Abstract
Exendin-4, a peptide analogue of glucagon-like peptide-1 (GLP-1), has been developed for treatment of type 2 diabetes. Herein, the secretive exendin-4 fusion protein, expressed by methanol induction in Pichia pastoris system, was purified to homogeneity by chromatography followed by enterokinase cleavage of the fusion protein and subsequent purification of the recombinant exendin-4. Purity of the recombinant exendin-4 was 95.6%. Bioactivity assay revealed that it had glucose-lowering and insulin-releasing action in vivo.
Keywords: Diabetes, Exendin-4, Glucagon peptide analogue, Pichia pastoris
Introduction
Exendin-4 is a 39 amino acid peptide (53% structural homology to GLP-1) and was first isolated from the salivary secretions of Gila monster lizard (Heloderma suspectum) (Eng et al. 1992). It shares many of the glucoregulatory actions with GLP-1 (Buse et al. 2004; Kendall et al. 2005) and has aroused great attention for its potential for the treatment of diabetes. Clinical and non-clinical studies have shown that exendin-4 has several beneficial anti-diabetic actions that include glucose-dependent enhancement of insulin secretion, glucose-dependent suppression of inappropriately high glucagon secretion, slowing of gastric emptying, reduction of food intake and body weight, and an increase in β-cell mass (Buse et al. 2004; Edwards et al. 2001; Kendall et al. 2005; Kolterman et al. 2003; Xu and Kaneto 2006).
For many years exendin-4 has been synthesized chemically at a high price and is thus unsuitable for mass production. It therefore needs to be produced in quantity by genetic recombinant technology. Exendin-4 has been successfully expressed in E. coli system and it proved having glucose-lowering action in vivo (Yi et al. 2006; Yin et al. 2005). However, no successful expression in other expression systems has been reported until now. For large-scale production, our laboratory recently constructed a recombinant Pichia pastoris that can produce exendin-4 constitutively and secrete it into the broth (Zhuang et al. 2007). In this study, the purification and bioactivity of recombinant exendin-4 expressed in P. pastoris are reported.
Materials and methods
Microorganism and expression of exendin-4 fusion protein
Pichia pastoris GS115 was transformed with plasmid pPIC9 containing the gene encoding exendin-4, which was obtained from GenBank database (Accession No. AAB22006). The details of the vector construction and transformation were described elsewhere (Zhuang et al. 2007). Recipes and fermentation procedures were all followed by the supplier’s protocol and were described briefly as follows. Fermentation of the pPIC9/exendin-4 transformant of was carried out using a 30 l fermentor. After 48 h of growth at 28°C, the transformant P. pastoris was induced by methanol for 72 h. The culture was centrifuged at 5,000g for 15 min to collect the supernatant which was used as the source of recombinant protein.
Purification of fusion protein
For the purification of exendin-4, the total protein in the supernatant was first concentrated by ultrafiltration using a 10 kDa molecular mass cutoff membrane. The fusion protein was subsequently purified by ion-exchange chromatography, hydrophobic interaction chromatography (HIC) and size-exclusion chromatography. The void volume and eluted fractions from the three chromatographic columns were monitored at 280 nm. Fractions were collected and subjected to SDS-PAGE analysis. The purification steps were shown in Table 1.
Table 1.
