Abstract
To gain insight into the neural basis of visual attention we combined transcranial magnetic stimulation (TMS) and event related potentials (ERP) during a visual search task. Single-pulse TMS over right posterior parietal cortex (rPPC) delayed response times to targets during conjunction search, and this behavioural effect had a direct ERP correlate. The early phase of the N2pc component that reflects the focusing of attention onto target locations in a search display was eliminated over the right hemisphere when TMS was applied there, but was present when TMS was delivered to a control site (vertex). This finding demonstrates that rPPC TMS interferes with attentional selectivity in remote visual areas.
Keywords: Attention, Event-related potentials, Transcranial magnetic stimulation, Neuroimaging, ERP–TMS combination, Visual search
INTRODUCTION
Numerous studies have used visual search to study the role of spatial attention in target selection when no advance spatial information is available (Treisman and Sato 1990). Functional brain imaging studies have suggested the involvement of a dorsal frontoparietal attentional network in visual search (Nobre et al. 2003). However, the specific functions of different parts of this network such as the frontal eye fields (FEFs) or posterior parietal cortex (PPC) are still poorly understood, and there is little direct evidence of the interactions of these areas with sensory cortex. Dissociating the roles of such areas requires methods that afford high-temporal resolution to capture the dynamics of cortico-cortical interactions, and to identify the effects of these areas on different stages of visual processing. Here, we combined event-related potentials (ERPs) and transcranial magnetic stimulation (TMS) to obtain new insight into how PPC contributes to selectivity in visual search. Previous TMS studies have provided evidence of the role of right PPC (rPPC) in visual search (Ashbridge et al. 1997; Fierro et al. 2001; Muri et al. 2002; Walsh et al. 1999). Single-pulse TMS over rPPC applied 100 ms after search array onset, for example, delays reaction times (RTs) to conjunction targets (Ashbridge et al. 1997). Previous ERP studies have shown that target detection in visual search gives rise to an enhanced N2 component at occipital electrodes contralateral to the side of a target (N2pc) that is typically elicited at latencies beyond 200 ms (Eimer 1996; Luck and Hillyard 1994; Woodman and Luck 1999). The N2pc originates from ventral occipital cortex in humans (Hopf et al. 2000) and is interpreted as a marker for a shift of attention to the search target location. In the present study, we combined TMS and EEG/ERP recordings in a task where targets were defined by a conjunction of colour and orientation. Single-pulse TMS was applied over rPPC, behavioural and ERP measures were obtained in parallel, and compared to blocks where TMS was applied to a control site (vertex). We expected RTs on target-present trials to be delayed with rPPC relative to vertex TMS. If this was due to a specific impairment of spatial attention shifts caused by rPPC stimulation, the N2pc should be delayed and/or attenuated when TMS is applied over rPPC, but not for vertex stimulation.
MATERIALS AND METHODS
Participants
Data from seven healthy right-handed volunteers (one female, aged 24–43 years) with normal or corrected-to-normal vision were analysed. Three other participants were excluded due to excessive eye movement artefacts. Subjects gave written informed consent. The study was approved by the Psychology Ethics Committee, Birkbeck College.
Stimuli and Procedure
Subjects were seated 57cm in front of an LCD monitor. The screen was divided into a virtual array of 36 positions (6 columns × 6 rows), with the innermost positions located 3° to the left and right of a black fixation cross (0.7° × 0.7° visual angle). On each trial, target and/or distractors appeared randomly in eight of these positions, with four stimuli to the left and four to the right of fixation. The target (a green vertical bar) and the distractors (green horizontal and blue vertical bars) subtended 1.5° × 0.5°of visual angle, and were presented against a grey background. Sixteen experimental blocks with 84 visual search trials per block were run. Search arrays were presented for 720 ms. Intertrial interval was 2426 ms. Subjects reported the presence or absence of a target as fast and accurately as possible by pressing the ‘1’ or ‘2’ key on a keyboard. Within blocks targets were presented with equal probability in the left or right visual field on 48 trials, and was absent in 24 trials. In half the trials, TMS was delivered 100 ms after search array onset; no TMS was applied in the other half. A TMS pulse was delivered without any visual stimulus on 12 randomly interspersed trials (TMS-only trials). The TMS coil was changed after every two blocks of trials to prevent overheating. A head and chin rest was used to minimize head movements, and a coil holding device was employed to ensure stable coil position.
