Abstract
Lateralised ERP components triggered during cued shifts of spatial attention (ADAN, LDAP) have been observed during visual, auditory, and tactile attention tasks, suggesting that these components reflect supramodal attentional control processes. This interpretation has recently been called into question by the finding that the ADAN is absent in response to auditory attention cues. Here we demonstrate that ADAN and LDAP components are reliably elicited in a purely unimodal auditory attention task where auditory cues are followed by auditory imperative stimuli. The fact that the ADAN is not restricted to task contexts where visual or tactile stimuli are relevant is consistent with the hypothesis that this component is linked to supramodal attentional control.
Investigations into the brain processes that mediate endogenous shifts of spatial attention have observed lateralised ERP components that are elicited in the interval between central symbolic attention cues and subsequently presented imperative stimuli (c.f., Nobre, Sebestyen & Miniussi, 2000; Hopf & Mangun, 2000; Eimer, Van Velzen, & Driver, 2002). An anterior directing attention negativity (ADAN), reflecting an enhanced negativity at anterior electrodes contralateral to the cued side of an attentional shift, was followed by a late directing attention positivity (LDAP), reflecting an enhanced posterior contralateral positivity. The ADAN is assumed to be generated in lateral premotor cortex and/or the frontal eye fields that are part of the frontoparietal attentional control network (Praamstra, Boutsen & Humphreys, 2005; Van der Lubbe, Neggers, Verleger, & Kenemans, 2006). The LDAP is assumed to originate in lateral occipital cortex (Praamstra et al., 2005), but has also been tentatively localized in the ventral intraparietal sulcus (Van der Lubbe et al., 2006).
One notable fact about ADAN and LDAP components is that they are not only elicited during cued shifts of visual-spatial attention, but also in experiments where visual or auditory cues direct attention towards the location of anticipated task-relevant auditory or tactile events (e.g., Eimer et al., 2002; Eimer, Van Velzen, Forster, & Driver, 2003). This observation has led us to suggest that these components may reflect supramodal attentional control processes that are triggered during shifts of spatial attention regardless of which stimulus modality is currently task-relevant. This interpretation has recently been called into question by Green, Teder-Sälejärvi, & McDonald (2005), and Green & McDonald (2006), who did not find an ADAN component when auditory cues were followed by auditory or visual targets, but only when visual cues were used instead. In contrast, the LDAP was triggered regardless of cue modality. Based on these findings, Green and colleagues suggested that while the LDAP may be associated with supramodal attentional control, the ADAN is more likely to be linked to the control of attention in visual space.
The absence of an ADAN in the experiments of Green et al. (2005) and Green & McDonald (2006) is puzzling, given that reliable ADAN components have previously been found during attentional shifts triggered by auditory cues (Eimer & Van Velzen, 2002; Eimer et al., 2003), both for sighted and congenitally blind people (Van Velzen, Eardley, Forster & Eimer, 2006). This latter finding demonstrates that the ADAN is not specifically linked to visually mediated attentional control processes, as suggested by Green et al. (2005).
Systematic differences between the attentional manipulations used by Green and colleagues, and those used in experiments where an ADAN was observed for auditory cues, may account for these discrepant results. In our earlier studies (Eimer et al., 2002, 2003; Eimer & Van Velzen, 2002; Van Velzen et al., 2006), we instructed participants to respond to infrequent target stimuli on the cued side only, and to ignore stimuli on the uncued side, in order to ensure that their attention was fully focussed. In contrast, Green and colleagues asked participants to respond to frequent “targets” regardless of their location, and to infrequent “probes” only when these were presented on the cued side. When perceptual discriminations and responses are frequently required to stimuli on the uncued side, the attentional focus is likely to be more diffuse than under conditions where uncued stimuli can be entirely ignored, and this may have resulted in the elimination of the ADAN to auditory attention cues.
