Abstract
Cognitive impairment remains a major complication of advanced human immunodeficiency virus (HIV) infection despite the wide spread use of anti-retroviral therapy. Diagnosis is made by exclusion making biomarkers of great potential use. Thus, we used an integrated proteomics platform to assess cerebrospinal fluid protein profiles from 50 HIV-1 seropositive Hispanic women. Nine of 38 proteins identified were unique in those patients with cognitive impairment. These proteins were linked to cell signaling, structural function, and antioxidant activities. This work highlights, in a preliminary manner, the utility of proteomic profiling for biomarker discovery for HIV-1 associated cognitive dysfunction.
Keywords: HIV-associated cognitive impairment, biomarkers, proteomics, CSF
1. Introduction
Cognitive, behavioral, and motor dysfunction induced as a consequence of HIV-1 infection remain chronic and debilitating despite the advent of anti-retroviral therapy (McArthur, 2004). In its most severe form, HIV-associated cognitive impairment is termed HIV-associated dementia (HAD). Anti-retroviral therapy has altered the magnitude of HIV-associated cognitive impairments as patients present milder forms of disease and show different patterns of mental dysfunction. This represents significant challenges in making a firm diagnosis to the exclusion of opportunistic brain infections, tumors, or psychiatric and behavioral disorders (Everall et al., 2005; Wojna and Nath, 2006; Ances and Ellis, 2007). In era of active antiretroviral therapy, the term HIV-associated cognitive impairment (CI) is preferred over HAD. Indeed, cognitive dysfunction is manifest with milder forms of impairment and present with increased CD4+ T cell counts (Navia and Rostasy, 2005; Wojna et al., 2006; Woods et al., 2007).
Viral and immune biomarkers associated with the development of HIV-associated cognitive impairments are neither diagnostic nor predictive (Anthony et al., 2005; St. Hillaire et al., 2005). More commonly, biomarkers associated with cognitive impairments are linked to a cascade of neuroinflammatory events perpetrated by HIV-1-infected and immune competent brain mononuclear phagocytes (MP; microglia and blood-borne macrophages) (Kadiu et al., 2005).
Cerebrospinal fluid (CSF) contains a number of immunoregulatory proteins (Yuan and Desiderio, 2005), making it a likely source for analysis of the brain’s microenvironment (Huhmer et al., 2006). Thus, we used CSF to test for biomarkers linked to CI during progressive HIV-1 disease. In this study, we used a proteomics platform consisting of surface enhanced laser desorption ionization time of-flight (SELDI-TOF), reverse phase high performance liquid chromatography (RP-HPLC) sample fractionation, 1D SDS PAGE electrophoresis, and liquid chromatography mass spectrometry (LC-MS/MS). This approach yielded signature spectra for HIV-associated CI on SELDI-TOF that coincided with specific protein identification. These results, while preliminary, support the use of proteomic protein profiling as an important tool in the biomarker discovery applied to NeuroAIDS.
2. Methods
2.1 Patient cohort
The study was conducted as part of the NeuroAIDS Specialized Neuroscience Research Program at the University of Puerto Rico Medical Sciences Campus (UPR-MSC). HIV-1 seropositive women were recruited from primary HIV clinics at the Puerto Rico Medical Center and the UPR-MSC. The study had the approval of the Institutional Review Board and was conducted with the informed consent of all participants. The inclusion criteria, recruitment, and evaluation have been described previously (Wojna et al., 2004a; Wojna et al., 2006). HIV-seropositive women with CD4+ T lymphocyte counts ≤500 cells/cubic mm and/or viral load > 1,000 copies/mL while on ART were characterized for neurological and neuropsychological functions. All participants had at least a 9th grade education and showed no evidence of systemic infection or concomitant neurological disorders (Wojna et al., 2004a). Plasma and CSF viral loads were determined with an Ultrasensitive RNA Roche Amplicor at an ACTG-certified laboratory with a detection range of 50 to 75000 copies of RNA/mL.
2.2 Neurological and neuropsychological evaluations
Neurological examination consisted of a mental status evaluation, testing of sensory (including speed of thought and language), behavior and mood functions as well as standard evaluations of cranial nerves, cerebellar, motor, reflexes, and sensation. The neuropsychological evaluations included evaluation to determine pre-morbid vocabulary and reading scores. The neurocognitive evaluations included tests of verbal memory (trial 5, delay recall, and recognition of the Rey Auditory Verbal Learning Test), frontal executive function (Stroop word/color and Trail Making B), psychomotor speed (Symbol Digit Modalities Test and visual and auditory reaction time non dominant hand), motor speed (Trail Making A and Grooved Pegboard dominant and non dominant hand), and Beck Depression Index. The neuropsychological evaluations have been described previously (Wojna et al., 2006), and are detailed in Table 1. All tests were conducted on all patients in Spanish. We calculated z-scores of the neuropsychological tests in 34 HIV-1 seronegative Puerto Rican women. These women did not differ from the HIV-infected group with regards to age, education, and socio-demographics status. The neurologist and the neuropsychologist were blinded to each other’s findings.
Table 1.
Neurocognitive examination of Hispanic women
| Neuropsychological domain | Test | Subtests |
|---|---|---|
| Verbal memory | Rey Auditory
Verbal Learning Test1 |
Trial 5
Memory recall Delayed recognition |
| Frontal executive | Stroop | Word/color |
| Trail Making B | Total score (seconds) | |
| Psychomotor speed | Digit Symbol
Modality Test |
Total score |
| Reaction Time | Visual & auditory non dominant hand | |
| Motor speed | Trail Making A | Total score (seconds) |
| Grooved Pegboard | Dominant & non dominant hand (seconds) | |
| Visuoconstruction | Cube | Copy |
) Spanish translation, standardized with a similar reference control group (see text description)
Cognitive impairment was determined using the American Academy of Neurology HIV-associated dementia criteria (AAN criteria) (American Academy of Neurology AIDS Task Force, 1991, 1996) modified to include an asymptomatic cognitively impaired group (m-AAN). An asymptomatic cognitively impaired group is defined as patients with abnormal neuropsychological tests (1 SD [on two or more tests] or 2 SD [on one or more tests] below the normal control group) but who presented neither functional/emotional nor neurological findings. According to the m-AAN, the patient groups were as follows: normal, asymptomatic cognitive impairment, minor cognitive motor disturbance (MCMD), and HAD (Wojna et al., 2006). For this study, patients with asymptomatic cognitive impairment, MCMD, or HAD were grouped together as CI and compared with patients having normal cognition (NC). Of the 50 patients included in this study, 13 had NC and 37 were CI. Three limited subsets of those 50 patients, e.g., n= 37, n=7, and n=18, were chosen for different assays dependent on availability of sample volume and protein concentration.
2.3. CSF collection and processing
Blood and CSF samples were collected from the patients on the day of Neurological and Neuropsychological evaluations. Blood was processed for monocyte isolation while CSF was immediately subjected to centrifugation in cold. Cell free CSF was mixed with 5% protease inhibitor cocktail (Sigma), and stored at -80°C for viral load and proteomics studies. CSF samples have been collected once a year and stored at −80°C within one hour of collection.
2.4. SELDI-TOF profiling
A pilot study was conducted to determine optimal experiment conditions, whole CSF was initially spotted in quadruplicates on three different protein chip array surfaces: CM10 (weak cation exchange), Q10 (strong anion exchange), and IMAC 30 (metal affinity). Each different surface property retains a specific subset of proteins from the sample. The binding buffers for the CM10 chip (0.1 M ammonium acetate, pH 4-10) and Q10 chip (50 mM Tris-HCl, pH 4-10) were optimized before analysis. Of the three surfaces tested (IMAC, CM10, and Q10) (Figure 1A), the ProteinChip® CM10 provided spectra with the best peak intensity and resolution. Therefore, CM10 was selected for analysis of whole and fractionated CSF. SELDI-TOF reproducibility of CSF samples spotted on CM10 chips is shown in Figure 1B.
Figure 1.
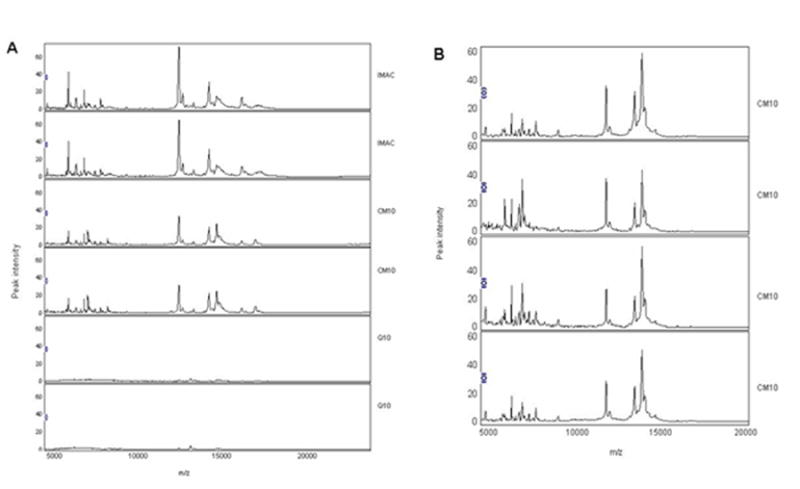
SELDI-TOF reproducibility of whole CSF on different chip arrays. Whole CSF was analyzed on three different types of Protein Chip array surfaces: IMAC chips (top two spectra), CM10 chips (middle two spectra), and Q10 chips (bottom two spectra) (panel A). Samples were run in quadruplicates and displayed in duplicate. A representative sample showing good reproducibility on CM10 is illustrated (panel B).