Summary of exendin-4 purification
| Purification step | Total protein (mg) | Protein of interesta (mg) | Recovery (%) | |
|---|---|---|---|---|
| Fusion exendin-4 | Broth supernatant | 1,640 | 580 | 100 |
| DEAE A-25 | 1,200 | 390 | 67 | |
| Phenyl FF | 760 | 285 | 49 | |
| G-75 | 410 | 214 | 37 | |
| Recombinant exendin-4 | G-25 | 214 | 26 | 4.5 |
The concentrated broth supernatant was dialyzed with buffer A (20 mM Tris/HCl, pH 6.0, 1 mM EDTA) and was applied to a DEAE Sephadex A-25 (DEAE A-25) (100 ml bed volume) equilibrated with buffer A beforehand, at 1 ml/min. The column was washed with 300 ml buffer A and then eluted with a linear gradient of 0 to 1 M NaCl in buffer A. The eluted fractions containing exendin-4 were pooled and dialyzed. The desalted protein was mixed with ammonium sulfate at 1 M and applied onto a phenyl-Sepharose Fast Flow (Phenyl FF) column (50 ml bed volume) pre-equilibrated with buffer B [20 mM Tris/HCl, pH 6.0, 1 M (NH4)2SO4, 1 mM EGTA] at 1 ml/min, the column was washed with 150 ml buffer B and eluted with a linear (NH4)2SO4 gradient from 1 to 0 M in buffer B. The peak with exendin-4 was concentrated and dialyzed with buffer C (50 mM PBS, pH 6.0, 10% (v/v) glycerol). The last step of purification was through a Sephadex G-75 column (1 × 50 cm) pre-equilibrated with buffer C at 0.5 ml/ min. For recombinant protein purification, the pool of fractions containing purified fusion protein from the three columns was dialyzed in 20 mM Tris/HCl (pH 6.0)
aThe amount of protein of interest was determined by quantifying the amount in each gel lane by densitometry (Totallab V1.11 software)
Cleavage of the fusion protein by enterokinase
After desalting, the fusion protein was incubated with enterokinase (1 U/1 mg fusion protein) at 37°C for 8 h to obtain the recombinant exendin-4. The reaction mixture was analyzed by Tricine SDS-PAGE. The recombinant exendin-4 was purified by Sepharose G-25 column and its purity was assayed by HPLC.
SDS-PAGE, Tricine SDS-PAGE, immunoblotting and protein determination
Proteins were analyzed by SDS-PAGE under reducing conditions using 12% (v/v) gels. Tricine SDS-PAGE was carried out with the supplier’s protocol (Amersham Bioscience). The separated proteins were stained with Coomassie Brilliant Blue R-250 or electroblotted to nitrocellulose membrane for western blotting. After blocking with 3% (v/v) non-fat milk in 0.05% Tween/PBS (PBST), the membrane was washed 3 times with PBST for 10 min and incubated further with a 1:2,000 dilution of polyclonal rat anti exendin-4 antibody for 1 h, followed by washing as was described above. The membrane was incubated with a 1:1,000 dilution of HRP-conjugated goat anti-rat IgG as the second antibody, washed with the same procedure as above and revealed with DAB substrate (Sigma). Protein concentration was determined by the method of Bradford using bovine serum albumin as a standard.
Biological activity assay
The effects of recombinant protein exendin-4 on plasma glucose concentration and insulin levels were examined using 8 week-old male Wistar rats. Animals were housed six per cage (three repetition) at 24°C environmental conditions with free access to food and water. They were allowed one week to adapt to their environment before the experiment. Food was withdrawn for 24 h before intraperitoneal injection of glucose (20 mM/kg body weight) alone or in combination with exendin-4 (10 nmol/kg body wt). Test solutions were administered in a final volume of 1 ml/kg body weight (O’Harte et al. 2000). Blood samples were collected at 15, 30 and 60 min after the injection from the eye socket with capillary into chilled heparin Eppendorf tubes. Blood samples were centrifuged and plasma samples were stored at −20°C before determination.
Plasma glucose and insulin level were assayed respectively by a glucose assay kit and insulin enzyme linked immunosorbent assay kit (Dingguo, Beijing). Results were expressed as mean ± SE and were analyzed by one-way analysis of variance (ANOVA) by SPSS 11.0 software. Differences were considered significant at P < 0.05.
Results
Expression and detection
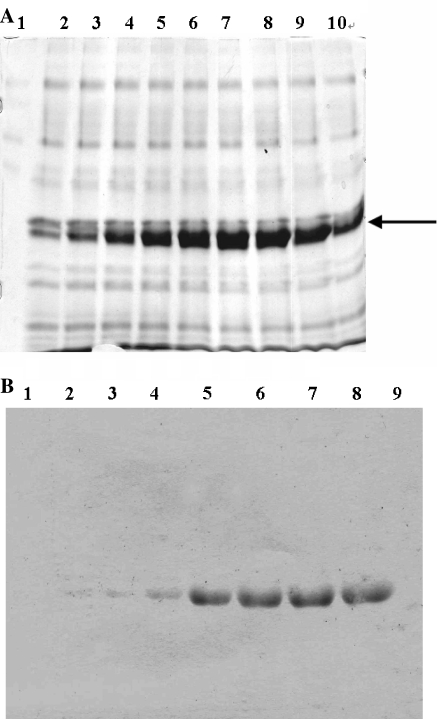
The results of expression and detection exendin-4 fusion protein are given in Fig. 1.