TMS
Single pulse TMS was delivered via a 70mm figure-of-eight coil connected to a Magstim Super Rapid Transcranial Magnetic Stimulator (Magstim, Dyfed, UK). A single TMS application time (100 ms after array onset; based on pilot data and on Ashbridge et al. 1997) was chosen to limit the number of conditions and number of trials required to compute reliable ERP waveforms. Stimulation sites were Vertex (Cz) and right posterior parietal cortex (rPPC). The rPPC site was identified within a 2 cm radius from electrode location P4 by using a hunting procedure as described previously by Ashbridge et al. (1997), and employed by Ellison et al. (2004). A recent study (Okamoto et al. 2004) which used cranio-cerebral projections to characterize the relationship between standard10-20 positions and underlying cortical structures has demonstrated that P4 is usually located above the right angular gyrus. PPC and vertex stimulation were delivered in separate halves of the experiment, in counterbalanced order across subjects. To compensate for the increased distance between coil and scalp necessitated by the EEG cap, TMS intensity was slightly higher (on average 85% of maximum stimulator output) than in previous behavioural studies.
EEG recording and analysis
EEG was recorded with a DC 32-channel-amplifier (1-kHz sampling rate; 250 Hz high cutoff frequency) from 25 Ag-AgCl electrodes with linked-earlobes reference. Horizontal EOG (HEOG) was recorded from electrodes positioned on the outer canthi of both eyes. Impedance was kept below 5kΩ. Because EEG acquisition was continuous, EEG waveforms included a TMS artefact induced by the magnetic pulse (18 ms duration, with a rebound residual after 30 ms). Artefacts were removed by cutting out 40 ms segments (from 2 ms prior to TMS onset to 38 ms after TMS onset) from EEG waveforms for all TMS trials and electrodes. EEG data points before and after each removed segment were then joined. This induced a random voltage step between the joined data points for single trials, which was eliminated by EEG averaging.
Only EEG data for TMS trials with correct responses and reaction times (RTs) between 150 ms and 1300 ms were analysed. EEG was epoched from 100 ms prior to search array onset to 740 ms after array onset (without the 40 ms segment removed through artefact cutting). Epochs with eye movements and muscle or movement artefacts (as indicated by HEOG activity exceeding ± 40 μV, and activity at other electrodes exceeding ± 80 μV) were excluded from analysis. ERPs were computed for different combinations of target location (left, right) and TMS condition (vertex, rPPC), relative to a 100 ms pre-stimulus baseline. To remove contribution of TMS-induced auditory and somatosensory activity to these ERPs, EEG recorded on TMS-only trials was averaged and then subtracted from ERPs on visual search trials with TMS, separately for blocks with TMS applied to rPPC or vertex (see Thut et al. 2005 for details of this artefact template subtraction technique). ERPs were then filtered using 0.01 Hz high-pass, 40 Hz low-pass, and 50 Hz notch filters. Differential effects of TMS applied to rPPC versus vertex on the N2pc were quantified on the basis of ERP mean amplitudes in two time windows (250-300 ms and 363-413 ms after array onset). Separate repeated measures analyses of variance (ANOVAs) were conducted for parieto-occipital electrodes over the right hemisphere where TMS was applied (P8, PO8), and over the left hemisphere (P7, PO7), for the factors electrode site, coil position (rPPC, vertex), and contralaterality (target contralateral versus ipsilateral to electrode). Greenhouse-Geisser epsilon adjustments for nonsphericity were applied where appropriate. Post-hoc paired t-tests were Bonferroni corrected for multiple comparisons.
RESULTS
Behavioural effects
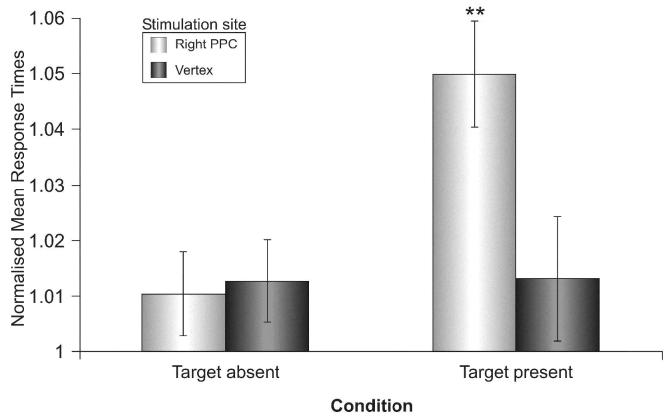
To control for interindividual RT differences, and for the order of TMS locations, RT data were normalized by computing the ratio between mean RTs for trials with and without TMS for different trial types. A main effect of coil position (rPPC versus vertex: F(1, 6)=7.1, p<.05) and a main effect of target (present versus absent: F(1, 6)=11.8, p<.05) showed that RTs were delayed with rPPC relative to vertex stimulation (see Fig.1). The interaction coil position × target was also significant (F(1, 6)=9.9, p<.05), Post-hoc comparisons showed that RTs on target-present trials were delayed with rPPC relative to vertex stimulation (p<.01). Further analyses were conducted for raw RTs. Mean raw RTs were delayed on target-present trials with rPPC stimulation relative to no-TMS trials (571 vs. 543 ms, p<.05), whereas no significant difference between TMS and no-TMS trials was found for blocks with vertex stimulation (546 vs. 540 ms). When rPPC stimulation was applied, RTs to left and right targets did not differ significantly (572 vs. 569 ms), thus ruling out the possibility that lateralised auditory or tactile effects of rPPC stimulation might have triggered automatic attention shifts towards the right. Errors were below 5% for all trial types.