Whole CSF from 37 patients (8 NC and 29 CI; Experiment 1) and fractionated CSF from 7 patients (2 NC and 5 CI; Experiment 2) were analyzed in quadruplicates by SELDI –TOF using CM10 chips. The spot surface of each CM10 chip was equilibrated with binding buffer (pH 4). An aliquot (25 μg) of whole CSF was applied to each spot and incubated in a bioprocessor (Ciphergen, Inc.) at room temperature for 30 minutes with shaking. Unbound proteins were removed by washing spots twice with binding buffer and HPLC-graded water. After drying the spot, 50% sinapic acid (SPA) was applied once and air-dried. SPA was prepared as a saturated solution containing 30% acetonitrile (ACN), 15% isopropanol, 0.5% trifluoroacetic acid, and 0.05% Triton X-100. The ionized proteins and their molecular mass/charge (m/z) ratios were detected using a ProteinChip® Series 4000 reader at Bio-Rad Biomarker Research Center (Malvern, PA) and analyzed using ProteinChip® software 3.2 (Ciphergen Biosystems, Inc.). Data were acquired in the range of 3,000-20,000 m/z with laser intensity set at 3000 and detector sensitivity at 100. The ProteinChip® Reader was externally calibrated for each analysis using six standard proteins: human ACTH peptide 1-24 (2,933.5 Da), bovine ubiquitin (5,733.6 Da), bovine cytochrome C (12,230.9 Da), bovine SOD (15,591.4 Da), equine cardiac myoglobin (16,951.5 Da), and beta-lactoglobulin (18,363.3 Da). Peaks were automatically detected using the Biomarker Wizard® provided within the ProteinChip® software 3.2. The following parameters for peak detection were used: first pass signal/noise (S/N) ratio=5, second pass S/N ratio=2, mass tolerance=0.5%. Estimated peaks were included in completion of clustering.
2.5 CSF fractionation
Subsequent to initial screening of whole CSF samples by SELDI-TOF (Experiment 1), CSF samples from 18 patients were fractionated by RP-HPLC. This procedure enhanced peak detection in the low mass range for SELDI-TOF analysis by making samples less complex, and facilitated protein isolation for sequencing. Pre-fractionation has been previously utilized to effectively separate complex biological samples, allowing isolation of specific low-abundance proteins and avoiding suppression by high-abundance proteins, such as albumin and immunoglobulin present in CSF. Indeed, previous studies demonstrated that CSF profiling was improved by pre-fractionation (Yuan and Desiderio, 2005; Ciborowski et al. 2007). This method does not remove high-abundance proteins, but separates them in different fractions, thereby effectively reducing their interference and allowing detection of low-abundant proteins. Briefly, CSF samples were fractionated using an Agilent 1100 Hewlett Packard HPLC system (Agilent Technologies, Palo Alto, CA). The mobile phases consisted of 100% HPLC-graded water with 0.1% formic acid (mobile phase A) and 100% HPLC-graded acetonitrile with 0.1% formic acid (mobile phase B). Chromatographic separations were achieved using a reverse phase 0.21 cm × 5 cm C8 column (Grace Vydac, Hesperia, CA). The flow rate was set at 0.1 mL/min. Chromatographic column was equilibrated with 100% HPLC-graded water before each run. A total of 100 μL of whole CSF was injected onto the column each time. This step was repeated five times for each patient sample. Since such a high volume of sample was necessary for fractionation and subsequent analysis, our sample size for subsequent assays was considerably reduced. After sample injection, an isocratic wash with mobile phase A to B ratio at 95:5 occurred for 20 minutes. A linear gradient of 90% mobile phase B was applied for 85 minutes to elute proteins. Four specific CSF fractions were obtained that contained sufficient amount of protein for further analysis. The fractions were eluted in 5-minute intervals: 50-55, 55-60, 60-65, and 65-70 minutes. The resultant fractions were dried using a speedvac and resuspended in 60 mL of HPLC-graded water. Each CSF fraction from NC and CI was pooled, one portion of which was tested by SELDI-TOF for detection of differentially expressed proteins in CI and NC patient groups, and the other portion was stored at -80°C prior to 1D SDS PAGE analysis.
2.6 1D SDS PAGE
Pooled CSF fractions from 18 patients were diluted with NuPAGE® LDS buffer (Invitrogen, Carlsbud, CA) and proteins were further separated by one dimensional electrophoresis. Duplicates of 20 μg of each fraction were loaded on a NuPAGE® Novex 10% Bis-Tris (Invitrogen) gel and after electrophoresis stained by Coomassie Brilliant Blue (BioRad, Hercules, CA). Protein bands corresponding to molecular weights of interest were cut out using a razor blade. Proteins in gel cubes were subjected to in-gel tryptic digestion as described previously (Ciborowski et al., 2007). Briefly, after distaining with 50% ACN, 50 mM NH4HCO3/50% ACN, and 10 mM NH4HCO3/50%, the gel slices were dried and then incubated with trypsin (Promega, Madison, WI) for 12-16 hours. All peptides extracted by 0.1% trifluoroacetic acid /60% ACN were pooled into a glass tube and dried prior to LC-MS/MS analysis. Resulting peptides were used for protein identification by LC-MS/MS peptide sequencing and database search.
2.7 Protein identification
The peptides were resuspended in 0.1% formic acid in HPLC-graded water and the ionized peptides were detected on a ProteomeX LC-MS/MS system (Thermo Electron Corporation, Waltham, MA) as previously described (Ciborowski et al., 2007). The amino acid sequence data from LC-MS/MS analysis were blasted against the protein database to search for the matching protein using BioWorks 3.1SR. Protein identifications were accepted as true positive on the basis of arbitrarily set up parameters as previously published (Omenn et al., 2005). Briefly, the criteria were: BioWorks® unified score was ≥3000, Xcorr for doubly charged precursor ion was ≥2.5, DeltaCn was ≥0.3, there were more than 60% of fragment ions per sequenced peptide, and at least two peptides per protein were identified.
2.8 Protein expression by Western blots
Western blot analysis was performed to examine the effect of CI on protein expression. Protein samples from ~20 to 80μg of CSF, from 6 patients with CI and 6 controls with normal cognition, were denatured and separated by SDS-PAGE on a 10% Tris-HCl Ready Gel in duplicates (Bio-Rad Laboratories) and transferred to nitrocellulose membranes (Bio-Rad Laboratories). Membranes were blotted overnight at 4°C either with or without a primary antibody. Immunoblot detection of candidate proteins was performed with the following antibodies: sheep polyclonal antibody against human Cu/Zn SOD-1 (Santa Cruz), polyclonal rabbit anti-human α-MIF (Santa Cruz); polyclonal rabbit anti-human α-VGF (Santa Cruz); and a monoclonal anti-human α-Galectin-7 (R&D Systems). All membranes were incubated for 1 hr at room temperature with horse radish peroxidase enzyme (HRP)-conjugated secondary antibody at a 1:2000 dilution. Secondary antibodies used were donkey anti-sheep (US Biologicals), goat anti rabbit, and donkey anti mouse (Jackson Immunoresearch). Expressed proteins were detected using an ultra sensitive HRP detection system (femtoLucent™ PLUS-HRP Reagent Kit, Geno Technology). The density of the bands was quantified using a scanning densitometer (VersaDoc Imaging System, Bio-Rad Laboratories). Uniformity of protein loading was confirmed by staining of the blots with Ponceau S staining (Sigma).
2.9. Statistical Analyses
Protein profiling spectra from whole CSF of 37 patients were normalized after baseline subtraction and calibration for mass accuracy. Peak detection and clustering was performed using Biomarker Wizard® software 3.2. Data were exported to Microsoft Excel for analysis using SAS® software (SAS Institute, Cary, NC). Protein CSF profiles obtained from patients with NC and CI were compared on the basis of peak intensity or normalized peak height. Repeated measures analysis of variance was used to identify peaks for which there was evidence of statistically significant differences in the distribution of intensity scores among the subjects. The raw intensity values were found to be asymmetrical and adjusted prior to analysis using the following transformation: Y = log2 (X + SQRT[X**2+ 1]), where ‘X’ is the observed intensity. This transformation has been previously used to stabilize intensity variance and make data more nearly normally distributed, and it has the advantage over a log-transformation of being able to handle negative intensities (Beyer et al., 2006). An “adjusted p-value” (a “q-value”) was computed to address the issue of multiple comparisons, by which the false discovery rate was controlled at 0.10 (i.e., no more than 10% expected false positives out of differentially expressed ones) (Storey et al., 2004). These comparisons were made using generalized estimating equation (GEE) statistics.
Data from fractionated CSF from 7 patients were analyzed similarly with the exception that the Bonferroni correction procedure was used instead of the false discovery rate due to the smaller number of comparisons. Following the recommendations of Yasui and coworkers (Yasui et al., 2003), only peaks identified in the m/z range of 3,000-20,000 were investigated in these analyses. The Random Forest technique was applied for determination of sensitivity and specificity of 20 differentially expressed peaks from whole CSF in discriminating between normal cognition and cognitive impairment as done previously (Luo et al., 2003). Briefly, this method can generate many classification trees and then estimate the importance of each variable (a peak in this case) by random permutation.
3. Results
3.1 Neuropsychological, immune, and viral load determinations
Fifty HIV-seropositive women characterized for cognitive function from the Hispanic Latino NeuroAIDS cohort were selected for this study. Of these, 13 patients had normal cognition and 37 were cognitively impaired. All of the patients selected were negative for hepatitis C and drug toxicology profile. CSF cell counts, protein and glucose determinations have been obtained for the first thirty patients entered into the cohort. All together as these measures were not altered by patient profiles, levels of immune dysfunction or viral load (data not shown).
