Abstract
Although non-syndromic hereditary gingival fibromatosis (HGF) is genetically heterogeneous, etiologic mutations have been identified only in the Son of Sevenless-1 gene (SOS1). To test evidence of increased cell proliferation, we studied histological, morphological, and proliferation characteristics in monolayer and three-dimensional cultures of fibroblasts with the SOS1 g.126,142-126,143insC mutation. Histological assessment of HGF gingiva indicated increased numbers of fibroblasts (30%) and increased collagen (10%). Cell proliferation studies demonstrated increased growth rates and 5-bromo-2-deoxyuridine incorporation for HGF fibroblasts. Flow cytometry showed greater proportions of HGF fibroblasts in the G2/M phase. Attachment of HGF fibroblasts to different extracellular matrix surfaces demonstrated increased formation of protrusions with lamellipodia. HGF fibroblasts in three-dimensional culture showed greater cell proliferation, higher cell density, and alteration of surrounding collagen matrix. These findings revealed that increased fibroblast numbers and collagen matrix changes are associated with mutation of the SOS1 gene in vitro and in vivo.
Keywords: hereditary gingival fibromatosis, Son of Sevenless-1, fibroblast, collagen
INTRODUCTION
Hereditary gingival fibromatosis (HGF) is a genetically heterogeneous condition characterized by a slowly progressive, benign fibrous enlargement of keratinized gingiva (Witkop, 1971; Hart et al., 1998; Gorlin et al., 2001; OMIM #135300). The clinical presentation of HGF is variable in distribution and severity of expression (Witkop, 1971; Jorgenson and Cocker, 1974; Raeste et al., 1978). Whether the increased gingiva reflects increased cell numbers, extracellular collagen, or other extracellular constituents is not always clear, and the underlying mechanism is unknown. Reported histological, morphological, and cellular characteristics of HGF gingival tissues differ (Johnson et al., 1986; Shirasuna et al., 1988; Hou, 1993; Barros et al., 2001; Araujo et al., 2003; Saygun et al., 2003; Tipton et al., 2004; Almeida et al., 2005; Gagliano et al., 2005; Martelli-Junior et al., 2005). Genotype-phenotype correlations are problematic, because it is unknown if reported differences between individuals with HGF are due to variable expression of a common gene mutation, allelic mutations, non-allelic mutations, or methodological differences (Hart et al., 2000; Xiao et al., 2000, 2001; Tipton et al., 2004; Ye et al., 2005). Although genetic studies demonstrate locus heterogeneity, mutation of only one gene, Son of sevenless-1 (SOS1), has been identified as etiologic for non-syndromic HGF (Hart et al., 2002; #135300). SOS1 is a guanine nucleotide-exchange factor that functions in the transduction of signals that control cell growth and differentiation (OMIM #182530). A single base insertion mutation (SOS1 g.126,142-126,143insC) introduces a frameshift, creating a premature stop codon, abolishing 4 functionally important proline-rich SH3 binding domains normally present in the carboxyl-terminal region of the SOS1 protein (Hart et al., 2002). The resultant protein chimera has enhanced activity, since it lacks the carboxyl-terminal domain that normally exerts negative alosteric control (Corbalan-Garcia et al., 1998). This mutation causes HGF in humans, and similar SOS1 deletion constructs are functional in animal models, and a transgenic mouse construct with a comparable SOS1 chimera produces a skin hypertrophy phenotype (Sibilia et al., 2000).
This study characterizes histological, morphological, and proliferation in monolayer and three-dimensional cultures of fibroblasts from HGF patients with the SOS1 mutation.
MATERIALS & METHODS
Histological and Quantitative Determination of Collagen Area Fraction
Maxillary anterior buccal gingiva was obtained from three normal control individuals and three HGF patients following informed consent and Human Subjects approval (University of Taubate and National Institutes of Health). HGF patients with gingiva covering greater than one-third of the clinical crowns carried an SOS1 gene mutation (Hart et al., 2002). Controls needing crown lengthening had clinically healthy gingiva. No individuals reported taking medications associated with gingival overgrowth (cyclosporine, phenytoin, calcium channel blockers). Gingival samples were fixed in 10% formalin and paraffin-embedded. Sections (5 μm) were stained with hematoxylin and eosin and with sirius red F3Ba (Junqueira et al., 1979). Fibroblast numbers and mean area fraction of collagen were determined from 6 different fields in each individual, as described previously (Séguier et al., 2000; Ejeil et al., 2003). Sections observed with polarized light were imaged and analyzed with ImageLab software (Diracom, Vargem Grande do Sul, SP, Brazil), which performed transformations of mathematical morphology and calculation of the area fraction occupied by collagen.