The present experiment was conducted to investigate this possibility. We used procedures analogous to Green et al. (2005, Experiment 4). Central auditory cues were followed by a target (white noise with or without a gap) or a probe (a cowbell) on the left or right side. Participants had to discriminate gap and non-gap targets on either side, and to respond to probes only when they were presented on the cued side. In one condition, probes were presented on one third of all trials (as in Green et al., 2005), while they appeared in two thirds of all trials in the other condition. Because task-relevant stimuli were less likely to appear at uncued locations, attention was expected to be more focused in the ‘frequent probe’ relative to the ‘infrequent probe’ condition. The critical question was whether an ADAN would be elicited in this condition in response to auditory cues.
Methods
Participants
Seventeen paid volunteers participated in the experiment. Three were excluded due to poor eye gaze control in the cue-target interval (see below), and two others were excluded due to an insufficient number of trials after artefact rejection. Thus twelve participants (7 females), aged 18-36 years (mean age: 26.3 years) remained in the sample. One participant was left handed, and one ambidextrous. All had normal or corrected to normal vision.
Stimuli, Apparatus, and Procedure
Participants were tested in a darkened room, and fixated a cross displayed continuously on a centrally located computer monitor. On each trial, an auditory cue (a 1000 or 1500 Hz tone presented for 100 ms at 74 dB SPL from a central loudspeaker) was followed after an empty interval of 900 ms by a target or a probe sound presented from one of two loudspeakers located 17° to the left or right of the central speaker). Target sounds were 100 ms bursts of white noise (20 ms rise and fall times, 71 db SPL) that were continuous or contained a 30 ms silent gap that started 40 ms after sound onset. A cowbell (100 ms duration, 64 dB SPL) served as probe sound.1 Intertrial interval was 1400 ms.
Eight blocks with 96 trials per block were run. Four successive blocks (infrequent probe condition) replicated the procedure used by Green et al. (2005, Experiment 4). Targets were presented on two thirds of all trials, and probes on one third, with equal probability on the left or right side. The task was to press a left response key for continuous targets, a right key for gap targets, and a middle response key when the probe was presented at the cued location (as indicated by the auditory cue, with mappings of cue frequency and side counterbalanced across participants). Response hand was changed after two blocks. The other four blocks (frequent probe condition) were identical, except that probes were now presented on two thirds of all trials, and targets on one third. The order of these two conditions was counterbalanced across participants.
Recording and Data Analysis
EEG was DC-recorded from 63 Ag-AgCl electrodes relative to a right earlobe reference (all impedances below 5 kΩ; 500 Hz sampling rate; 40 Hz upper cut-off frequency). EEG was digitally re-referenced to the average of the left and right earlobe, and epoched into 1000 ms periods, starting 100 ms prior to cue onset and ending 900 ms after cue onset. Trials with eyeblinks (Fpz exceeding ±80 μV relative to baseline), horizontal eye movements (HEOG exceeding ±30 μV relative to baseline), or other artefacts (a voltage exceeding ±80 μV at any electrode location relative to baseline) were excluded. Averaged HEOG waveforms to auditory cues directing attention to the left versus right side were scored for systematic deviations of eye position, indicating residual eye movements towards the cued location. HEOG deviations exceeding ±3.5 μV led to the disqualification of three participants.
The EEG obtained in the cue-target interval was averaged for all combinations of condition (infrequent versus frequent probe) and cued side (left versus right). The ADAN was quantified on the basis of mean amplitude values at lateral anterior sites (F3/4, F5/6, FC3/4, FC5/6), and the LDAP at lateral posterior sites (P5/6, P7/8, PO7/O8, P9/10) during the 300-500 ms and 600-900 ms post-cue intervals, respectively. Mean amplitudes were analysed by repeated measures ANOVAs for the factors electrode site, condition, lateralization (electrode ipsilateral versus contralateral to the cued side), cued side (left vs. right) and condition order (frequent and infrequent condition presented first).
Results
Behavioural performance
Participants missed probes on the cued side on 7% and 4% of these trials in the infrequent and frequent probe conditions, respectively. False Alarm rates to uncued probes were 9% and 6% in these two conditions. As expected, reaction times (RTs) to probes were faster in the frequent relative to the infrequent probe condition (598 ms vs. 772 ms; t(11)=6.4, p<.001). A repeated measures ANOVA for RTs to target sounds revealed a main effect of cueing, F(1,11)=8.5, p<.02, with faster RTs for targets on the cued relative to the uncued side (771 ms vs. 787 ms), but no main effect of condition, and no condition x cueing interaction. Incorrect responses occurred on 3.8% of all target trials. The order of conditions did not influence the RT results obtained for the probe, F<1, or target sounds, F<1.