The demographics of the patients included in this study by cognitive status category are described in Table 2. The overall group mean of age was as follows (mean ± SD): 38 ± 6 years old, with current CD4 count of 380 + 248 cells/mm3, CD4 nadir of 217 ± 27 cells/mm3, plasma viral load of 45,061 ± 144,760 copies/mL, and CSF viral load of 2,502 ± 8,334 copies/mL. Using analysis of variance, there were no significant differences between age (p=0.425), CD4 cell count (p=0.159), CD4 nadir cell count (p=0.753), plasma HIV RNA (p=0.189), or CSF HIV RNA (p=0.977) between patients with NC and CI. Among patients without cognitive impairment reporting therapy use (12/13 (92.3%) reported using HAART. This was not significantly different than the distribution of therapy among those with asymptomatic cognitive impairment (11/15 HAART, 2/15 mono, and 2/15 naive), those with MCMD (2/2 HAART), and those with HIV-D (11/17 HAART, 1/17 naive, and 1/17 ART) (p=0.616).
Table 2.
Demographics, viral and immune parameters in HIV-seropositive women
| m-AAN criteria1 (n=50) | |||||
|---|---|---|---|---|---|
| Normal Cognition NC | Cognitive Impairment CI | p-value2 | |||
| Normal
n=13 |
Asymptomatic
n=18 |
MCMD
n=2 |
HAD
n=17 |
||
|
Age
Range |
37 ± 5
29-44 |
36 ± 6
22-44 |
36 ± 1
35-37 |
39 ± 7
29-53 |
p= 0.425 |
|
CD4 3
Range |
307.5 ± 147.9
60-596 |
327.6 ± 243.3
4-949 |
271.5 ± 92.6
206-337 |
482.9 ± 288.5
35-477 |
p= 0.159 |
|
CD4 nadir 3
Range |
209.1 ± 139.5
28-309 |
256.1 ± 274.5
4-1058 |
181.5 ± 201.5
39-324 |
209.1 ± 139.5
35-477 |
p=0.753 |
|
Plasma HIV RNA 4
Range |
18,871 ± 38,225
50-128,444 |
122,052 ± 257,624
50-892,916 |
13,857 ± 8,376
7,934-19,780 |
14,029 ± 23,401
50-72,329 |
p=0.189 |
| CSF HIV RNA4
Range |
2,242-4,740
50-13,928 |
2,334 ± 6,028
50-23,616 |
1,170 ± 1,584
50-2,290 |
3,343 ± 12,795
50-51,319 |
p=0.977 |
| Treatment | p=0.616 | ||||
| •No treatment | 7.6% (1/13) | 13.3% (2/15) | 0 | 5.9% (1/17) | |
| •ART | 0 | 13.3% (2/15) | 0 | 5.9% (1/17) | |
| •HAART | 92.3% (12/13) | 73.3% (11/15) | 100.00% (2/2) | 88.2% (15/17) | |
) m-AAN, modified American Academy of Neurology HIV-associated dementia; MCMD, HIV-associated minor cognitive-motor disturbance; HAD, HIV-associated dementia; ART, antiretroviral treatment; HAART, highly active antiretroviral treatment.
) ANOVA statistics.
) CD4 cells/mm3.
) HIV RNA or viral load in copies/mL.
Figure 2 shows the distribution of scores on the cognitive domains. There were significant differences in neuropsychological performance (NP) on all the cognitive domains: verbal memory (p=0.009) frontal executive function (p=0.000), psychomotor speed (p=0.000), motor speed (p=0.00), and NPZ (p=0.000) between normal cognition and CI group (A, MCMD and HAD). These differences in NP have been reported previously in a larger group of patients (Wojna et al., 2006).
Figure 2.
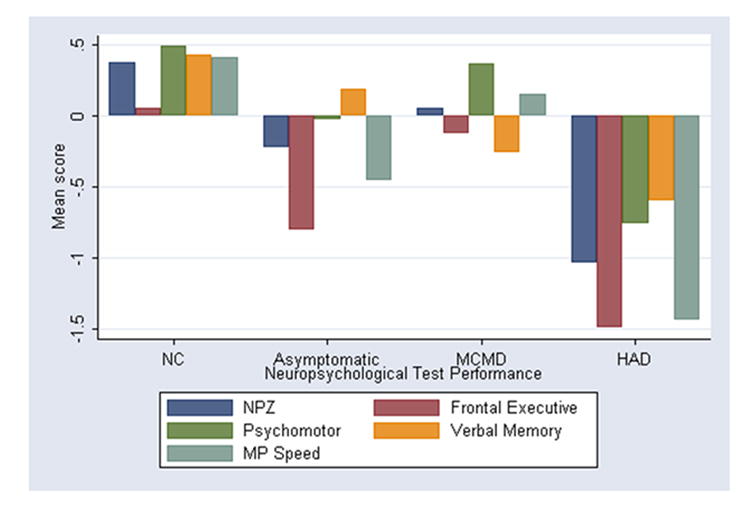
Neuropsychological performance by cognitive domain among women with HIV infection by modified American Academy of Neurology diagnostic criteria for HIV-associated dementia (mAAN). Neuropsychological performance was significantly different in CI group (A, MCMD, and HAD) compared to normal cognition by ANOVA (p<0.001). A, asymptomatics; MCMD, HIV-associated minor cognitive-motor disorder; HAD, HIV-associated dementia.
3.1 Protein profiling
Whole CSF from 37 patients was analyzed on a CM10 chip and tested in quadruplicate sets. One hundred forty-eight spectra, which included biological and technical replicates, were analyzed. Of the 357 detected peaks, five showed significantly lower intensities (q≤0.10) and 14 peaks showed significantly higher intensities in CI when compared to NC (Table 3). The differences in intensities between NC and CI is illustrated in Figure 3A.
Table 3.
Differentially expressed peaks in whole CSF of NC (n=8) and CI (n=29) patients
| Peak Number | Median m/z | q-value1 | NC | CI |
|---|---|---|---|---|
| 1 | 3101 | 0.0571 | HIGH | LOW |
| 2 | 3363 | 0.0860 | HIGH | LOW |
| 3 | 3520 | 0.0571 | HIGH | LOW |
| 4 | 5316 | 0.0213 | HIGH | LOW |
| 5 | 6436 | 0.0545 | LOW | HIGH |
| 6 | 6974 | 0.0571 | LOW | HIGH |
| 7 | 7048 | 0.0832 | LOW | HIGH |
| 8 | 12121 | 0.0928 | LOW | HIGH |
| 9 | 13919 | 0.0213 | LOW | HIGH |
| 10 | 13945 | 0.0928 | LOW | HIGH |
| 11 | 13967 | 0.0911 | LOW | HIGH |
| 12 | 14074 | 0.0389 | LOW | HIGH |
| 13 | 14096 | 0.0213 | LOW | HIGH |
| 14 | 14113 | 0.0213 | LOW | HIGH |
| 15 | 14131 | 0.0213 | LOW | HIGH |
| 16 | 14162 | 0.0489 | LOW | HIGH |
| 17 | 14268 | 0.0213 | LOW | HIGH |
| 18 | 14387 | 0.0213 | LOW | HIGH |
| 19 | 14448 | 0.0213 | LOW | HIGH |
| 20 | 16636 | 0.0661 | HIGH | LOW |
) The adjusted p-value ≤ 0.10, after correcting for multiple comparisons (Storey and Sigmund, 2004).
Figure 3.
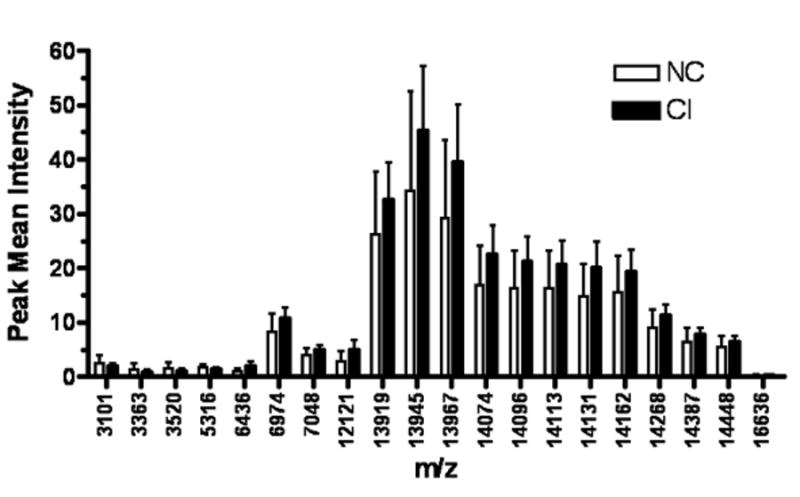
Differentially expressed protein peaks of whole CSF from NC and CI groups. One hundred and forty-eight spectra from CSF of 29 CI patients and 8 patients with NC were compared using generalized estimating equations. This analysis revealed significant differences (p<0.10) between the peak intensities of both patient groups.
Random Forest statistical analyses were performed on whole CSF to determine the sensitivity and specificity of the protein peaks in distinguishing NC and CI. We found that the 20 peaks analyzed had either high sensitivity and low specificity, or low sensitivity and high specificity (Table 4A). Four most important peaks were selected by Random Forest analysis (3101, 3520, 5316 and 16636) that differentiated NC and CI groups. The sensitivity and specificity values of these peaks are included in Table 4B. The peaks 16636 and 3520 had relatively low sensitivity values. However, a combination of these two peaks was necessary to obtain high sensitivity (90%) and specificity (100%) (Table 4B). Unfortunately, these peaks showed very low intensities to detect them with naked eye most probably due to high abundant proteins present in whole CSF samples.
Table 4A.