Fig. 1.
Expression and detection of exendin-4 fusion protein. (a) SDS-PAGE analysis of the expression level of fusion exendin-4 at different time in hours. After 56 h, the amount of the fusion protein expression reached the maximum (580 mg/l). Fifteen micro liter of supernatant were applied to a 12% gel and then stained with R-250. Lane 1, control transformant GS155/pPIC9 after 72 h culture; Lanes 2–10, recombinant GS115/pPIC9/exendin-4 after 8, 16, 24, 32, 40, 48, 56, 64 and 72 h of culture; The position of the target proteins induced by methanol is indicated by arrow. (b) Western blotting analysis of expressed protein in P. pastoris. Lanes 1–8 expressed protein of recombinant GS115/pPIC9/exendin-4 after 0, 8, 16, 24, 32, 40, 48 and 56 h, lane 9 expressed protein of control transformant GS115/pPIC9
Purifying the fusion protein and obtaining the recombinant exendin-4 by enterokinase cleave
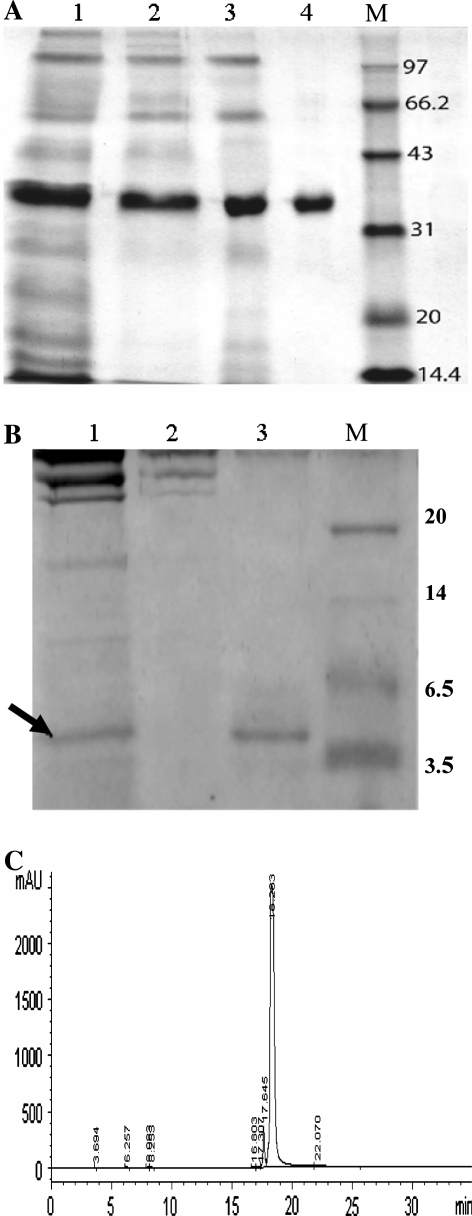
Purification of the fusion protein and production of the recombinant exendin-4 by enterokinase cleavage are given in Table 1 and further details were shown in Fig. 2.
Fig. 2.