Fig. 1.
Mean normalised response times (RT ± S.E.) for target-absent and target-present trials with TMS applied over rPPC or vertex.
ERP effects
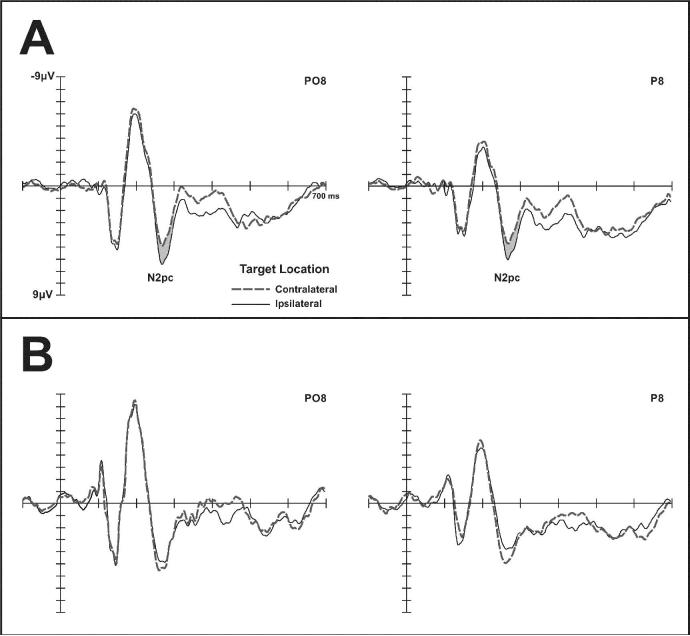
Fig. 2 shows ERPs at right posterior electrodes P8 and PO8 in response to arrays with contralateral (left) or ipsilateral (right) targets, when TMS was delivered to the vertex (A) or to rPPC (B). The early phase of the N2pc component (250-300 ms post-stimulus) was present with vertex TMS, but absent for rPPC stimulation, as reflected by a contralaterality × coil position interaction (F(1, 6)=7.1, p<.05). Post-hoc comparisons revealed a significant N2pc (1.270 μV, p<.01) with vertex TMS, and no N2pc (− 0.621 μV, p= ns) for rPPC stimulation. In the late N2pc time window (363–413 ms post-stimulus), a main effect of contralaterality (F(1, 6)=6.0, p<.05), but no contralaterality × coil position interaction was present at P8 and PO8, suggesting that later phases of the N2pc were not affected by TMS (see Fig.2). At left posterior electrodes (P7, PO7, not shown in Fig.2), no significant contralaterality × coil position interactions were present for either time window.
Fig. 2.
Grand-averaged ERPs elicited at right posterior electrodes P8 and PO8 in response to arrays containing a target in the contralateral (left; dashed lines) or ipsilateral (right, solid lines) visual field. A: TMS over vertex. B: TMS over rPPC.
DISCUSSION
To investigate the role of rPPC for target selection in a conjunction search task, we used a combined TMS/ERP procedure where single pulse TMS was delivered 100 ms after search array onset over rPPC or a control site (vertex). RTs on target-present trials were delayed with rPPC relative to vertex stimulation. This impairment of search performance was reflected by systematic TMS effects on the N2pc component over the right hemisphere where TMS was delivered. For trials with rPPC stimulation, this component was absent between 250 and 300 ms post-stimulus, and only appeared in a later time window (363-413 ms). In contrast, the N2pc was present in both time windows with vertex stimulation. No differential effects of TMS on N2pc amplitudes were found over the unstimulated (left) hemisphere. Magnetoencephalographic results (Hopf et al. 2000) have suggested that the initial portion of magnetic equivalent of the N2pc reflects neuronal activity in the posterior parietal lobe that is linked to processes involved in the initiation of attention shifts, whereas the later portion of the N2pc reflects attentional modulation of visual processing in extrastriate occipito-temporal areas. Our observation that TMS over rPPC affects early, but not later phases of the N2pc could thus be interpreted as evidence for a disruption of posterior parietal attentional control processes. However, the N2pc as seen in ERP waveforms appears to be primarily generated by occipito-temporal sources (Hopf et al. 2000), and is usually interpreted as a result of re-entrant feedback signals from regions involved in higher-order attentional control processes (such as PPC) to extrastriate ventral visual regions (Woodman and Luck 1999). The differential effects of rPPC versus vertex stimulation during the early phase of the N2pc are therefore more likely to reflect a delay of spatially selective processing in extrastriate visual cortex that is caused by TMS-induced disruptions of attentional control mechanisms in PPC (see Rushworth et al. 2005 for details of connections between posterior parietal regions and ventral visual areas in the temporal lobe).