Diagnostic value of the 20 differentially expressed peaks between NC and CI
| Peak in m/z
(mean intensity) |
HIV-CI vs. HIV-NC | |
|---|---|---|
| Sensitivity (%) | Specificity (%) | |
| 3101 (<2.453)1 | 93 | 63 |
| 3363 (<1.333) | 83 | 63 |
| 3520 (<1.119) | 55 | 100 |
| 5316 (<1.776) | 97 | 63 |
| 6436 (>=0.906) | 90 | 50 |
| 6974 (>=8.233) | 83 | 63 |
| 7048 (>= 4.222) | 62 | 88 |
| 12121 (>=4.196) | 45 | 100 |
| 13919 (>=27.15) | 62 | 88 |
| 13945 (>=37.1) | 66 | 75 |
| 13967 (>=37.03) | 41 | 100 |
| 14074 (>=20.09) | 41 | 100 |
| 14096 (>=18.86) | 45 | 100 |
| 14113 (>=18.77) | 45 | 100 |
| 14131 (>=16.5) | 48 | 100 |
| 14162 (>=18.3) | 45 | 100 |
| 14268 (>=10.15) | 48 | 100 |
| 14387 (>=6.996) | 62 | 88 |
| 14448 (>=5.791) | 66 | 88 |
| 16636 (<0.219) | 69 | 100 |
) For this entry, the m/z value is 3101 and the patient with mean intensity less than 2.453 is classified as CI
Table 4B.
Comparisons of most important protein peaks for diagnostic value1
| Peak (m/z) | HIV-CI vs. HIV-NC | |
|---|---|---|
| Sensitivity (%) | Specificity (%) | |
| Single peak | ||
| 5316 | 97 | 63 |
| 16636 | 69 | 100 |
| 3101 | 93 | 63 |
| 3520 | 55 | 100 |
| Combined peak | ||
| 16636 + 3520 | 90 | 100 |
) Four most important peaks were selected based on Random Forest statistical analysis
Protein profiles from whole CSF from patients with NC and those CI show multiple peaks with limited resolution in the lower mass range (Figure 4). Thus, 1D SDS PAGE was performed on CSF samples to assess the possibility that high abundant proteins present in all samples, could saturate the ProteinChip® detector and reduce peak intensities. These samples showed concordant distribution of up to 50% of the total protein content with immunoglobulin and albumin present among patient groups (data not shown). To improve resolution of proteins that could be differentially expressed amongst the CI and NC groups in SELDI-TOF analysis, we next performed CSF fractionation. Each of the four fractions obtained by RP-HPLC was analyzed on a CM10 chip and tested in quadruplicate sets as described in methods. Representative SELDI-TOF spectra are displayed for each fraction and for whole CSF (Figure 5). Twenty eight spectra, which included biological and technical replicates, were analyzed. These spectra represent RP-HPLC fractionated (fraction 2) CSF samples from 5 CI subjects and 2 subjects with NC. In fractionated CSF, of the 26 peaks detected, only the protein peaks with m/z of 4320, 5389, 5597 and 6982 were significantly lower (q<0.10) in CI, whereas the protein peaks with m/z of 6695 6799, 13376, 13579, 13811 were significantly higher (q<0.10) in CSF of patients with CI (Figure 6).
Figure 4.
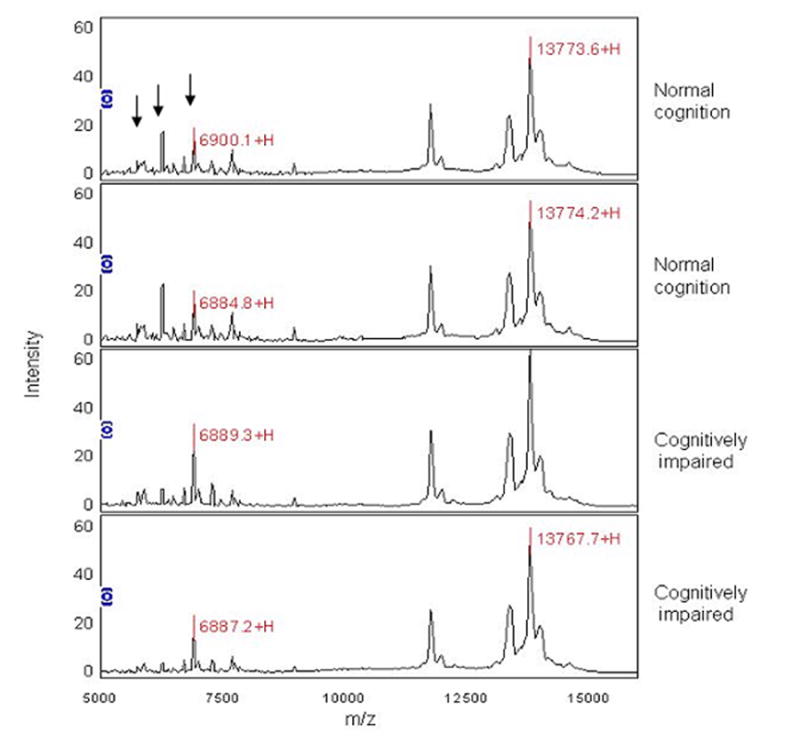
Representative SELDI-TOF profile of whole CSF from NC and CI. SELDI-TOF spectra on CM10 chips show peaks at the higher end of the spectra and peaks with low intensities at lower m/z values. Samples were run in quadruplicates and displayed in duplicates to illustrate reproducibility. The first spectrum has three arrows that point out several differences in peak intensities between NC and CI.
Figure 5.
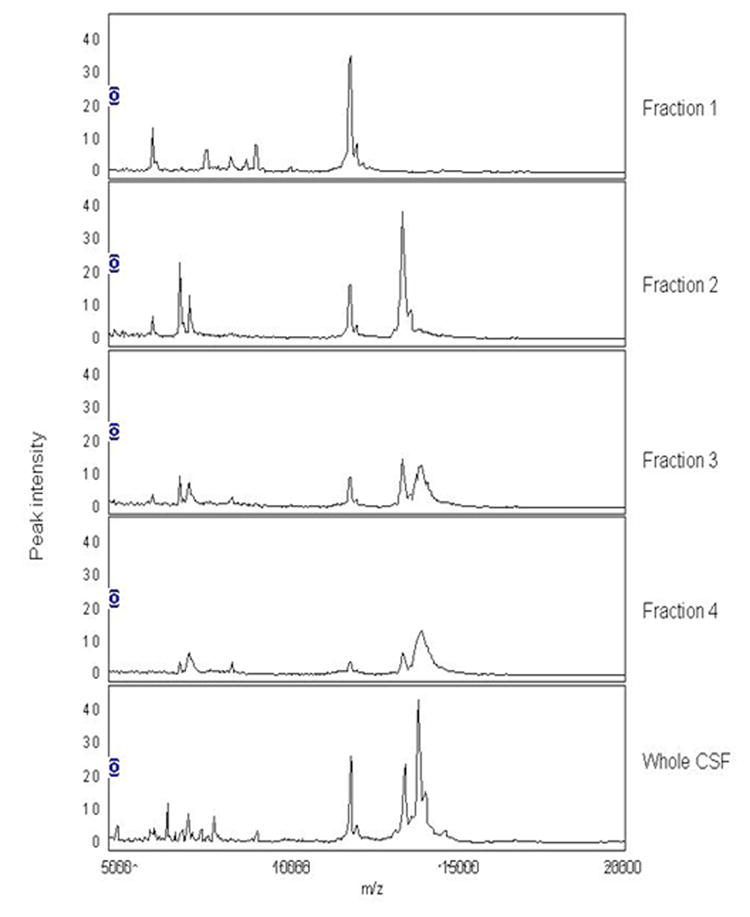
Representative SELDI-TOF spectra for CSF fractions and whole CSF. Four fractions were obtained from RP-HPLC with elution times between 50-55, 55-60, 60-65, and 65-70 min. Each of the fractions was prepared on a CM 10 chip and run in quadruplicate sets. Only fraction 2 contained sufficient amount of protein for further analysis.
Figure 6.
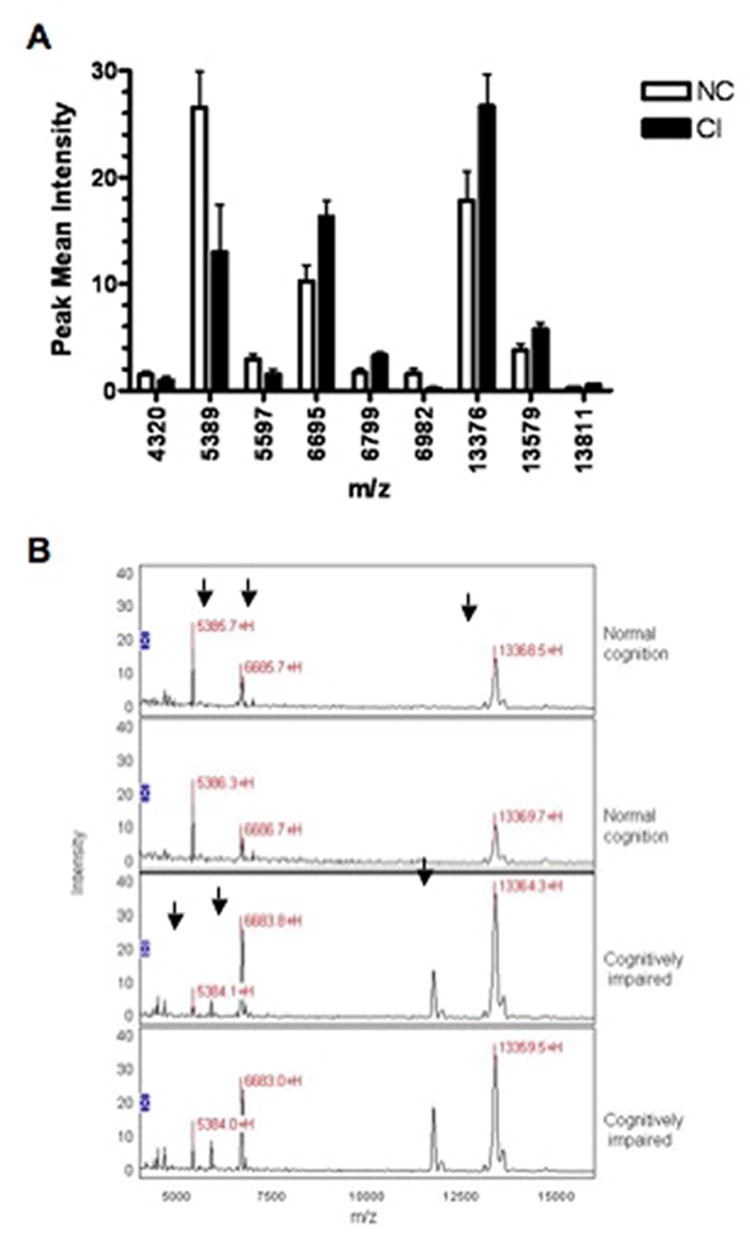
Differentially expressed peaks in CSF of NC and CI. Twenty-eight spectra from CSF fraction 2 of 2 CI patients and 5 patients with NC were analyzed. Nine protein species were differentially expressed between the two groups (p<0.10; panel A). Representative spectra of showing several differentially expressed peaks (black arrows) from NC (upper spectra) and CI (bottom spectra) in duplicates to illustrate reproducibility (panel B).
