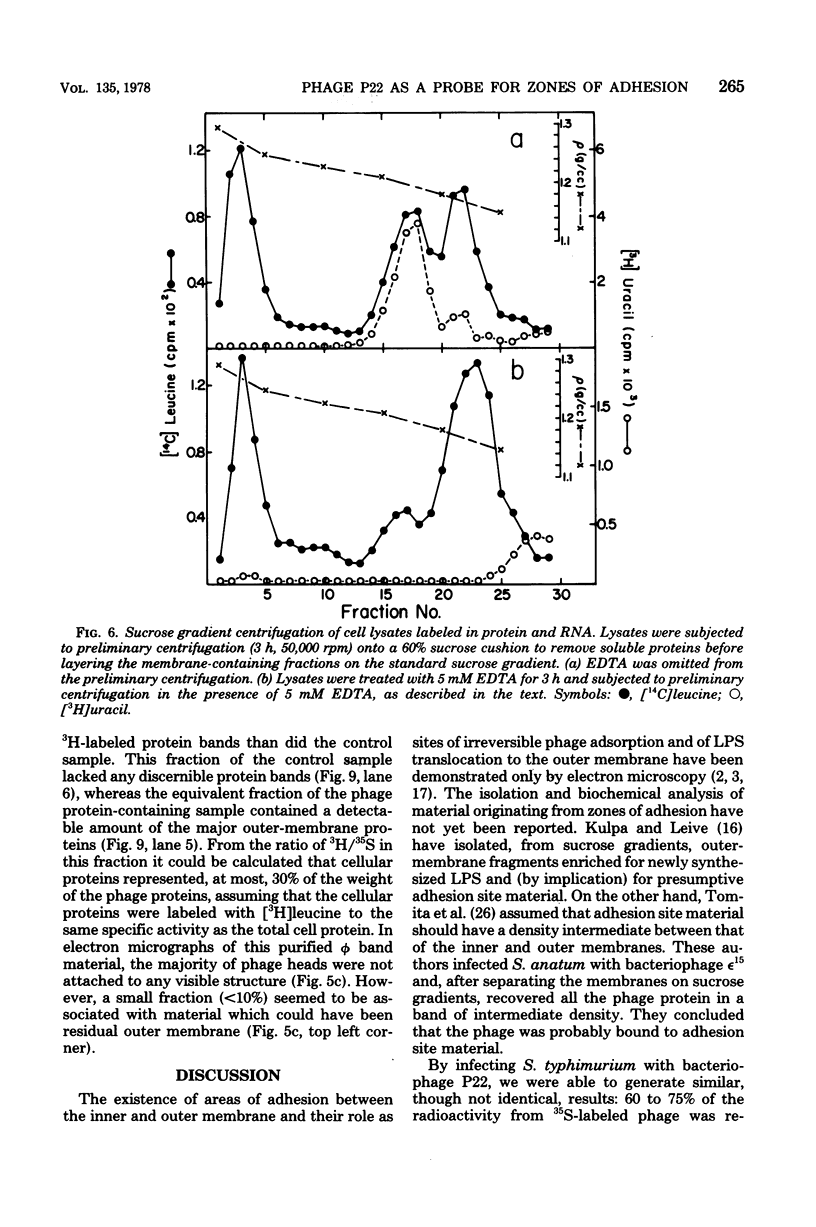
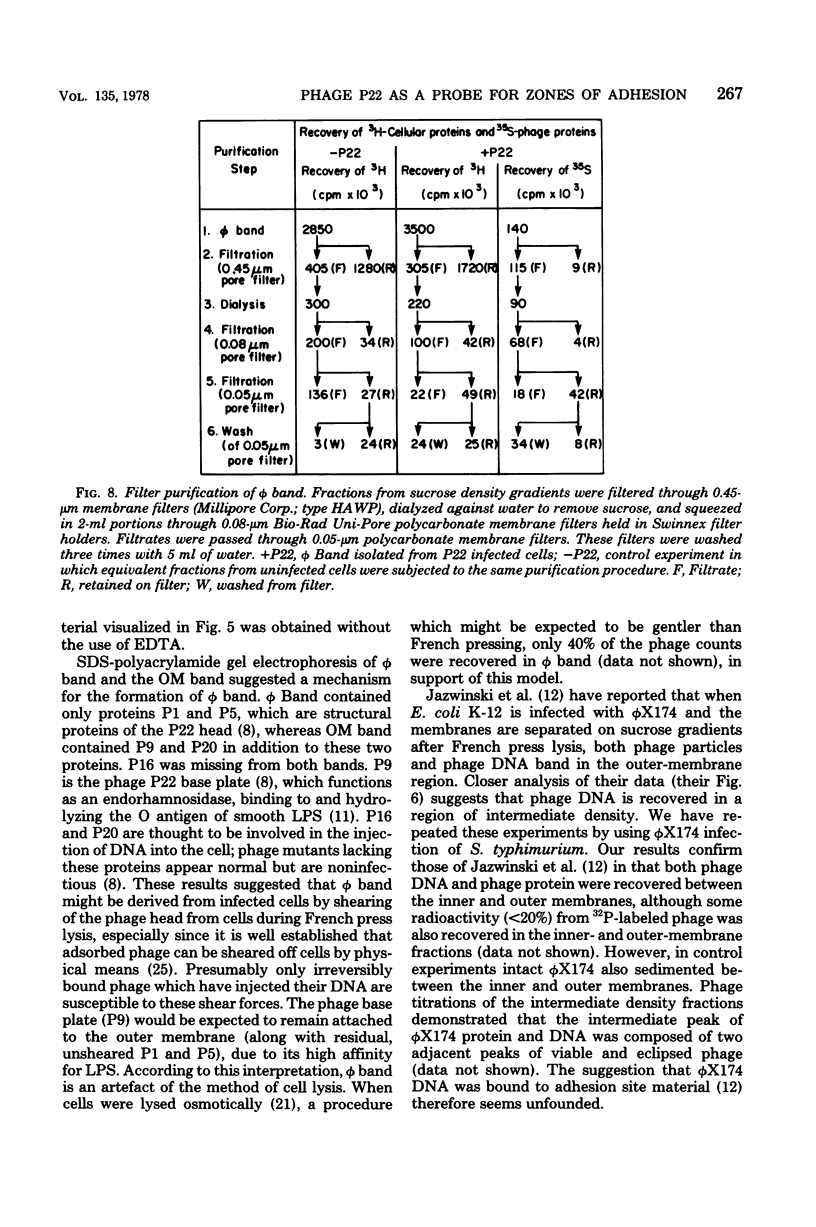
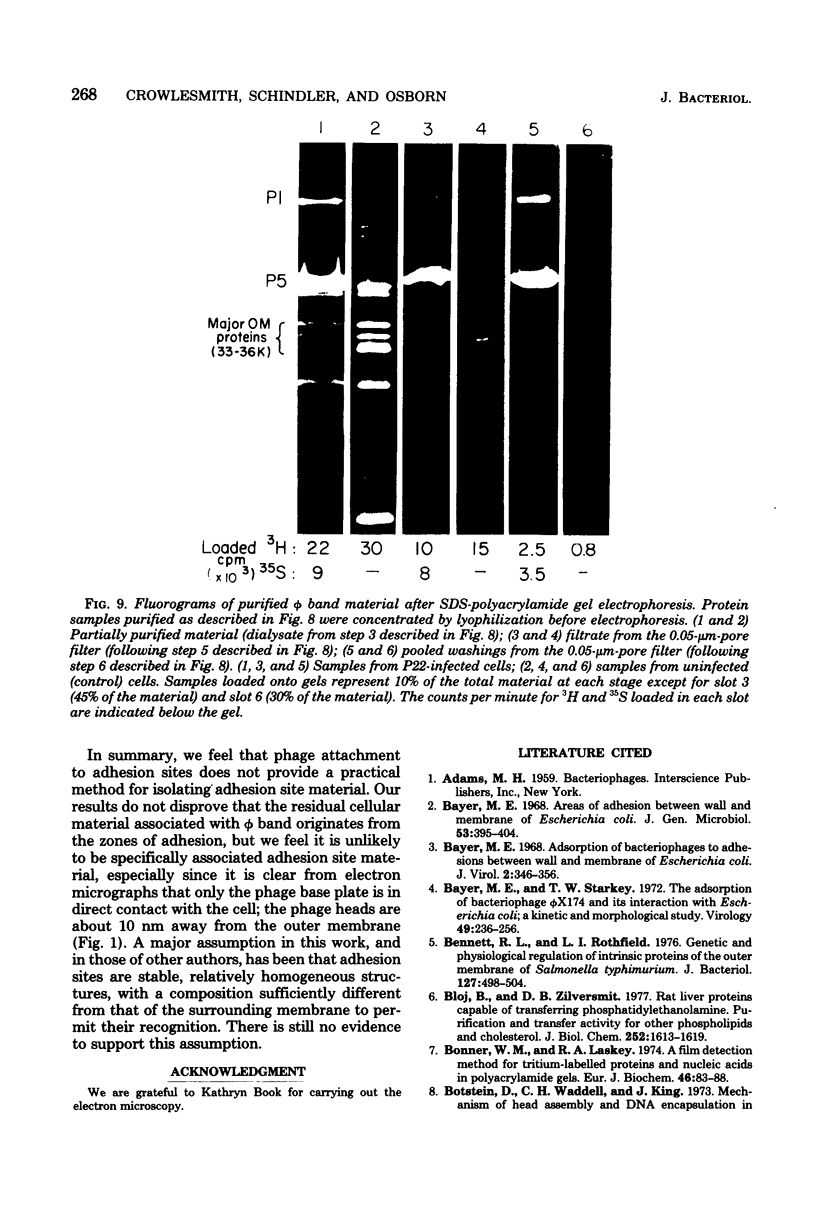
Thin-section electron micrographs of plasmolyzed Salmonella typhimurium infected with bacteriophage P22 demonstrated that phage adsorbed to cells over sites of inner- and outer-membrane contact. Efforts were made to isolate such adsorption sites by infection of cells with 35S- and 32P-labeled phage and by separation of the membranes on sucrose gradients. At 37 degrees C, about 75% of the 35S radioactivity could be recovered in a region of intermediate density between the inner and outer membranes. This region (phi band) did not contain 32P. The gradient profile was independent of the multiplicity of infection (between 0.2 and 50) and of the presence or absence of chloramphenicol, dinitrophenol, or cyanide. However, ethylenediaminetetraacetate, when present during the infection step, prevented the formation of phi band. The density of phi band was at least 1.30 g/cm3, as demonstrated by prolonged centrifugation on a D2O-sucrose gradient. phi Band was shown by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electron microscopy to contain empty phage heads and contaminating cellular debris. In purified preparations, phage heads were the only structures, visible by negative staining, and very little cellular phospholipid or protein was associated with the phage proteins (less than 2% and 30% by weight, respectively, as determined by using [3H]glycerol or [3H]leucine). The residual cellular protein included all of the major outer-membrane proteins rather than any one specific protein. These results are interpreted as indicating that phi band probably does not contain adhesion site material stably associated with phage heads.