Lateralised ERP components in the cue-target interval
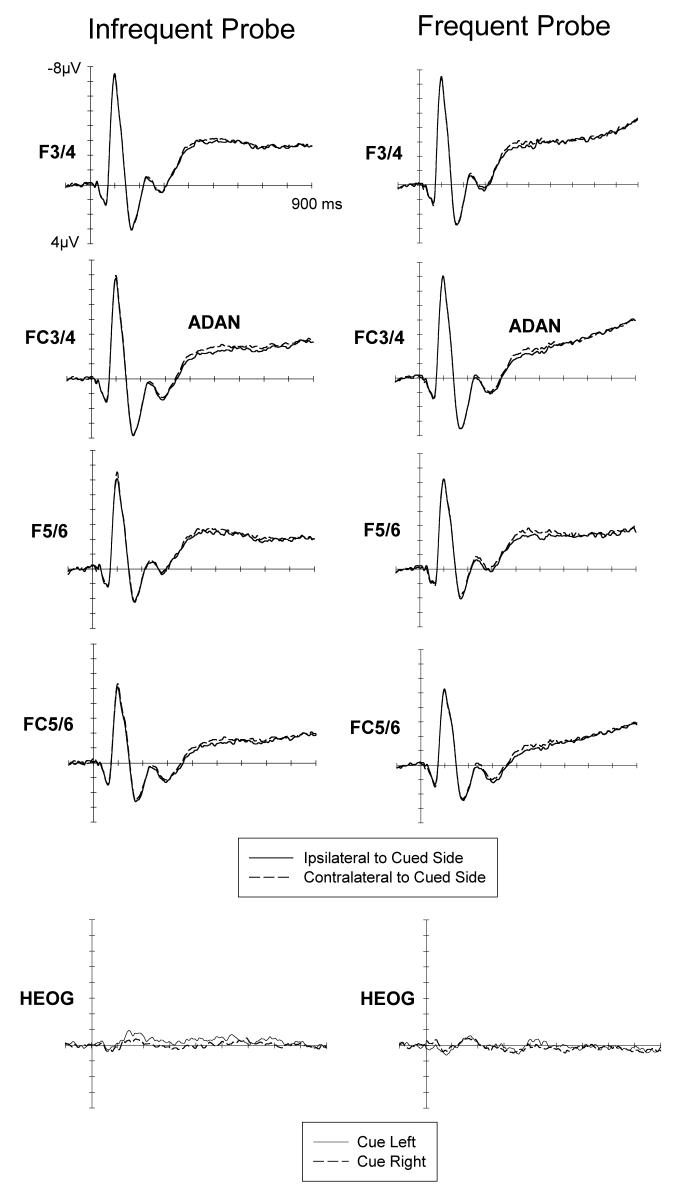
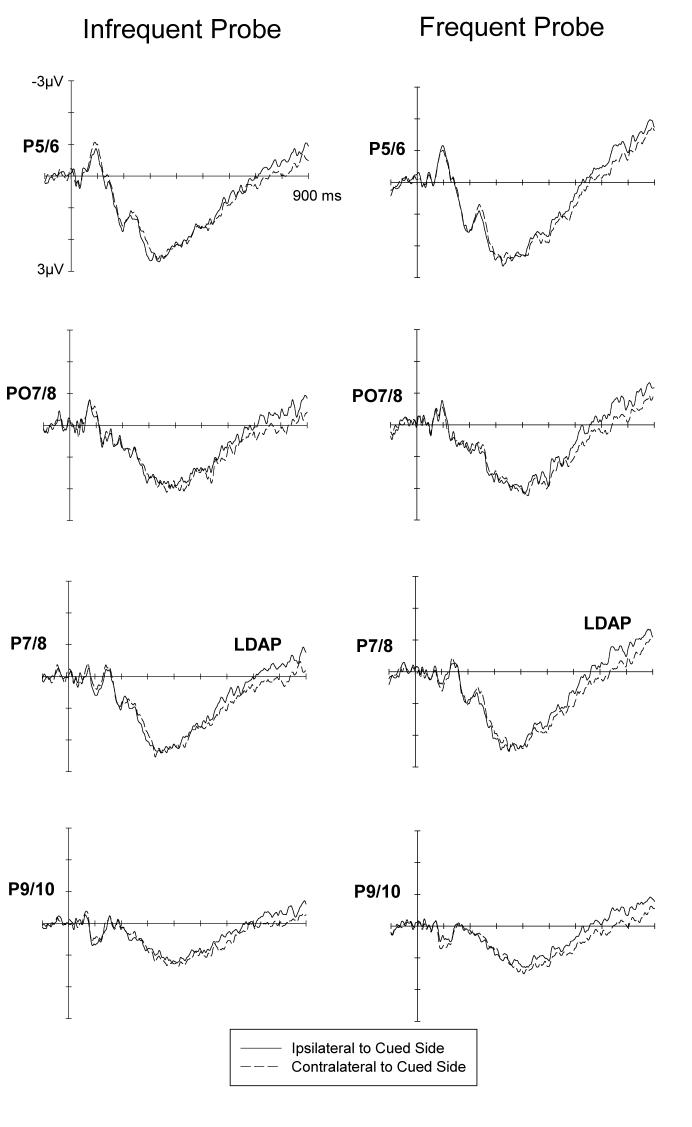
Figures 1 and 2 show ERPs to auditory cues at lateral anterior and posterior electrodes ipsilateral and contralateral to the cued side, for the infrequent (left) and frequent (right) cue condition. Both the ADAN (Figure 1) and the LDAP (Figure 2) appear to be present in both task conditions, and this was confirmed by statistical tests. At lateral anterior electrodes, a main effect of lateralization during the 300-500 ms post-cue interval, F(1,11)=7.6, p<.02, reflected the presence of the ADAN. The main effect of cue was not significant, F<1. There was no condition x lateralization interaction, F<1, as ADAN amplitudes did not differ between the infrequent and frequent probe condition. In the 600-900 ms interval, a main lateralization effect, F(1,11)=16.0, p<.002, demonstrated that an LDAP was present. The main effect of cue was not significant (F<1). Again, no condition x lateralization interaction was obtained, F<1, as LDAP amplitudes were equivalent in blocks with frequent and infrequent probes.
Figure 1.
Grand-averaged ERPs elicited in the 900 ms interval following cue onset at lateral anterior electrodes ipsilateral and contralateral to the cued side, for the infrequent and frequent probe conditions. ADAN: Anterior Directing Attention Negativity. Bottom: Grand-averaged HEOG waveforms in response to left and right