3.2 Protein identification
Subsequently, all four fractions derived from RP-HPLC fractionated CSF of 18 patients were separated in duplicates by 1D SDS PAGE gels with equivalent protein content. Bands corresponding to regions of interest in SELDI-TOF spectra and others corresponding to higher molecular weights were excised and proteins digested with trypsin. The derived peptides were sequenced by the LC-MS/MS, and eventually proteins were identified. Sixteen proteins were found in CSF from NC and CI meeting all five criteria for protein identification (Table 5). Nine proteins were identified as unique to CSF of CI. These were: soluble superoxide dismutase (SOD 1; related to amyotrophic lateral sclerosis (ALS)), migration inhibitory factor (MIF) -related protein 14, macrophage capping protein, neurosecretory protein VGF, galectin-7, L-plastin, acylphosphatase 1, and a tyrosine 3/tryptophan 5-monooxygenase activation protein. Eleven proteins were identified as unique to CSF of NC (Table 5). Although these proteins were matched only with one peptide, they satisfied four of the five criteria. We accepted these proteins as “true positive” identifications on the basis of results published by the Plasma Proteome Project performed by the Human Proteome Organization (Omenn et al., 2005), which showed that as many as 25% identifications based on the sequence of one peptide were eventually confirmed as “true positive.” It is important to note that we performed a careful inspection of MS/MS data for these peptides and increased a requirement of the number of fragment ions from >60% to >80%.
Table 5.
Identified proteins in fractionated CSF of CI and NC patients.
| Protein | NC | CI | NCBIa | SwissProtb | MW | Proposed function | Peptides |
|---|---|---|---|---|---|---|---|
| Acylphosphatase 1 | - | + | 1082173 | P07311 | 11.3 | Catalyzes the hydrolysis of the carboxyl-phosphate bond of acylphosphates | 1 |
| Albumin | + | + | 23307793 | Q56G89 | 69.1 | Regulation of the colloidal osmotic pressure of blood | 7 |
| Alpha-1-antitrypsin | + | + | 24438 | P01009 | 46.7 | Inhibitor of serine proteases. | 5 |
| Amyloid beta A4 protein precursor | + | + | 28558768 | P05067 | 86.9 | Cell surface receptor relevant to neurite growth, neuronal adhesion and axonogenesis. | 2 |
| Antithrombin | + | + | 4502261 | Q5M7T5 | 52.2 | Serpin; serine protease inhibitor. | 2 |
| Apolipoprotein A-I (and precursor) | + | + | 37499465 | P02647 | 30.8 | Promotes cholesterol efflux from tissues and acts as a cofactor for the lecithin cholesterol acyltransferase. . | 5 |
| Apolipoprotein A-IV (and precursor) | + | + | 37499461 | P06727 | 45.4 | Major component of HDL and chylomicrons and a potent activator of lecithin-cholesterol acyltransferase in vitro. | 8 |
| Apolipoprotein D | + | + | 1246096 | P05090 | 21.3 | Occurs in the macromolecular complex with lecithin-cholesterol acyltransferase. | 1 |
| Apolipoprotein E (and precursor) | + | + | 13097699 | P02649 | 36.2 | Mediates the binding, internalization, and catabolism of lipoprotein particles. | 5 |
| Apolipoprotein H (Beta-2-Glycoprotein-I) | + | - | 543826 | P02749 | 38.3 | May prevent activation of the intrinsic blood coagulation cascade by binding to phospholipids on the surface of damaged cells. | 1 |
| Apolipoprotein J (Clusterin) | + | + | 32891795 | P10909 | 52.5 | Associated with the clearance of cellular debris and apoptosis. | 5 |
| Brain-specific angiogenesis inhibitor 2 | + | - | 21928571 | O60241 | 171.1 | Might be involved in angiogenesis inhibition. | 1 |
| Cell surface glycoprotein MUC18 | + | - | 33989399 | P43121 | 71.6 | CD146. Plays a role in cell adhesion, and in cohesion of the endothelial monolayer at intercellular junctions in vascular tissue. | 2 |
| Chromogranin B precursor (secretogranin 1) | + | - | 4502807 | P05060 | 78.2 | Neuroendocrine secretory granule protein. | 1 |
| Cystatin C (and precursor) | + | + | 296643 | P01034 | 15.8 | Inhibitor of cysteine proteinases. | 5 |
| Dermcidin precursor | + | + | 16751921 | P81605 | 11.3 | Antimicrobial activity. | 1 |
| Extracellular superoxide dismutase (Cu, Zn) | + | - | 4507151 | P08294 | 25.9 | SOD 3, destroys radicals which are normally produced within the cells and which are toxic to biological systems. | 1 |
| Fibrinogen beta chain | + | - | 399492 | P02675 | 55.9 | Yields monomers that polymerize into fibrin and acts as a cofactor in platelet aggregation. | 1 |
| Galectin-7 | - | + | 3891470 | P47929 | 15.1 | Pro-apoptotic protein upstream of JNK activation and cytochrome c release. | 2 |
| Gelsolin | + | + | 4504165 | P06396 | 85.7 | Calcium-regulated, actin-modulating protein that binds to actin monomers or filaments, preventing monomer exchange | 1 |
| Hypothetical protein U55B | - | + | 51874279 | P52518 | 49.6 | Herpes virus 7. | 1 |
| Kallikrein-6 | + | - | 15930186 | Q92876 | 26.9 | Serine endopeptidase. | 1 |
| L-plastin | - | + | 14043359 | P13796 | 70.3 | Actin-binding protein. | 1 |
| Macrophage Capping Protein | - | + | 21730367 | P40121 | 38.5 | Calcium-sensitive protein which reversibly blocks the barbed ends of actin filaments but does not sever preformed actin filaments. | 1 |
| MAP/ERK kinase kinase 4 | + | - | 50741728 | Q9Y6R4 | 181.6 | Activates the CSBP2, P38 and JNK MAPK pathways, but not the ERK pathway. | 1 |
| Migration inhibitory factor-related protein 14 | - | + | 4506773 | P06702 | 13.2 | Expressed by macrophages in acutely inflammated tissues and in chronic inflammations. Inhibitor of protein kinases. | 1 |
| N-acetyllactosaminide beta-1,3-N-acetylglucosaminyltransferase | + | - | 18314366 | O43505 | 47.1 | Can initiate the synthesis or the elongation of the linear poly-N-acetyllactosaminoglycans. | 1 |
| Neurosecretory protein VGF (precursor) | - | + | 20140360 | O15240 | 67.3 | May be involved in the regulation of cell-cell interactions or in synatogenesis during the maturation of the nervous system (By similarity). | 1 |
| Olfactory receptor 1B1 | + | - | 52219202 | Q8NGR6 | 35.2 | G-protein-coupled receptor responsible for the recognition and G protein-mediated transduction of odorant signals. | 1 |
| Orosmucoid -1 and -2 | + | + | 55958974, 4505529 | P02763, P19652 | 23.5 | Appears to function in modulating the activity of the immune system during the acute-phase reaction. | 1 |
| Osteopontin precursor | + | + | 22761565 | P10451 | 35.4 | Acts as a cytokine involved in enhancing production of interferon-gamma and interleukin-12 and reducing production of interleukin-10 and is essential in the pathway that leads to type I immunity.. | 1 |
| Prostaglandin D2 synthase | + | + | 54696706 | P41222 | 21.2 | Involved in a variety of CNS functions, such as sedation, NREM sleep and PGE2-induced allodynia, and may have an anti-apoptotic role in oligodendrocytes. | 3 |
| Protein FAM3C | + | - | 7661714 | Q92520 | 24.7 | Cytokine activity. Secreted protein. | 1 |
| Soluble superoxide dismutase (Cu, Zn; amyotrophic lateral sclerosis 1) | - | + | 31615967 | P00441 | 15.9 | SOD 1, destroys radicals which are normally produced within the cells and which are toxic to biological systems. | 1 |
| Transferrin (and precursor) | + | + | 4557871 | Q9XT72 | 76.5 | Glycoprotein that transports iron from the intestine, reticuloendothelial system, and liver parenchymal cells to all proliferating cells in the body. | 9 |
| Transthyretin (prealbumin) | + | + | 48145933 | P02766 | 15.9 | Thyroid hormone-binding protein. Probably transports thyroxine from the bloodstream to the brain. | 3 |
| Tyrosine 3/tryptophan 5 -monooxygenase activation protein | - | + | 4507953 | P63104 | 27.7 | Protein Kinase C inhibitor. | 2 |
| Tyrosine-protein phosphatase non-receptor type substrate 1 precursor | + | - | 21619841 | P78324 | 54.8 | Immunoglobulin-like cell surface receptor for CD47. May play a key role in intracellular signaling during synaptogenesis and in synaptic function. Mediates negative regulation of phagocytosis, mast cell activation and dendritic cell activation. | 2 |
| Vitamin D Binding Protein | + | + | 34785355 | P02774 | 52.9 | Carries vitamin D sterols and prevents polymerization of actin by binding its monomers. | 2 |
NCBI Accession Numbers.