Isolation of Gingival Fibroblasts and Cell Cultures
Gingival tissues were washed with phosphate-buffered saline containing 1X antibiotic-antimycotic solution (Invitrogen, Carlsbad, CA, USA), minced, and incubated overnight (4°C) with dispase (2.5 mg/mL; Worthington Biochemical, Lakewood, NJ, USA). Following epithelial separation, connective tissues were washed with phosphate-buffered saline, digested in collagenase (0.5%, w/v; Worthington Biochemical), passed through a cell strainer (70 μm, BD Falcon, Franklin Lakes, NJ, USA), and centrifuged. Pellets were re-suspended in Dulbecco's Modified Eagle's Medium containing 10% FBS and antibiotic-antimycotic solution (Invitrogen). All cells underwent fewer than 10 passages.
Proliferation Measurements
Fibroblasts were plated (48-well culture dishes; 500 cells/well), and cell numbers from triplicate wells were determined daily for 7 days by means of a Z2 Coulter Particle Count and Size Analyzer (Beckman Coulter, Fullerton, CA, USA). All data are from at least 3 separate experiments. To monitor DNA synthesis, we plated fibroblasts in 48-well culture dishes (500 cells/well, 37°C). At each time-point, cells were incubated with 5-bromo-2-deoxyuridine (20 μM) in its growth medium (37°C) for 4 hrs prior to fixation. DNA incorporation was quantified by ELISA (Roche, Indianapolis, IN, USA).
Flow Cytometry Analysis
For cell cycle studies, from 70 ∼ 80 × 104 fibroblasts were plated in 100-mm tissue culture dishes and maintained in growth media until 90% confluent. After being washed with phosphate-buffered saline, cultures were serum-starved overnight with Dulbecco's Modified Eagle's Medium containing 20 mM HEPES (starving medium), to synchronize cell cycles. Three culture conditions were evaluated (see Fig. 2 legend). Cells were detached by trypsinization, and total cell numbers were determined. Cells were fixed and labeled with propidium iodide (Guo et al., 2005) and analyzed by flow cytometry by means of a FACSCalibur (Becton Dickinson, San Jose, CA, USA). Data were analyzed with Cell Quest analysis software. The averaged results are presented as mean ± standard deviation. Differences between groups were analyzed by Student's t test, with P-values < 0.05 considered significant.
Figure 2.
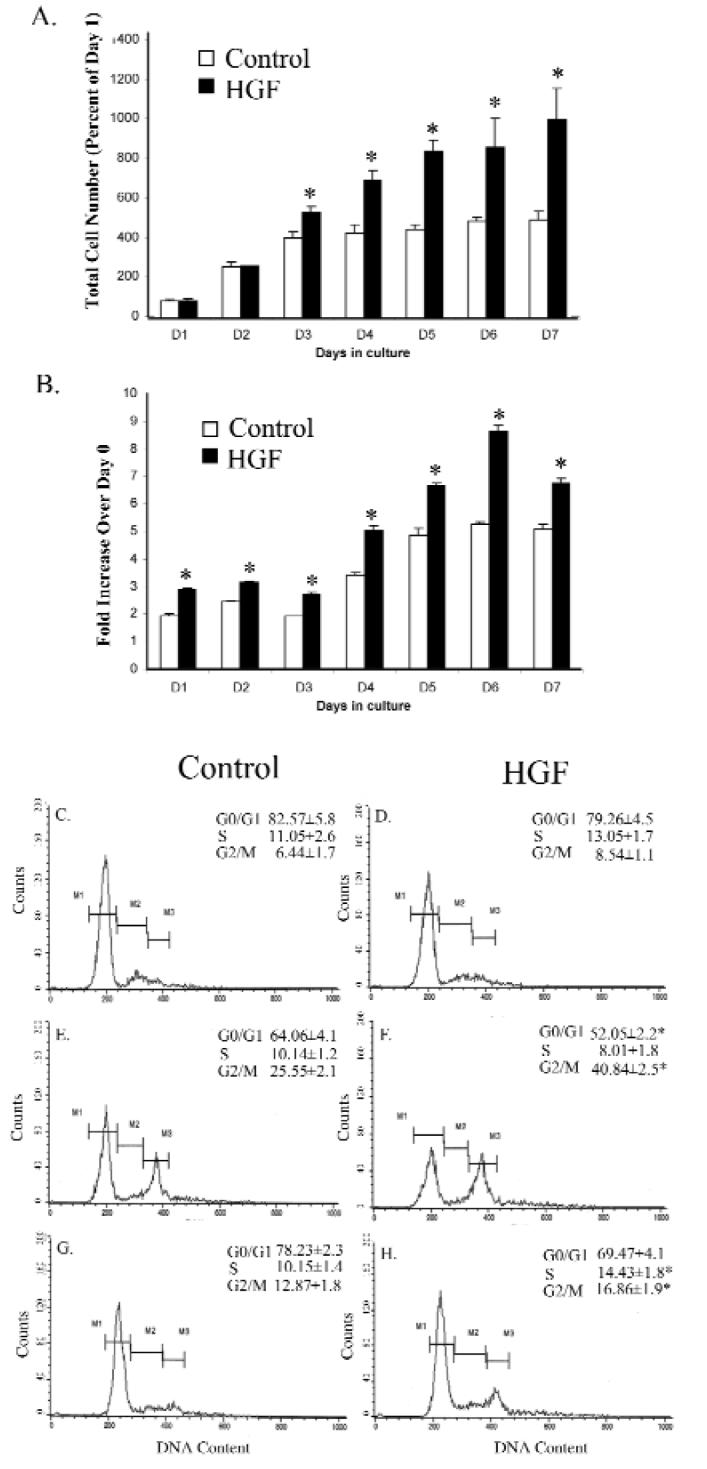
Cell proliferation, 5-bromo-2-deoxyuridine uptake, and cell cycle profiles in fibroblasts. Fibroblasts from normal control (N = 3 individuals) and HGF patients (N = 3 patients) were compared. At indicated time-points, total cell numbers (A) or 5-bromo-2-deoxyuridine incorporation into DNA (B) were determined from triplicate samples. The data are presented as an average with standard deviations; * indicates P < 0.05. (C-H) Fibroblast cultures were prepared under 3 different conditions as described below. Panels