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E. Adsorption of bacteriophages to adhesions between wall and membrane of Escherichia coli. J Virol. 1968 Apr;2(4):346–356. doi: 10.1128/jvi.2.4.346-356.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Areas of adhesion between wall and membrane of Escherichia coli. J Gen Microbiol. 1968 Oct;53(3):395–404. doi: 10.1099/00221287-53-3-395. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Starkey T. W. The adsorption of bacteriophage phi X174 and its interaction with Escherichia coli; a kinetic and morphological study. Virology. 1972 Jul;49(1):236–256. doi: 10.1016/s0042-6822(72)80026-6. [DOI] [PubMed] [Google Scholar]
- Bennett R. L., Rothfield L. I. Genetic and physiological regulation of intrinsic proteins of the outer membrane of Salmonella typhimurium. J Bacteriol. 1976 Jul;127(1):498–504. doi: 10.1128/jb.127.1.498-504.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloj B., Zilversmit D. B. Rat liver proteins capable of transferring phosphatidylethanolamine. Purification and transfer activity for other phospholipids and cholesterol. J Biol Chem. 1977 Mar 10;252(5):1613–1619. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Inouye H., Beckwith J. Synthesis and processing of an Escherichia coli alkaline phosphatase precursor in vitro. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1440–1444. doi: 10.1073/pnas.74.