SwissProt Accession Numbers.
3.3 Protein validation by Western Blots
Western blots were performed initially for SOD-1 with 11 samples (5 NC and 6 with CI). SOD was recognized by specific-antibodies at the expected size of 16kDa thus demonstrating that it was expressed in CSF of HIV-infected individuals with and without CI. Relative abundance of SOD was higher in CI than NC patients (Figure 7). Subsequent Western blots assays were performed on 13 additional CSF samples from NC (3) and CI (10) for MIF, and galectin-7. Results for these proteins were generally increased in CI but not consistently so (data not shown).
Figure 7.
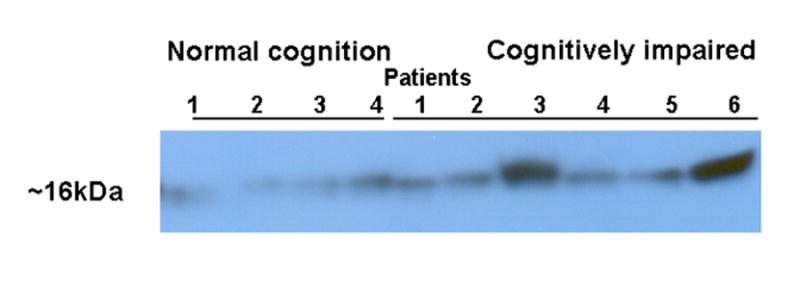
Western blot analysis of Cu/Zn SOD-1 in CSF. A representative Western blot demonstrating SOD-1 (16KDa) in CSF of 11 of HIV-1 infected individuals with and without CI (6 CI and 5 NC).
4. Discussion
To our knowledge this report is one of two comprehensive proteomic platform analyses performed on CSF of HIV-1 seropositive patients with or at risk for CI (Berger et al. 2005). We identified unique spectral differences amongst CI and NC patient groups with putative proteins identified by tandem mass spectrometry. Nonetheless, identification of CSF protein biomarkers using a proteomics platform was completed for other neurodegenerative diseases including Alzheimer’s disease (Carrette et al., 2003), ALS (Ranganathan et al., 2005) and frontotemporal dementia (Pasinetti et al., 2006). In those studies, SELDI-TOF provided important information as to differential expression of proteins between patients with neurodegenerative diseases and healthy individuals. Proteomics has also been applied successfully by our group to fingerprint macrophages from the Hispanic cohort and from in vitro HIV infected macrophages (Luo et al., 2003; Wojna et al., 2004a; Ciborowski et al. 2007). We have used the current platform to differentiate macrophage populations from different tissues including those of spleen, bone marrow, and brain (Enose et al., 2005) and response of mouse microglia to injured nerve (Glanzer et al. 2007). Differentially expressed proteins were found in the three distinct macrophage populations by combining SELDI-TOF, RP-HPLC, and peptide sequencing. We extended this proteomic platform to study CSF protein profiles and identify possible biomarkers for HIV-associated cognitive impairment. As part of this platform, we initially performed SELDI-TOF profiling of 37 whole CSF samples from women evaluated for cognitive function from the Hispanic women cohort (Experiment 1). This method yielded 20 differentially expressed protein peaks, of which 15 were over expressed and 5 under expressed in women with CI. A combination of protein peaks was necessary to obtain increased sensitivity and specificity of protein peaks for diagnostic studies. These included the 16.6 + 3.5 kDa protein peaks for 90% sensitivity and 100% specificity. Following RP-HPLC fractionation, 9 differentially expressed protein peaks were detected, 5 of which were over expressed and 4 under expressed in CI (Experiment 2). Of these, 2 protein peaks with m/z of ~5.3 and 6.9 kDa were detected in both whole CSF and fractionated CSF. In case of the 6.9 kDa peak we obtained inconsistent results. Of the protein peaks detected in fractionated CSF, we noticed that two similar peaks with m/z of 6.8 and 5.4 kDa have been observed as up-regulated and down-regulated in previous studies of peripheral blood monocyte-derived macrophages of one patient with CI, who was also included in this study (Wojna et al., 2004b).
One prior study used SELDI-TOF to profile CSF from HAD patients. However, the study was limited in number of patients evaluated in that six HIV-seropositive men, two with NC, two with mild dementia, and two with HAD (Berger et al., 2005). Using metal binding chips (IMAC), those investigators found a protein peak with m/z of 6.7 kDa that was under expressed in CI, and a peak with m/z of 8.9 kDa that was over expressed in CI. Using anionic chips WCX2 (equivalent to the CM10), they found a peak with an m/z of 2.0 kDa that was over expressed in CI and a peak with m/z of 4.9 kDa that was under expressed in CI. In this our study, we also detected a peak with m/z of 6.7 kDa as differentially expressed in fractionated CSF of NC compared to and CI. However, this peak was found using anionic chips CM10, as over expressed in the CSF of women with CI. The protein found by Berger and coworkers (2005) could be distinct from what we observed in this study since it was metal binding (negatively charged) and they did not detect it in the WCX2 chips. The differences between the two studies could be due to variations in the patient classification criteria for cognitive dysfunction, the cohort composition (proportion of men and women vs. women only), or ethnicity (Meléndez et al., 2007).
In an attempt to identify these differentially expressed proteins, we performed LC MS/MS of pooled CSF fractions. Samples were pooled by fractions within each patient group. Interestingly, five of the nine proteins identified in the CSF of CI patients (migration inhibitory factor (MIF) -related protein 14, neurosecretory protein VGF, galectin-7, acylphosphatase 1, and a tyrosine 3/tryptophan 5-monooxygenase activation protein) are important in cell signaling. One of these, MIF is secreted by macrophages during inflammation and is an inhibitor of protein kinases. MIF was originally discovered as a lymphokine involved in delayed hypersensitivity and various macrophage functions, including phagocytosis, spreading, and tumoricidal activity (Nishihira, 2000). To determine if MIF could increase with the development of CI, we performed Western blots. Indeed, after densitometry analyses MIF showed a biphasic mode where it was increased in HAD. Additional studies will be pursued with increased number of patients to confirm the validity of MIF as a biomarker for stages of CI associated with HIV. However, the ideal biomarker will be one that increases with the degree of CI.
VGF has been reported as decreased in ALS (Pasinetti et al., 2006), Alzheimer’s disease (Carrete et al., 2003), and frontotemporal dementia (Rüetschi et al., 2005). VGF is a nerve growth factor that could be produced in response to neuronal damage during the development of CI. We performed Western blots on additional CSF samples from the cohort using a polyclonal antibody against VGF. However, this antibody reacted with a higher mw band (~98 kDa) and lower molecular masses (~49 and 62 kDa) than the theoretical band expected from mass spectrometry analyses (~67kDa). Despite this cross reactivity, we could not detect differences between the groups.
Galectin-7 is involved in cell-cell and/or cell-matrix interactions necessary for normal growth control and is pro-apoptotic; an event that is known to occur during HIV associated CI (Kaul and Lipton, 2006). We pursued Western blot analyses on Galectin-7 using a monoclonal antibody that recognized a ~72kDa and 42kDa bands, but nothing in the expected size (15-17kDa). This may represent a truncated fragment of Galectin-7 not detectable by the monoclonal antibody.
Two of the nine proteins identified in CSF of CI patients have a structural role in actin filaments (macrophage capping protein and L-plastin). Macrophage capping protein is calcium sensitive, and has an important role in macrophage function by regulating cytoplasmic and nuclear structures. Both L-plastin and human macrophage capping protein are associated with phagocytosis, an important macrophage function that leads to the release of inflammatory mediators. Disruption of these structural filaments is well known to alter macrophage function (Hartwig and Yin, 1988). Indeed, we identified L-plastin in macrophages infected in vitro with HIV isolates from CI, and it was not present in uninfected controls or in macrophages infected with other isolates (Toro et al., manuscript in preparation). We could not confirm L-plastin in the CSF samples from the cohort by Western blot analyses at the moment as there is no antibody commercially available to test L-plastin.
One protein was found to be related to antioxidant activity, SOD 1, a variant of Cu/Zn superoxide dismutase related to ALS, is the primary enzyme in the antioxidant pathways and was identified only in CSF of CI patients. This finding was intriguing since this mutation is mostly associated with familial ALS, a genetic neurodegenerative disease. However, several reports show an ALS-like syndrome occurring in association with HIV infection (von Giesen et al., 2002; Cone et al., 2002; Ahmad, 2001; MacGowan et al., 2001; Moulingnier et. al., 2001; Verma and Berger, 2006). Cu/Zn SOD 1 is a cytosolic enzyme produced during an inflammatory response to neutralize reactive oxygen species such as superoxide anions that induce oxidative damage. Elevated SOD 1 was found in a subset of 6 CSF samples from patients with HAD by Western blot. When attempting to correlate this result with the SELDI-TOF experiments, we found that in whole CSF there was a peak with an m/z of 16636 Da that decreased with CI (Table 3). We believe that this apparent discrepancy could be due to distinct proteins, that SOD was modified, or that the protein did not bind well to the chip’s surface under conditions used in this study. Such differences are not uncommon in proteomics analyses and the most likely conclusions are that Table 3 and Fig. 7 are both correct but represent different proteins. Ongoing studies at our laboratories suggest that there may be changes in SOD related to the degree of CI. High SOD1 was previously reported in HIV-1-infected macrophages present in brain tissues of patients who died with HAD (Treitinger et al., 2000; Boven et al., 1999; Mollace et al., 2001). As the primary infected cells in the brain, HIV-infected macrophages can respond to oxidative stress by producing a number of viral and cellular factors that affect disease pathogenesis. Ongoing studies on SOD-1 expression and activity are active in our laboratory from body fluids derived from our patient cohort and from in vitro infected macrophages. Preliminary studies suggest that SOD expression is decreased during monocyte-macrophage differentiation.