C, E, and G are normal control fibroblasts, and panels D, F, and H are HGF fibroblasts. (C,D) Cultures under starving media conditions for 2 days. (E,F) Cultures under starving conditions for 1 day and switched to growth media for one day. (G,H) Cultures under starving conditions for 1 day and switched to growth media for 3 days. The percentages of total cells contained in different cell cycle phases are indicated. M1 represents the “Go/G1” phase, M2 denotes the “S” phase, and M3 denotes the “G2/M” phase. Significant difference between control and HGF is marked with asterisks (P < 0.05).
Attachment Assays
To compare fibroblast attachment on different extracellular matrices, we coated four-chamber coverglass slides (Lab-Tak, BD Biosciences, Bedford, MA, USA) with type I collagen (100 μg/mL), fibronectin (50 μg/mL), or poly-D-lysine (50 μg/mL) (Sigma, St. Louis, MO, USA). Fibroblasts (30,000/0.5 mL) were plated in each chamber and incubated (37°C, 30 min). Unattached cells were removed, and attached cells were rinsed with phosphate-buffered saline and fixed with 4% paraformaldehyde. Cultures were imaged by inverted microscopy (Olympus, IX71, Center Valley, PA, USA), and images were processed with Adobe Photoshop CS software (San Jose, CA, USA).
Construction of Three-dimensional Collagen Cultures
To monitor three-dimensional cell proliferation in vitro, we prepared a three-dimensional collagen matrix (Igarashi et al., 2003). After 5 days, cultures were fixed (10% formalin, 37°C, 30 min) and stained with Diff-quik (Med-Ox Diagnostics, Inc., Orlando, FL, USA). To monitor cell proliferation, we released cells by collagenase digestion and determined cell numbers by means of a Coulter Counter. The matrix was also fixed with 10% formalin solution and stained with 0.1% sirius red (Sigma), and collagen fibrils were examined under a microscope. Each experiment was conducted in triplicate.
Statistical Analyses
The mean area fractions occupied by collagen, fibroblast numbers, and proliferation measures for HGF and controls were compared by the Student's t test. Values of p < 0.05 were considered significant.
RESULTS
Mean ages of HGF patients (24.83 ± 8.03 yrs) and controls (24.66 ± 13.5 yrs) were not different. Clinical and histological findings are shown in Fig. 1. HGF gingiva showed increased connective tissue projections into the epithelium (Fig. 1Bb), compared with controls (Fig. 1Ab). HGF connective tissue contained more fibroblasts per unit area (67.54 ± 8.69) (Fig. 1Bc), compared with controls (47.52 ± 5.41) (Fig. 1Ac). The fractional area of collagen in controls, 63.30% ± 0.89%, was significantly less (p < 0.05) than that in the HGF group, 69.65% ± 1.57% (Fig. 1Ad). Collagen in the HGF groups displayed a less parallel fiber orientation compared with controls (Fig. 1Bd).