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita S., Kanegasaki S. Smooth specific phage adsorption: endorhamnosidase activity of tail parts of P22. Biochem Biophys Res Commun. 1973 Nov 16;55(2):403–409. doi: 10.1016/0006-291x(73)91101-7. [DOI] [PubMed] [Google Scholar]
- Jazwinski S. M., Lindberg A. A., Kornberg A. The lipopolysaccharide receptor for bacteriophage phiX174 and S13. Virology. 1975 Jul;66(1):268–282. doi: 10.1016/0042-6822(75)90197-x. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Interaction of Salmonella typhimurium with phospholipid vesicles. Incorporation of exogenous lipids into intact cells. J Biol Chem. 1977 Oct 25;252(20):7398–7404. [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Translocation of phospholipids between the outer and inner membranes of Salmonella typhimurium. J Biol Chem. 1977 Oct 25;252(20):7405–7412. [PubMed] [Google Scholar]
- Kanegasaki S., Tomita T. Mutants of Salmonella anatum that block bacteriophage epsilon infection at early stages. J Bacteriol. 1976 Jul;127(1):7–13. doi: 10.1128/jb.127.1.7-13.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulpa C. F., Jr, Leive L. Mode of insertion of lipopolysaccharide into the outer membrane of escherichia coli. J Bacteriol. 1976 Apr;126(1):467–477. doi: 10.1128/jb.126.1.467-477.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlradt P. F., Menzel J., Golecki J. R., Speth V. Outer membrane of salmonella. Sites of export of newly synthesised lipopolysaccharide on the bacterial surface. Eur J Biochem. 