Additional proteins found common to NC and CI, such as Apo E, APP, and cystatin C have been associated with HAD and other neurodegenerative diseases such as Alzheimer’s, multiple sclerosis (MS), and ALS (Vehmas et al., 2004; Irani et al., 2006; Pasinetti et al., 2006, Pocernish et al., 2004). The findings of increased enzymes in the CSF is consistent with the reported high lysosomal expansion that occurs during HAD (Gelman et al., 2005). Although found common to NC and CI, it is still possible that they may differ in abundance between both groups. A peak of 13.4 kDa detected by SELDI-TOF and corresponding to cystatin C was decreased in ALS and MS (Pasinetti et al., 2006; Irani et al., 2006). Interestingly, we found that this peak was significantly higher in CSF of patients with CI analyzed in this study. In a parallel study, we recently found increased expression of cystatin B, the non-secreted form of cystatin C, to be related to the levels of viral replication in macrophages (Luciano-Montalvo et al., manuscript in preparation). Cystatin family members are cysteine proteinase inhibitors of cathepsins, which are released during pain and inflammation and can interfere with antigen processing by monocytes (Mannes et al., 2003; Greiner et al., 2003). Therefore, increased cystatin C may play a protective role against neuronal apoptosis during HIV-cognitive impairment.
PTP was found among the 11 proteins unique to the NC group. The finding of this protein, CD45, in macrophages suggests another linkage of these cells to CSF of women with normal cognition. Current efforts in our laboratories are directed at detecting the proteins common to CSF and MDM from selected members of the Hispanic women cohort.
Overall, our study objective was to apply a proteomics platform to the study of CSF to identify proteins that would differentiate CI from NC. We were able to successfully identify several candidate biomarkers related to signaling, macrophage function, and redox, namely, MIF, SOD 1, cystatin C, and VGF among others. Validation of these proteins will require additional efforts to find the correct experimental conditions and disease state that is linked to CI. Our study has several limitations that need to be addressed. These include the small number of patients with NC compared to CI and the low protein concentration in CSF. These are inherent obstacles towards the precise identification, and validation of all protein candidates. Nonetheless, these may be overcome, in part, by increasing the CSF sample volume collection. Additional experiments are being conducted to improve this platform to minimize the amount of protein required for identification. Combining measures of enzymes and their activity with other CSF biomarkers uncovered through such screening may offer complementary information on the underlying pathology and prognosis in an individual patient who is susceptible to virus-associated brain disease. It may ultimately provide unique insights into the timing and mechanism of brain injury. The search for biomarkers in patients under HAART must continue. Ultimately a better understanding of immune responses to viral infection will lead not only to a better understanding of disease and disease mechanisms but also improved measures to diagnose, follow, and treat a most devastating complication of progressive infection in the human host.
Acknowledgments
The authors thank the patients for their time and effort supporting this research. Tania Ginebra and Tania de la Torre facilitated patient outreach, and Dr. Rosa Hechavarría performed neuropsychological testing. The authors also thank Amanda Bulman from Bio-Rad for technical assistance in rerunning our protein chip arrays. We acknowledge the contributions of Drs. James Anderson and Fred Ullrich in statistical analyses of the SELDI-TOF data. We acknowledge the help of Richard Skolasky with the statistical analyses of the cohort. We thank Robin Taylor for editorial support. We thank Drs. Carmen Zorrilla and Hermes García (our clinic directors) for referring patients. We are grateful to the RCMI-Clinical Research Center for providing staff and supplies for laboratory sample collections. Dr. Edmundo Kraiselburd’s continuous support for this project and the Puerto Rico Specialized Neuroscience Program in NeuroAIDS in general are greatly appreciated.
Footnotes
Disclosure: 1) The authors have reported no conflicts of interest. No redundant publications. All authors agreed to conditions noted on the Author Disclosure form.
2) Presented, in part, at the 5th and 6th Annual Conference of Specialized Neuroscience Research Programs, Maryland, June 2, 2005, the 12th Annual Meeting of the Society on Neuroimmune Pharmacology, New Mexico (abstract TP-25), and the 8th International Conference of Neuroimmunology (abstract WS08-05).
3) Financial support: Supported by the National Institutes of Health grants NINDS U54 NS4301, RCMI-CRC-1P20RR11126, R01 NS34239, R37 NS36136 and NCRR-RCMI G12 RR-03051 and the Carol Swarts, M.D. Laboratory for Emerging Neuroscience Research supported this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad K. HIV may underlie ALS-like condition. Lancet Infect Dis. 2001;1:217. doi: 10.1016/S1473-3099(01)00108-6. [DOI] [PubMed] [Google Scholar]
- American Academy of Neurology AIDS Task Force Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Report of a working group of the American Academy of Neurology AIDS task force. Neurol. 1991;41:778–785. doi: 10.1212/wnl.41.6.778. [DOI] [PubMed] [Google Scholar]
- American Academy of Neurology AIDS Task Force Clinical confirmation of the American Academy of Neurology algorithm for HIV-1-associated cognitive/motor disorder. The Dana Consortium on Therapy for HIV Dementia and Related Cognitive Disorders. Neurol. 1996;47:1247–1253. doi: 10.1212/wnl.47.5.1247. [DOI] [PubMed] [Google Scholar]
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol. 2005;64:529–536. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- Berger JR, Avison M, Mootoor Y, Beach C. Cerebrospinal fluid proteomics and human immunodeficiency virus dementia: preliminary observations. J Neurovirol. 2005;11:557–562. doi: 10.1080/13550280500385237. [DOI] [PubMed] [Google Scholar]
- Beyer S, Walter Y, Hellmann J, Kramer PJ, Kopp-Schneider A, Kroeger M, Ittrich C. Comparison of software tools to improve the detection of carcinogen induced changes in the rat liver proteome by analyzing SELDI-TOF-MS spectra. Journal of Proteome Research. 2006;5(2):254–261. doi: 10.1021/pr050279o. [DOI] [PubMed] [Google Scholar]
- Boven LA, Gomes L, Hery C, Gray F, Verhoef J, Portegies P, Tardieu M, Nottet HSLM. Increased peroxynitrite activity in AIDS dementia complex: implications for the neurogenesis of HIV-infection. J Immunol. 1999;162:4319–4327. [PubMed] [Google Scholar]
- Carrette O, Burgess JA, Burkhard PR, Lang C, Cote M, Rodrigo N, Hochstrasser DF, Sanchez JC. A panel of cerebrospinal fluid potential biomarkers for the diagnosis of Alzheimer’s disease. Proteomics. 2003;3:1486–1494. doi: 10.1002/pmic.200300470. [DOI] [PubMed] [Google Scholar]
- Ciborowski P, Kadiu I, Rozek W, Smith L, Bernhardt K, Fladseth M, Ricardo-Dukelow M, Gendelman HE. Investigating the human immunodeficiency virus type 1-infected monocyte-derived macrophage secretome. Virology. doi: 10.1016/j.virol.2007.01.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone LA, Nazemi R, Cone MO. Reversible ALS-like disorder in HIV infection. Reversible ALS-like disorder in HIV infection. An ALS-like syndrome with new HIV infection and complete response to antiretroviral therapy. Neurol. 2001;59:474–475. [PubMed] [Google Scholar]
- Enose Y, Destache CJ, Mack AL, Anderson JR, Ullrich F, Ciborowski PS, Gendelman HE. Proteomic fingerprints distinguish microglia, bone marrow, and spleen macrophage populations. Glia. 2005;51:161–172. doi: 10.1002/glia.20193. [DOI] [PubMed] [Google Scholar]
- Gelman B, Soukup VM, Holzer CE, Fabian RH, Schuenke KW, Keherly MJ, Richey FJRN, Lahart CJ. Potential role for white matter lysosome expansion in HIV-associated dementia. JAIDS. 2005;39:422–425. doi: 10.1097/01.qai.0000164250.41475.f2. [DOI] [PubMed] [Google Scholar]
- Glanzer JG, Enose Y, Wang T, Kadiu I, Gong N, Rozek W, Liu J, Schlautman JD, Ciborowski PS, Thomas MP, Gendelman HE. Genomic and Proteomic Microglial Profiling: Pathways for Neuroprotective Inflammatory Responses Following Nerve Fragment Clearance and Activation. J Neurochem. doi: 10.1111/j.1471-4159.2007.04568.x. in press. [DOI] [PubMed] [Google Scholar]
- Greiner A, Lautwein A, Overkleeft HS, Weber E, Driessen C. Activity and subcellular distribution of cathepsins in primary human monocytes. J Leukoc Biol. 2003;73:235–242. doi: 10.1189/jlb.0802398. [DOI] [PubMed] [Google Scholar]