Figure 1.
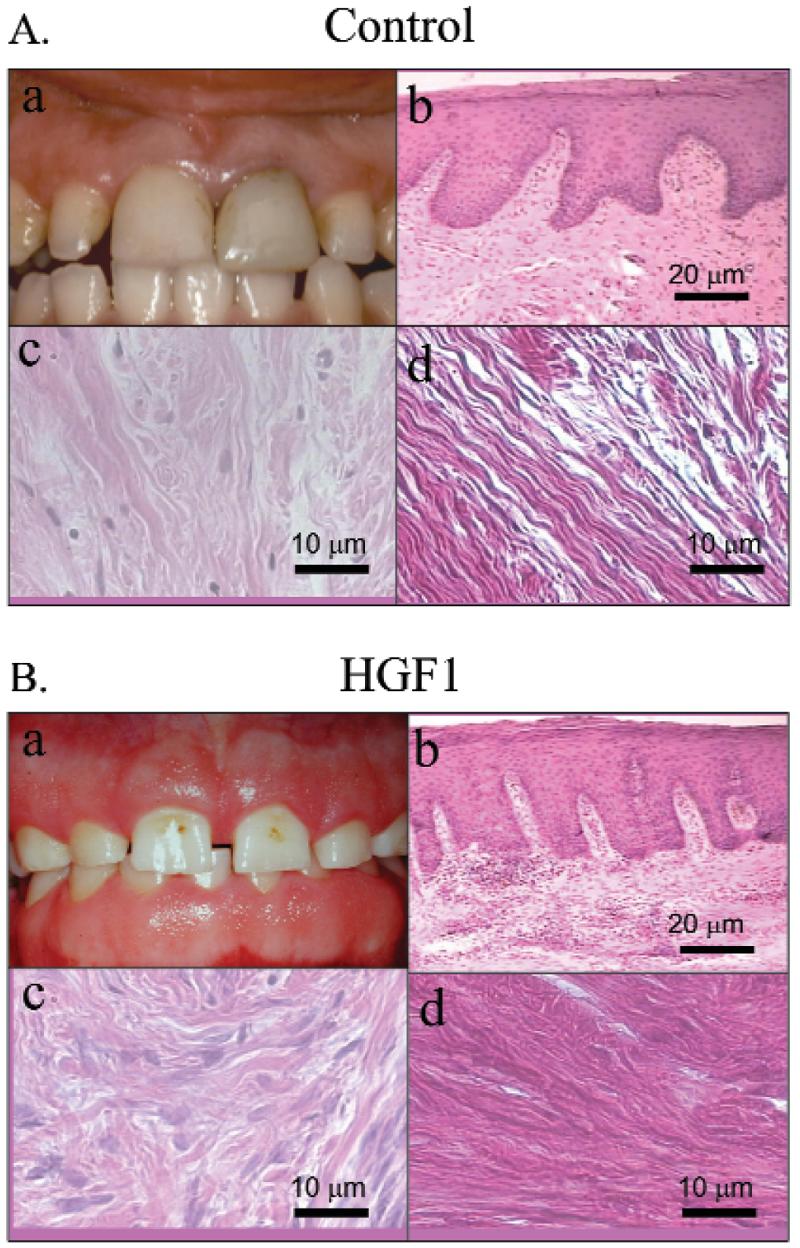
Clinical appearance and histology. (A) Control gingiva. (a) Clinical aspect before the crown extension surgery; (b) histological section showing the normal tissue (hematoxylin and eosin, magnification of 5x; bar is 100 μm); (c) connective tissue section (magnification of 40x; bar is 20 μm); (d) area of connective tissue stained with sirius red F3Ba (magnification of 40x; bar is 20 μm). (B) HGF patient. (a) Clinical aspect before the surgery; (b) histological section showing the epithelial rete pegs into underlying corneum (hematoxylin and eosin, magnification of 5x; bar is 100 μm); (c) connective tissue section (magnification of 40x; bar is 20 μm); (d) area of connective tissue stained with sirius red F3Ba (magnification of 40x; bar is 20 μm).