cues in the infrequent and frequent probe condition. Scalp distribution map of the ADAN showing a maximum activation over lateral frontocentral electrode sides in the time interval between 400 and 500 ms (maximum effect) after cue onset. This map represents the difference between ipsi- and contralateral brain acticity and was constructed by spherical spline interpolation (see Perrin, Pernier, Bertrand, & Echallier, 1989) after mirroring the difference waveforms to the contralateral hemisphere to obtain symmetrical, but inverse, voltage values for both hemispheres. Amplitudes range between −0.6 to 0.6μV; each line represents an increment of 0.1μV.
Figure 2.
Grand-averaged ERPs elicited in the 900 ms interval following cue onset at lateral posterior electrodes ipsilateral and contralateral to the cued side, for the infrequent and frequent probe conditions. LDAP: Late Directing Attention Positivity. Scalp distribution map of the LDAP showing a maximum over lateral posterior electrode sides in the time interval between 700 and 800 ms (maximum effect) after cue onset. This map represents the difference between ipsi- and contralateral brain activity. It was constructed in the same was as for the ADAN. As a result of the mirroring procedure, the LDAP appears as negative voltage (blue) over the left hemisphere and as positive voltage over the right hemisphere (red). Amplitudes range between from −0.6 and0.6μV; each line represents an increment of 0.1μV.