- Huhmer AF, Biringer RG, Amato H, Fonteh AN, Harrington MG. Protein analysis in human cerebrospinal fluid: Physiological aspects, current progress and future challenges. Dis Markers. 2006;22:3–26. doi: 10.1155/2006/158797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall IP, Hansen LA, Masliah E. The Shifting Patterns of HIV Encephalitis Neuropathology. Neurotox Res. 2005;8:51–61. doi: 10.1007/BF03033819. [DOI] [PubMed] [Google Scholar]
- Hartwig JH, Yin HL. The organization and regulation of the macrophage actin skeleton. Cell Motil Cytoskeleton. 1988;10:117–125. doi: 10.1002/cm.970100116. [DOI] [PubMed] [Google Scholar]
- Irani DN, Anderson C, Gundry R, Cotter R, Moore S, Kerr D, McArthur JC, Sacktor N, Pardo CA, Jones M, Calabresi P, Nath A. Cleavage of Cystatin C in the cerebrospinal fluid of patients with multiple sclerosis. An Neurol. 2006;59:237–247. doi: 10.1002/ana.20786. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton A. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res. 2006;4:307–318. doi: 10.2174/157016206777709384. [DOI] [PubMed] [Google Scholar]
- Kadiu I, Glanzer JG, Kipnis J, Gendelman HE, Thomas MP. Mononuclear phagocytes in the pathogenesis of neurodegenerative diseases. Neurotox Res. 2005;8:25–50. doi: 10.1007/BF03033818. [DOI] [PubMed] [Google Scholar]
- Luo X, Carlson KA, Wojna V, Mayo R, Biskup TM, Stoner J, Anderson J, Gendelman HE, Melendez LM. Macrophage proteomic fingerprinting predicts HIV-1-associated cognitive impairment. Neurol. 2003;60:1931–1937. doi: 10.1212/01.wnl.0000064396.54554.26. [DOI] [PubMed] [Google Scholar]
- MacGowan DJ, Scelsa SN, Waldron M. An ALS-like syndrome with new HIV infection and complete response to antiretroviral therapy. Neurol. 2001;57:1094–1097. doi: 10.1212/wnl.57.6.1094. [DOI] [PubMed] [Google Scholar]
- Mannes AJ, Martin BM, Yang HY, Keller JM, Lewin S, Gaiser RR, Iadarola MJ. Cystatin C as a cerebrospinal fluid biomarker for pain in humans. Pain. 2003;102:251–256. doi: 10.1016/S0304-3959(02)00403-7. [DOI] [PubMed] [Google Scholar]
- McArthur JC. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2004;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- Meléndez LM, Luciano C, Mayo-Santana R, Wojna V. Ethnicity and neuro-AIDS conditions in the HAART Era. In: Goodkin K, Shapshak P, Verma A, editors. The Spectrum of NeuroAIDS Disorders: Pathophysiology, Diagnosis, and Treatment. ASM Press; Washington DC: In press. [Google Scholar]
- Mollace V, Nottet HS, Clayette P, Turco MC, Muscoli C, Salvemini D, Perno CF. Oxidative stress and neuroAIDS: triggers, modulators, and novel antioxidants. TRENDS Neurosci. 2001;24:411–416. doi: 10.1016/s0166-2236(00)01819-1. [DOI] [PubMed] [Google Scholar]
- Moulignier A, Moulonguet A, Pialoux G, Rozenbaum W. Reversible ALS-like disorder in HIV infection. Neurol. 2001;57:995–1001. doi: 10.1212/wnl.57.6.995. [DOI] [PubMed] [Google Scholar]
- Navia BA, Rostasy K. The AIDS dementia complex: clinical and basic neuroscience with implications for novel molecular therapies. Neurotox Res. 2005;8:3–24. doi: 10.1007/BF03033817. [DOI] [PubMed] [Google Scholar]
- Nishihira J. Macrophage migration inhibitory factor (MIF): its essential role in the immune system and cell growth. J Interferon Cytokine Res. 2000;20:751–762. doi: 10.1089/10799900050151012. [DOI] [PubMed] [Google Scholar]
- Omenn GS, States DJ, Adamski M, Blackwell TW, Menon R, Hermjakob H, Apweiler R, Haab BB, Simpson RJ, Eddes JS, Kapp EA, Moritz RL, Chan DW, Rai AJ, Admon A, Aebersold R, Eng J, Hancock WS, Hefta SA, Meyer H, Paik YK, Yoo JS, Ping P, Pounds J, Adkins J, Qian X, Wang R, Wasinger V, Wu CY, Zhao X, Zeng R, Archakov A, Tsugita A, Beer I, Pandey A, Pisano M, Andrews P, Tammen H, Speicher DW, Hanash SM. Overview of the HUPO Plasma Proteome Project: results from the pilot phase with 35 collaborating laboratories and multiple analytical groups, generating a core dataset of 3020 proteins and a publicly-available database. Proteomics. 2005;5:3226–3245. doi: 10.1002/pmic.200500358. [DOI] [PubMed] [Google Scholar]
- Pasinetti GM, Ungar LH, Lange DJ, Yemul S, Deng H, Yuan X, Brown RH, Phil D, Cudkowicz ME, Newhall K, Peskind E, Marcus S, Ho L. Identification of potential CSF biomarkers in ALS. Neurology. 2006;66:1218–1222. doi: 10.1212/01.wnl.0000203129.82104.07. [DOI] [PubMed] [Google Scholar]
- Pocernish CB, Sultana R, Hone E, Turchan J, Martin RN, Calabrese V, Nath A, Butterfield DA. Effects of apolipoprotein E on the human immunodeficiency virus protein Tat in neuronal cultures and synaptosomes. J Neurosci Res. 2004;77:532–539. doi: 10.1002/jnr.20182. [DOI] [PubMed] [Google Scholar]
- Ranganathan S, Williams E, Ganchey P, Gopalakrishnan V, Lacomis D, Urbinelli L, Newhall K, Cudkowicz ME, Brown RH, Jr, Bowser R. Proteomic profiling of cerebrospinal fluid identifies biomarkers for amyotrophic lateral sclerosis. J Neurochem. 2005;95:1461–1471. doi: 10.1111/j.1471-4159.2005.03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüetschi U, Zetterberg H, Podust VN, Gottfries J, Li S, Simonsen AH, McGuire JM, Karlsson M, Rymo L, Davies H, Minthon L, Blennow K. Identification of CSF biomarkers for frontotemporal dementia using SELDI-TOF. Exp Neurol. 2005;196:273–281. doi: 10.1016/j.expneurol.2005.08.002. [DOI] [PubMed] [Google Scholar]
- SAS/STAT Software 8.1. SAS Institute Inc; Cary, NC: 2000. [Google Scholar]
- St. Hillaire C, Vargas D, Pardo CA, Gincel D, Mann J, Rothstein JD, McArthur JC, Conant K. Aquaporin 4 is increased in association with human immunodeficiency virus dementia: implications for disease pathogenesis. J Neurovirol. 2005;11:535–543. doi: 10.1080/13550280500385203. [DOI] [PubMed] [Google Scholar]
- Storey JD, Taylor JE, Siegmund D. Strong control, conservative point estimation, and simultaneous conservative consistency of false discovery rates: A unified approach. Journal of the Royal Statistical Society, Series B. 2004;66:187–205. [Google Scholar]
- Treitinger A, Spada C, Verdi JC, Miranda AF, Oliveira OV, Silveira MV, Moriel P, Abadía DS. Decreased antioxidant defense in individuals infected by the human immunodeficiency virus. Eur J Clin Invest. 2000;30:454–459. doi: 10.1046/j.1365-2362.2000.00642.x. [DOI] [PubMed] [Google Scholar]
- Vehmas A, Lieu J, Pardo CA, McArthur J, Gartner S. Amyloid precursor protein expression in circulating monocytes and brain macrophages from patients with HIV-associated cognitive impairment. J Neuroimmunol. 2004;157:99–110. doi: 10.1016/j.jneuroim.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Verma A, Berger JR. ALS syndrome in patients with HIV-1 infection. J Neurological Sci. 2006;240:59–64. doi: 10.1016/j.jns.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Von Giesen HJ, Kaiser R, Koller H, Wetzel K, Arendt G. Reversible ALS-like disorder in HIV infection. An ALS-like syndrome with new HIV infection and complete response to antiretroviral therapy. Neurol. 2006;59:474–475. doi: 10.1212/wnl.59.3.474. [DOI] [PubMed] [Google Scholar]
- Wojna V, Nath A. Challenges to the diagnosis and management of HIV Dementia. AIDS Reader. 2006;16:615–632. [PubMed] [Google Scholar]
- Wojna V, Carlson KA, Luo X, Mayo R, Melendez LM, Kraiselburd E, Gendelman HE. Proteomic fingerprinting of human immunodeficiency virus type 1-associated dementia from patient monocyte-derived macrophages: A case study. J Neurovirol. 2004b;10:74–81. doi: 10.1080/753312756. [DOI] [PubMed] [Google Scholar]
- Wojna V, Gerena V, Ginebra T, Skolasky R, García H, Gendelman HE, Kraiselburd E, McArthur J, Nath A, Zorrilla C, Melendez L. Prevalence of HIV Dementia in a group of Hispanic women at risk. J Neurovirol. 2004a;10:88–89. [Google Scholar]
- Wojna V, Skolasky R, Hechavarria R, Mayo R, Selnes O, McArthur J, Melendez L, Maldonado E, Zorrilla C, Garcia H, Kraiselburd E, Nath A. Prevalence of human immunodeficiency virus-associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. J Neurovirol. 2006;12:356–364. doi: 10.1080/13550280600964576. [DOI] [PubMed] [Google Scholar]
- Woods SP, Carey CL, Moran LM, Dawson MS, Letendre SL, Grant I The HIV Neurobehavioral Research Center (HNRC) Group. Frequency and predictors of self-reported prospective memory complaints in individuals infected with HIV. Arch Clin Neuropsychol. 2007;22:187–195. doi: 10.1016/j.acn.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y, McLerran D, Adam BL, Winget M, Thornquist M, Feng Z. An automated peak identification/calibration procedure for high-dimensional protein measures from mass spectrometers. J Biomed Biotechnol. 2003;2003:242–248. doi: 10.1155/S111072430320927X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Desiderio DM. Proteomics analysis of human cerebrospinal fluid. J Chromatogr. 2005;815:179–189. doi: 10.1016/j.jchromb.2004.06.044. [DOI] [PubMed] [Google Scholar]