Proliferation, 5-bromo-2-deoxyuridine Incorporation, and Cell Cycle Profile
Cell proliferation was compared between control and HGF fibroblasts in monolayer culture. Total cell numbers increased at similar rates for the first 2 days (Fig. 2A), after which growth rates increased only in HGF fibroblasts. DNA synthesis was monitored through 5-bromo-2-deoxyuridine incorporation. In the first 3 days, incorporation was 25% higher in HGF fibroblasts (Fig. 2B). After that, 5-bromo-2-deoxyuridine uptake increased gradually and plateaued after day 5 in control fibroblasts, while uptake increased through day 6 in HGF fibroblasts. Cell cycle profiles were evaluated with 3 different culture conditions (see Fig. 2 legend). With flow cytometry, serum starving for 2 days resulted in a major cell population in the G0/G1 phase (Figs. 2C, 2D). A slightly greater percentage of cells was found in S and G2/M phases in HGF fibroblasts (Fig. 2D) than in controls (Fig. 2C). When serum-starved for 1 day and switched to regular growth medium for 1 day, no difference was found in S-phases between control and HGF fibroblasts; however, a greater proportion of cells entered the G2/M phase in HGF fibroblasts (41% vs. 25%, p < 0.05, Figs. 2E, 2F), indicating greater HGF fibroblast proliferation. When cultures were maintained in regular growth medium for another 2 days, the percentage of the cell population remained higher in both S and G2/M phases in HGF fibroblasts (Figs. 2G, 2H).
Comparison of Cell Attachment
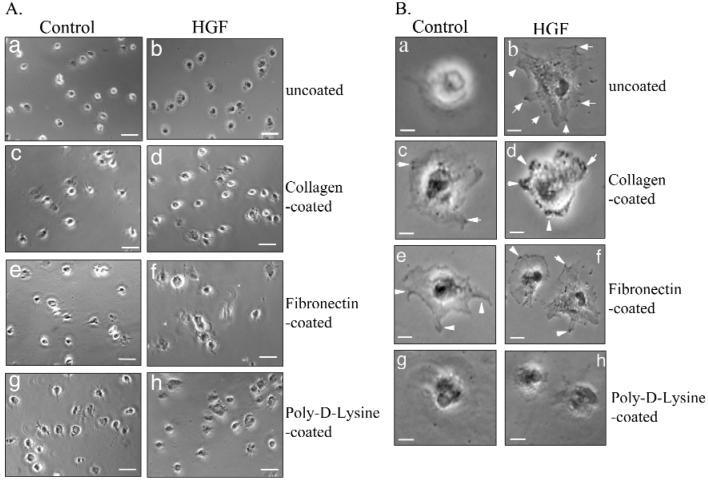
Cell attachment can affect cell growth in culture systems. To study if cell attachment plays a role in the higher growth rate of HGF fibroblasts, we performed attachment assays. Three commonly used extracellular matrices were coated onto culture dishes. Time-course studies determined that > 80% of cells were attached within 30 min (data not shown). HGF and control fibroblasts displayed different degrees of cell protrusion when plated on different extracellular matrix-coated plates (Fig. 3A). For control fibroblasts, > 90% of attached cells already showed extension on the cell periphery in poly-D-lysine-coated plates (Figs. 3Ag). Less than 10% of attached cells did so in uncoated plates (Fig. 3Aa). Almost all HGF fibroblasts showed cell extensions in both uncoated and poly-D-lysine-coated plates (Figs. 3Ab, 3Ah). Similar proportions (∼ 60%) of attached cells showed cell extensions for both control and HGF fibroblasts on collagen- and fibronectin-coated plates (Figs. 3Ad, 3Af). Under higher magnification, control fibroblasts showed only attachment on uncoated and poly-D-lysine-coated surfaces (Figs. 3Ba, 3Bg). Control fibroblasts displayed membrane ruffles, with some protrusion on collagen- and fibronectin-coated dishes (Fig. 3B, arrows in Figs. 3Bc, 3Be). HGF fibroblasts already showed protrusion with lamellipodia and membrane ruffles with possible focal contact formation on uncoated, collagen-, and fibronectin-coated matrices (arrows in Figs. 3Bb, 3Bd, 3Bf), but not on poly-D-lysine-coated surfaces (h). Since fibroblast cultures were usually plated and maintained in uncoated tissue culture plates, the different proportions of cells showing protrusion, < 30% for control fibroblasts and > 90% for HGF fibroblasts (Figs. 3Aa, 3Ab), could account for higher in vitro HGF fibroblast growth rates.
Figure 3.
Cell attachment on different extracellular matrices. The chamber slides were coated with either collagen (c,d), fibronectin (e,f), or poly-D-lysine (g,h), and uncoated (a,b) was the control. After being plated for 30 min, cells were fixed and examined under an inverted microscope. At least 20 different fields were examined, and 1 representative image is shown. (A) Low magnification (100X). Bar is 20 μm. (B) High magnification (960X). Bar is 5 μm.