1973 Jun 15;35(3):471–481. doi: 10.1111/j.1432-1033.1973.tb02861.x. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Newbold J. E., Sinsheimer R. L. The process of infection with bacteriophage phiX174. XXXII. Early steps in the infection process: attachment, eclipse and DNA penetration. J Mol Biol. 1970 Apr 14;49(1):49–66. doi: 10.1016/0022-2836(70)90375-x. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J., ROSEN S. M., ROTHFIELD L., HORECKER B. L. Biosynthesis of bacterial lipopolysaccharide. I. Enzymatic incorporation of galactose in a mutant strain of Salmonella. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1831–1838. doi: 10.1073/pnas.48.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Site of synthesis of lipopolysaccharide. J Biol Chem. 1972 Jun 25;247(12):3973–3986. [PubMed] [Google Scholar]
- ROTHFIELD L., OSBORN M. J., HORECKER B. L. BIOSYNTHESIS OF BACTERIAL LIPOPOLYSACCHARIDE. II. INCORPORATION OF GLUCOSE AND GALACTOSE CATALYZED BY PARTICULATE AND SOLUBLE ENZYMES IN SALMONELLA. J Biol Chem. 1964 Sep;239:2788–2795. [PubMed] [Google Scholar]
- Tomita T., Iwashita S., Kanegasaki S. Role of cell surface mobility on bacteriophage infection: translocation of Salmonella phages to membrane adhesions. Biochem Biophys Res Commun. 1976 Dec 6;73(3):807–813. doi: 10.1016/0006-291x(76)90881-0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wright A., Barzilai N. Isolation and haracterization nonconverting mutants of bacteriophage epsilon 34. J Bacteriol. 1971 Mar;105(3):937–939. doi: 10.1128/jb.105.3.937-939.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]