To further explore the surprising fact that a reliable ADAN component was obtained in the infrequent probe condition, whereas Green et al. (2005) did not find an ADAN under identical circumstances, follow-up analyses were conducted for this condition only. Although a reliable ADAN, F(1,11)=11.5, p<.006, was observed when ERPs were analysed across all four anterior electrode pairs, the ADAN was not significant when only the electrode pair used by Green et al. (2005) to quantify the ADAN (F5/6) was considered, F(1,11)=2.3, p>.15. When ERP differences between electrodes contralateral and ipsilateral to the cued side were tested within successive 20 ms time windows for the 300-500 ms post-cue interval for F5/6 (analogous to Green et al., 2006), significant differences (p<.05) were only observed for two out of ten intervals, suggesting that the ADAN was not reliably elicited in the infrequent probe condition at F5/6. To strengthen our argument, we analysed the ADAN in the infrequent cueing condition only for the subset of participants that performed this condition before the frequent cueing condition. This analysis was conducted to exclude the explanation that the ADAN in the infrequent cueing condition is caused by carry over effects between conditions. This explanation could be ruled out. A reliable ADAN was observed for this subset of participants when ERPs were analysed across all 4 anterior electrode sites, F(1,6)=17.0, p=.006. However, the ADAN was not significant when only F5/6 were used in the analysis, F(1,6)=2.8, p=.14.
The ADAN in the infrequent probe condition is not modulated by the condition order, both, in the analysis with all four electrode pairs (interaction lateralisation x task order: F<1) and in the analysis with the F5/6 only (F<1).
Discussion
We used attentional cueing procedures analogous to those employed by Green and colleagues (2005, 2006) to investigate the claim that the ADAN component is absent when shifts of attention are signalled by auditory cues. The central finding was that an ADAN was reliably elicited regardless of whether probes were presented infrequently (as in Green et al., 2005), or more frequently, so that stimuli at uncued locations were less likely to be task-relevant. The presence of an ADAN in the frequent probe condition demonstrates that this component can be observed under purely unimodal auditory attention conditions. Its presence in the infrequent probe condition is surprising, as it is inconsistent with results by Green et al. (2005, Experiment 4), who found no ADAN under virtually identical conditions. One possible explanation for this discrepancy is that Green et al. (2005) quantified the ADAN exclusively for electrodes F5/6. In the present experiment, the ADAN was very small at F5/6 in the infrequent probe condition (Figure 1), and in fact non-significant during the critical 300-500 ms interval. However, Green & McDonald (2006) found no reliable ADAN for auditory cues (upward or downward frequency sweeps) followed by visual targets even when all four anterior electrode pairs included in the present analysis were considered. The fact that the ADAN observed in the present experiment, although reliable, was considerably smaller than the ADAN observed previously for auditory cues signalling the location of task-relevant visual or tactile events (Eimer & van Velzen, 2002; Eimer et al., 2003; Van Velzen et al., 2006) suggests that this component may be attenuated in purely unimodal auditory attention tasks, which could have contributed to the negative results reported by Green and colleagues.
It is unlikely that the ADAN observed here resulted from undetected lateral eye movements. As can be seen in the grand-averaged HEOG waveforms (Figure 1, bottom), eye movement rejection and the exclusion of three participants with poor gaze control ensured that there were no systematic eye gaze deviation during the cue-target interval. The presence of ADAN components in the present experiment demonstrates that this component is triggered in response to auditory cues in a unimodal auditory attention task, and is consistent with previous observations that an ADAN is elicited by auditory cues during visual or tactile attention tasks (Eimer & Van Velzen, 2002; Eimer et al. 2003), and is also reliably observed in the congenitally blind when auditory cues direct attention to the location of task-relevant tactile events (Van Velzen et al., 2006). Green and colleagues have argued against an interpretation of the ADAN in terms of supramodal attentional control because this component was absent with auditory cues, and suggested that the ADAN is linked to the control of attention in visual (and possibly tactile) space. The present demonstration that this component can be reliably elicited in a unimodal cued auditory attention task provides strong evidence against this argument, and thus supports a supramodal interpretation.