Proliferation of Fibroblasts and Change of Collagen Structure in Three-dimensional Matrix
Results of fibroblast proliferation assays in monolayer cultures were consistent with histological observations indicating greater fibroblast cell numbers in HGF gingiva (Figs. 1Ac, 1Bc). Since it is difficult to monitor the proliferation of gingival fibroblasts in vivo, we developed a three-dimensional culture system to compare growth rates and to study whether fibroblasts would alter the structure of collagen as observed in HGF gingival tissue sections (Figs. 1Ad, 1Bd). We used Type-I collagen to construct three-dimensional matrices, since similar proportions of cell extensions were observed in control and HGF fibroblasts plated on collagen-1-coated plates (Figs. 3Bc, 3Bd), and because it is the major extracellular matrix component of gingiva. To evaluate fibroblast proliferation in three-dimensional matrices, we mixed equal numbers of cells, at 2 different densities, in collagen-1 matrices during three-dimensional culture preparation. After 48 hrs' incubation, cells were released from the matrix by collagenase digestion, and cell numbers were determined. Regardless of the initial cell-plating density, an increased cell density was observed for both control and HGF fibroblasts (Fig. 4A). However, the increase in HGF fibroblasts was greater in both the low- (37% increase vs. control) and high-density (31% increase vs. control) platings, indicating significantly greater HGF fibroblast growth rates (p < 0.05). Diff-Quik staining also illustrated the increase of cell populations in HGF fibroblasts compared with controls (Figs. 4B, 4C).
Figure 4.
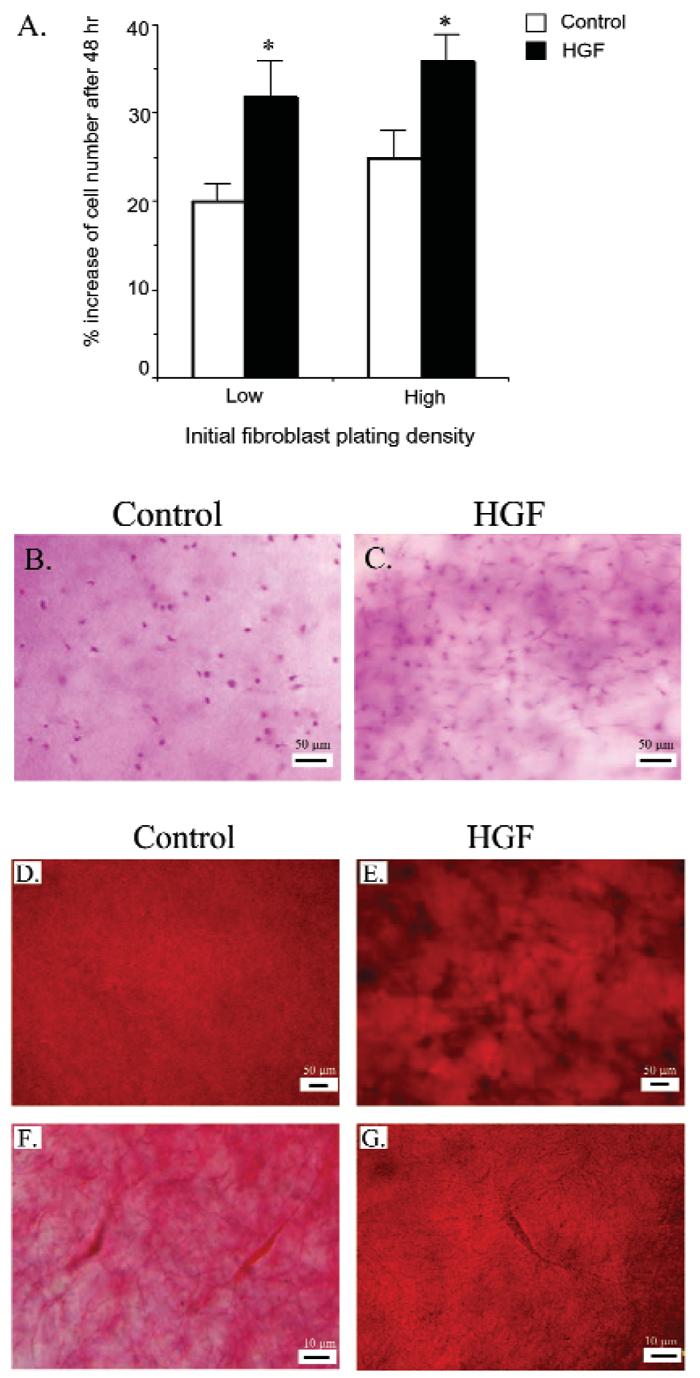
Proliferation of fibroblasts and change of collagen matrix in three-dimensional cultures. The three-dimensional collagen matrix containing normal control fibroblasts or HGF fibroblasts was prepared at 2 different densities (low, 15,000 cells/mL; and high, 20,000 cells/mL). (A) After 2 days, fibroblasts were released from 1 set of three-dimensional cultures as described in MATERIALS & METHODS. Total cell numbers were determined, and data are presented as mean ± standard deviations from triplicate samples for controls (N = 3) and HGF patients (N = 3); * indicates P < 0.05. Another set of three-dimensional cultures (20,000 cells/mL), maintained in regular growth medium for an additional 3 days, was stained with Diff-quik to show cells (B,C). The three-dimensional cultures were fixed and stained with picro-sirius red to show collagen. Panels D and F demonstrate the ordered spreading (D) and density (F) from control cells. Irregularly clumped deposition (E) and increased collagen density (G) were observed from HGF cells in three-dimensional culture. Panels D and E are low-magnification (100X) images; panels F and G are high-magnification (960X) images. Bar is 50 μm in D and E, 10 μm in F and G.