(a) Purification of the exendin-4 fusion protein by three chromatography steps. Lane 1, broth supernatant; lane 2, DEAE A-25; lane 3, Phenyl FF; lane 4, G-75; M is protein marker (KDa). (b) Purification of the recombinant protein exendin-4 by a G-25 column. Lane 1, reaction mixture of fusion protein cleaved by enterokinlase. Recombinant exendin-4 (4.3 kDa) is marked by arrow; lane 2, the non-target protein eluted from the G-25 column; lane 3, purified recombinant peptide exendin-4 from G-25 column. (c). The purity analyzed by HPLC was 95.6% which was performed on a 250 mm × 4.6 mm C5 column. The column was eluted with the linear gradient of acetonitrile (90–0%) in 0.1% trifluoroacetic acid for 35 min at 0.5 ml/min. The target peak was at 18.3 min
Bioactivity of exendin-4 in vivo
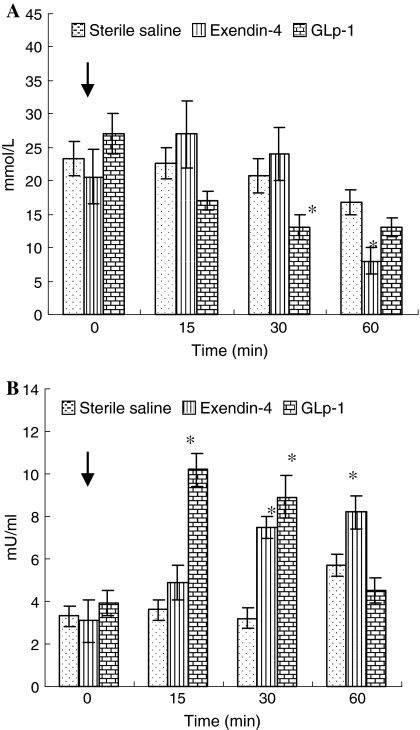
Figure 3 showed the results of bioactivity of exendin-4 in vivo. Compared with the control group, plasma glucose concentration in GLP-1 group was significantly reduced (P < 0.05) at 30 min, and the same phenomenon was observed after 60 min in exendin-4 group (Fig. 3a). There was no difference between the glucose-lowering capabilities of treatment group and control group before 15 min. The capabilities of stimulating insulin secretion showed significant response to GLP-1 and exendin-4 supplementation. The maximal increase in insulin in rat treated with GLP-1 was 2 times higher than the control group before 30 min. The plasma insulin concentration of exendin-4 treated group was also significantly raised (P < 0.05) at 30 min compared with the control group and the capabilities of stimulating insulin secretion by exendin-4 could keep to 60 min (Fig. 3b).
Fig. 3.
Biological activity assay of exendin-4 in rats. The capabilities of glucose-lowering (a) and insulin-releasing (b) after intraperitoneal glucose alone (20 mM/kg) (control group), or glucose in combination with either exendin-4 (trial group) or GLP-1 (positive group) (10 nmol/kg). The time of injection is indicated by the arrow (0 min). Values are mean ± SE (n = 6). Asterisk denotes statistically significant differences (P < 0.05) between control and trial (or positive) groups by one-way ANOVA
Discussion
For many years, the production of exendin-4 was by chemical synthesis which necessitated a high cost. Recently, bioengineering methods of producing exendin-4 have appeared. Yi et al (2006) and Yin et al (2005) cloned the exendin-4 gene and obtained stable expression in E. coli. However, the E. coli-expressed exendin-4 existed initially in inclusion bodies and bioactivity was realized only after renaturation, which made the purification process complicated and led to a low yield. Furthermore, clinical application of bacterially produced products may be affected by the possible presence of endotoxins that sometimes contaminate protein preparations expressed by E. coli. We have several reasons to select P. pastoris as an alternative recombinant expression host for exendin-4 synthesis. P. pastoris has the advantages of large production, genetically stable expression strains, the potential to secrete recombinant proteins into culture medium, and simple inexpensive culture conditions (Patrick et al. 2005). In this study, the highest expression level of fusion protein exendin-4 was estimated at about 580 mg/l culture, which was higher than E. coli system. In addition, Pichia pastoris-synthesized recombinant exendin-4 was effectively purified from the culture medium through four chromatograph steps in this study. The whole purification process was simple and easy. These properties make P. pastoris a superior system to E. coli for preparation of exendin-4.
To the best of our knowledge, this is the first study to directly investigate the effects of recombinant exendin-4 expressed in P. pastoris on glucose-lowering and insulin-releasing in vivo. The results suggested that the ability of native peptide GLP-1 to reduce plasma glucose to a certain level was at 30 min, while the activity of exendin-4 appeared at 60 min (Fig. 3a). It indicated that the exendin-4 has a longer duration of action and a greater potency than GLP-1 (Fig. 3a). Insulin is one of the most important hormonal immune responses and is linked to glucose-lowering responses. Although reports on influence of administration of exenatides on insulin activity are not very consistent, this function can be enhanced by injection administration of exendin-4 or its analogue (Gedulin et al. 2007; Kolterman et al. 2003; Nielsen et al. 2004; Xu and Kaneto 2006). The present study observed similar phenomenon. Furthermore, we observed that the capabilities of stimulating insulin secretion by GLP-1 was maintained up to 30 min, while exendin-4 was up to 60 min (Fig. 3b). Thus the half-life of exendin-4 in plasma is longer than GLP-1. There are two main reasons for this. One reason is that the exendin-4 lacks many of the neutral endopeptidase substrate sites present in GLP-1 and has a nine-AA sequence at the C-terminus absent from GLP-1 (Hupe et al. 1995). Another one is that the N-terminal sequence (His:Gly:Glu) of exendin-4 is not recognized by dipeptidylpeptidase (IV) (DPP (IV)), which rapidly cleaves the His:Ala:Glu sequence found on the N-terminus of GLP-1 (O’Harte et al. 2000). In our opinion, we should not rule out the presumption that the exendin-4 (4.3 kDa) has higher molecular weight than GLP-1 (3.1 KDa), which can reduce the glomerular filtration in vivo.