In summary, the current results show for the first time that a TMS-induced disruption of attentional control mechanisms in right posterior parietal cortex not only impairs performance in a visual search task, but also delays the onset of the N2pc component. The observation that TMS over rPPC can affect the spatially selective processing of visual stimuli in remote extrastriate areas within 250 ms after stimulus onset provides new evidence for cortico-cortical links that implement top-down attentional modulations of sensory processing.
Acknowledgements
This work was supported by a grant from the Wellcome Trust. GF is a member of the Marie Curie RTN:LAB (MRTN-CT-2004-512141). VW is supported by the Royal Society. ME holds a Royal Society-Wolfson Research Merit Award.
REFERENCES
- Ashbridge E, Walsh V, Cowey A. Temporal aspects of visual search studied by transcranial magnetic stimulation. Neuropsychologia. 1997;35:1121–1131. doi: 10.1016/s0028-3932(97)00003-1. [DOI] [PubMed] [Google Scholar]
- Eimer M. The N2pc component as an indicator of attentional selectivity. Electroencephalog Clin Neurophysiol. 1996;99:225–234. doi: 10.1016/0013-4694(96)95711-9. [DOI] [PubMed] [Google Scholar]
- Ellison A, Schindler I, Pattison LL, Milner AD. An exploration of the role of the superior temporal gyrus in visual search and spatial perception using TMS. Brain. 2004;127:2307–2315. doi: 10.1093/brain/awh244. [DOI] [PubMed] [Google Scholar]
- Fierro B, Brighina F, Piazza A, Oliveri M, Bisiach E. Timing of right parietal and frontal cortex activity in visuo-spatial perception: a TMS study in normal individuals. Neuroreport. 2001;12:2605–2607. doi: 10.1097/00001756-200108080-00062. [DOI] [PubMed] [Google Scholar]
- Hopf JM, Luck SJ, Girelli M, Hagner T, Mangun GR, Scheich H, Heinze HJ. Neural sources of focused attention in visual search. Cereb Cortex. 2000;10:1233–1241. doi: 10.1093/cercor/10.12.1233. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Spatial filtering during visual search: Evidence from human electrophysiology. J Exp Psychol Hum Percept Perform. 1994;20:1000–1014. doi: 10.1037//0096-1523.20.5.1000. [DOI] [PubMed] [Google Scholar]
- Muri RM, Buhler R, Heinemann D, Mosimann UP, Felblinger J, Schlaepfer TE, Hess CW. Hemispheric asymmetry in visuospatial attention assessed with transcranial magnetic stimulation. Exp Brain Res. 2002;143:426–430. doi: 10.1007/s00221-002-1009-9. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Coull JT, Walsh V, Frith CD. Brain activations during visual search: Contributions of search efficiency versus feature binding. Neuroimage. 2003;18:91–103. doi: 10.1006/nimg.2002.1329. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Dan H, Sakamoto K, Takeo K, Shimizu K, Kohno S, Oda I, Isobe S, Suzuki T, Kohyama K, Dan I. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10-20 system oriented for transcranial functional brain mapping. Neuroimage. 2004;21:99–111. doi: 10.1016/j.neuroimage.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Behrens TEJ, Johansen-Berg H. Connection patterns distinguish 3 regions of human parietal cortex. Cerebral Cortex. 2005 November 28; doi: 10.1093/cercor/bhj079. Advance Access online publication. [DOI] [PubMed] [Google Scholar]
- Thut G, Ives JR, Kampmann F, Pastor MA, Pascual-Leone A. A new device and protocol for combining TMS and online recordings of EEG and evoked potentials. J Neurosci Methods. 2005;141:207–217. doi: 10.1016/j.jneumeth.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Treisman A, Sato S. Conjunction search revisited. J Exp Psychol Hum Percept Perform. 1990;16:459–478. doi: 10.1037//0096-1523.16.3.459. [DOI] [PubMed] [Google Scholar]
- Walsh V, Ellison A, Ashbridge E, Cowey A. The role of the parietal cortex in visual attention - hemispheric asymmetries and the effects of learning: a magnetic stimulation study. Neuropsychologia. 1999;37:245–251. doi: 10.1016/s0028-3932(98)00099-2. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Electrophysiological measurement of rapid shifts of attention during visual search. Nature. 1999;400:867–869. doi: 10.1038/23698. [DOI] [PubMed] [Google Scholar]