In addition to increased fibroblast numbers, greater shadow areas were observed around HGF fibroblasts, indicating changes of the collagen matrix. Picro-sirius red staining of collagen demonstrated even spreading in three-dimensional matrix containing control fibroblasts (Fig. 4D). In contrast, an uneven collagen distribution with darker and cluster regions was associated with HGF fibroblasts (Fig. 4E). While light staining with loose collagen fibers was displayed around control fibroblasts (Fig. 4F), dense staining with compact collagen fibers was present around HGF fibroblasts (Fig. 4G). These results indicate that growth of HGF fibroblasts in the three-dimensional collagen culture system can lead to a structural change of the surrounding collagen matrix.
DISCUSSION
Increased gingival volumes reported in HGF appear chiefly due to increased connective tissue. Studies generally have reported increases of extracellular matrix, primarily collagen (Coletta et al., 1998; Saygun et al., 2003; Gagliano et al., 2005; Martelli-Junior et al., 2005). Our histological analysis of HGF gingiva indicated increased collagen (approximately 10%) as well as denser, less-regularly-ordered patterns of collagen deposition (Fig. 1). We found more fibroblasts present (approximately 30%) at all levels of the connective tissue. Previous studies indicated increased proliferation rates for epithelial cells and fibroblasts in HGF gingiva. Immunohistochemical assays for epidermal growth factors, epidermal growth factor receptor, proliferating cell nuclear antigen, and pKi-67 generally reported increased epithelial cell proliferation in HGF tissue (Araujo et al., 2003; Saygun et al., 2003; Almeida et al., 2005; Martelli-Junior et al., 2005). Increased fibroblast proliferation has also been reported with 5-bromo-2-deoxyuridine incorporation into DNA (Tipton et al., 1997, 2004; Almeida et al., 2005). Others using Ki-67 immunohistochemistry reported no increased fibroblast proliferation in HGF (Saygun et al., 2003). Our assays for total cell number and 5-bromo-2-deoxyuridine incorporation into actively growing fibroblasts demonstrated significantly higher HGF fibroblast proliferation rates (p < 0.05; Fig. 2). Interestingly, whereas cell proliferation for control fibroblasts plateaued after 3 days, HGF fibroblasts continued to proliferate through day 7. Flow cytometry experiments indicated that when released from starvation conditions, a significantly greater proportion of HGF fibroblasts entered the G2/M cell cycle phase (Figs. 2E, 2F), consistent with an increased rate of cell proliferation, findings consistent with those of Coletta et al. (1999).
Since cells behave differently in three-dimensional tissues than in the monolayer, we evaluated HGF and control fibroblasts in three-dimensional matrices. Regardless of initial cell density, there was an increase (∼ 31-37%) of HGF fibroblasts compared with controls, again indicating higher proliferation rates for HGF fibroblasts. Additionally, whereas control fibroblasts were associated with a uniform spreading of collagen in the three-dimensional matrix assay, collagen associated with HGF fibroblasts appeared uneven, with dense staining indicating clustering of collagen fibers. These findings are consistent with histological observations of gingiva from our HGF patients. Because the ability of cells to attach to surfaces involves formation of focal adhesions and focal contacts which affect cell migration, growth, and survival through integrin signaling pathways, we compared the ability of fibroblasts to attach to different extracellular matrix surfaces (Zamir and Geiger, 2001; Martin et al., 2002). HGF fibroblasts demonstrated greater protrusions with lamellipodia and membrane ruffles under all surface conditions tested, except on poly-D-lysine-coated surfaces (Fig. 3). This difference in HGF fibroblast behavior may be related to their increased proliferation rates and to the changes in the collagen matrix observed in three-dimensional cultures (Fig. 4).