It is important to note that in the three independent experiments, the recombinant exendin-4 did not have obvious effects on one or two rats of the six. It was probably because of individual differences. The results of the three independent experiments were comparable. According to the research, the recombinant exendin-4 may also have effects at smaller doses but remains to be proved.
In summary, we have achieved high-level secretion of biologically active exendin-4 in P. pastoris. The result of the study indicated that although the heterogeneity-expressed exendin-4 in P. pastoris was slightly different from native GLP-1 in biological value, it still maintained good bioactivity. High purity and stability of the recombinant peptide of exendin-4 after purification makes it possible to be a therapeutical drug in the future. In the next step, we will focus on structure analysis and evaluation of the safety of exendin-4.
Acknowledgements
This work was supported by National Basic Research Program (973 Program No. 2007CB714303) and the grant from the “Key Project of the Technology Research and Development” (Shanghai municipal Program. No.047252058).
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Buse JB, Henry RR, Han J et al (2004) Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea treated patients with type 2 diabetes. Diabetes Care 27:2628–2635 [DOI] [PubMed]
- Edwards CM, Stanley SA, Davis R et al (2001) Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 281:155–161 [DOI] [PubMed]
- Eng J, Kleinman WA, Singh L et al (1992) Isolation and characterization of exendin-4, an exendin-3 analogue from Heloderma suspectum venom. J Biol Chem 267:7402–7405 [PubMed]
- Gedulin BR, Nikoulina SE, Smith PA et al (2007) Exenatide (exendin-4) improves insulin sensitivity and β-cell mass in insulin-resistant obese fa/fa zucker rats independent of glycemia and body weight. Endocrinology 146(4):2069–2076 [DOI] [PubMed]
- Hupe SK, Mcgregor GP, Bridenbaugh R et al (1995) Characterisation of the processing by human neutral endopeptidase 24.11 of GLP-1 (7–36) amide and comparison of the substrate specificity of the enzyme for other glucagon-like peptides. Regul Pept 58:149–156 [DOI] [PubMed]
- Kendall DM, Riddle MC, Rosenstock J et al (2005) Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes mellitus treated with metformin and a sulfonylurea. Diabetes Care 28:1083–1091 [DOI] [PubMed]
- Kolterman OG, Buse JB, Fineman MS et al (2003) Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 88:3082–3089 [DOI] [PubMed]
- Nielsen LL, Young AA, Parkes DG (2004) Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept 117:77–88 [DOI] [PubMed]
- O’Harte FPM, Mooney MH, Lawlor A et al (2000) N-terminally modified glucagons-like peptide-1 (7–36) amide exhibits resistance to enzymatic degradation while maintaining its antihyperglycaemic activity in vivo. Biochim Biophys Acta 1474:13–22 [DOI] [PubMed]
- Patrick SM, Fazenda ML, Mcneil B et al (2005) Heterologous protein production using the Pichia pastoris expression system. Yeast 22:249–270 [DOI] [PubMed]
- Xu G, Kaneto H (2006) GLP-1/exendin-4 facilitates β-cell neogenesis in rat and human pancreatic ducts. Diabetes Res Clin Pract 73:107–110 [DOI] [PubMed]
- Yi LA, Yin XP, Wei DZ et al (2006) Expression and purification of exendin-4 dimer in Eschelichia Coli and its interaction with GLP-1 receptor in vitro. Protein Pep Lett 13:823–827 [DOI] [PubMed]
- Yin XP, Wei DZ, Yi LN et al (2005) Expression and purification of exendin-4, a GLP-1 receptor agonist in Escherichia Coli. Protein Expr Purif 41:259–265 [DOI] [PubMed]
- Zhuang YP, Wang H, Chu J et al (2007) Construction, expression and culture methods of a new glucose-lowering peptide in Pichia pastoris. Chinese Patent 2,007,100,371,336 Mar 2007