The present results and the results by Green et al. (2005) and Green & McDonald (2006) converge with respect to the posterior LDAP component. This component was consistently present, demonstrating that the LDAP is not confined to visual and tactile attention tasks, but is also triggered in a unimodal auditory context. While this is consistent with the hypothesis that this component is linked to supramodal attentional control, other interpretations remain possible. The fact that the LDAP is eliminated during shifts of tactile attention in the congenitally blind (Van Velzen et al., 2006) suggests that this component may be associated with the availability of visually mediated spatial information. Given the superior spatial acuity of vision, visual-spatial coordinates may be used whenever available to anchor shifts of attention in external space, even when other modalities are task-relevant, and the LDAP might emerge as a result of such visually mediated attention shifts.
In summary, the present study has shown that an ADAN can be observed during shifts of auditory attention that are triggered by symbolic auditory cues. This demonstrates that this component is not specific to tasks where visual or tactile stimuli are relevant, and is consistent with the hypothesis that the ADAN reflects supramodal attentional control mechanisms.
Acknowledgments
This research has been supported by grants from the Wellcome Trust, the Economic and Social Research Council (ESRC), and the Medical Research Council (MRC), UK. M.E. holds a Royal Society-Wolfson Research Merit Award.
Footnotes
Our thanks to Jessica Green and John McDonald providing us with the cowbell sound file employed in their experiments.
References
- Eimer M, Van Velzen J. Crossmodal links in spatial attention are mediated by supramodal control processes: Evidence from event-related potentials. Psychophysiology. 2002;39:437–449. doi: 10.1017.S0048577201393162. [DOI] [PubMed] [Google Scholar]
- Eimer M, Van Velzen J, Driver J. Crossmodal interactions between audition, touch and vision in endogenous spatial attention: ERP evidence on preparatory states and sensory modulations. Journal of Cognitive Neuroscience. 2002;14:254–271. doi: 10.1162/089892902317236885. [DOI] [PubMed] [Google Scholar]
- Eimer M, Van Velzen J, Forster B, Driver J. Shifts of attention in light and in darkness: An ERP study of supramodal attentional control and crossmodal links in spatial attention. Cognitive Brain Research. 2003;15:308–323. doi: 10.1016/s0926-6410(02)00203-3. [DOI] [PubMed] [Google Scholar]
- Green J, McDonald JJ. An event-related potential study of supramodal attentional control and crossmodal attention effects. Psychophysiology. 2006;43:161–171. doi: 10.1111/j.1469-8986.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- Green J, Teder-Sälejärvi WA, McDonald JJ. Control mechanisms mediating shifts of attention in auditory and visual space: A spatio-temporal ERP analysis. Experimental Brain Research. 2005;166:358–369. doi: 10.1007/s00221-005-2377-8. [DOI] [PubMed] [Google Scholar]
- Hopf JM, Mangun GR. Shifting visual attention in space: An electrophysiological analysis using high spatial resolution mapping. Clinical Neurophysiolology. 2000;111:1241–1257. doi: 10.1016/s1388-2457(00)00313-8. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Sebestyen GN, Miniussi C. The dynamics of shifting visuospatial attention revealed by event-related brain potentials. Neuropsychologia. 2000;38:964–974. doi: 10.1016/s0028-3932(00)00015-4. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Boutsen L, Humphreys GW. Frontoparietal control of spatial attention and motor intention in human EEG. Journal of Neurophysiology. 2005;94:764–774. doi: 10.1152/jn.01052.2004. [DOI] [PubMed] [Google Scholar]
- Van der Lubbe RH, Neggers SF, Verleger R, Kenemans JL. Spatiotemporal overlap between brain activation related to saccade preparation and attentional orienting. Brain Research. 2006;1072:133–152. doi: 10.1016/j.brainres.2005.11.087. [DOI] [PubMed] [Google Scholar]
- Van Velzen J, Eardley AF, Forster B, Eimer M. Shifts of attention in the early blind: An ERP study of attentional control processes in the absence visual spatial information. Neuropsychologia. 2006;44:2533–2546. doi: 10.1016/j.neuropsychologia.2006.03.025. [DOI] [PubMed] [Google Scholar]