These findings demonstrated increased numbers of fibroblasts associated with mutation of the SOS1 gene, both in vitro and in vivo. SOS1 functions as a guanine-exchange factor and plays a critical role (in Ras signaling pathways) that affects cell proliferation, movement, and differentiation (Bar-Sagi and Hall, 2000). Fibroblasts carrying the SOS1 mutation demonstrated increased cell proliferation rates, an altered ability to attach, and an enhanced propensity to form protrusions with lamellipodia. These interactions may relate to the alterations of cell numbers and collagen observed in HGF gingiva.
ACKNOWLEDGMENTS
We thank Dr. Ok Hee Ryu for isolation of fibroblasts, and acknowledge support from the Intramural Program of the NIDCR, National Institutes of Health, Bethesda, MD 20892, USA.
REFERENCES
- Almeida JP, Coletta RD, Silva SD, Agostini M, Vargas PA, Bozzo L, et al. Proliferation of fibroblasts cultured from normal gingiva and hereditary gingival fibromatosis is dependent on fatty acid synthase activity. J Periodontol. 2005;76:272–278. doi: 10.1902/jop.2005.76.2.272. [DOI] [PubMed] [Google Scholar]
- Araujo CS, Graner E, Almeida OP, Sauk JJ, Coletta RD. Histomorphometric characteristics and expression of epidermal growth factor and its receptor by epithelial cells of normal gingiva and hereditary gingival fibromatosis. J Periodontal Res. 2003;38:237–241. doi: 10.1034/j.1600-0765.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D, Hall A. Ras and Rho GTPases: a family reunion. Cell. 2000;103:227–238. doi: 10.1016/s0092-8674(00)00115-x. [DOI] [PubMed] [Google Scholar]
- Barros SP, Merzel J, de Araujo VC, de Almeida OP, Bozzo L. Ultrastructural aspects of connective tissue in hereditary gingival fibromatosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:78–82. doi: 10.1067/moe.2001.115026. [DOI] [PubMed] [Google Scholar]
- Coletta RD, Almeida OP, Graner E, Page RC, Bozzo L. Differential proliferation of fibroblasts cultured from hereditary gingival fibromatosis and normal gingiva. J Periodontal Res. 1998;33:469–475. doi: 10.1111/j.1600-0765.1998.tb02346.x. [DOI] [PubMed] [Google Scholar]
- Coletta RD, Almeida OP, Ferreira LR, Reynolds MA, Sauk JJ. Increase in expression of Hsp47 and collagen in hereditary gingival fibromatosis is modulated by stress and terminal procollagen N-propeptides. Connect Tissue Res. 1999;40:237–249. doi: 10.3109/03008209909000702. [DOI] [PubMed] [Google Scholar]
- Corbalan-Garcia S, Margarit SM, Galron D, Yang SS, Bar-Sagi D. Regulation of Sos activity by intramolecular interactions. Mol Cell Biol. 1998;18:880–886. doi: 10.1128/mcb.18.2.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejeil AL, Igondjo-Tchen S, Ghomrasseni S, Pellat B, Godeau G, Gogly B. Expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in healthy and diseased human gingiva. J Periodontol. 2003;74:188–195. doi: 10.1902/jop.2003.74.2.188. [DOI] [PubMed] [Google Scholar]
- Gagliano N, Moscheni C, Dellavia C, Masiero S, Torri C, Grizzi F, et al. Morphological and molecular analysis of idiopathic gingival fibromatosis: a case report. J Clin Periodontol. 2005;32:1116–1121. doi: 10.1111/j.1600-051X.2005.00811.x. [DOI] [PubMed] [Google Scholar]
- Gorlin RJ, Cohen MM, Levi LS. Syndromes of the head and neck. In: Gorlin RJ, Cohen MM Jr, Hennekam RCM, editors. Syndromes of the head and neck. Oxford University Press; New York: 2001. pp. 1093–1106. [Google Scholar]
- Guo J, Sheng G, Warner BW. Epidermal growth factor-induced rapid retinoblastoma phosphorylation at Ser780 and Ser795 is mediated by ERK1/2 in small intestine epithelial cells. J Biol Chem. 2005;280:35992–35998. doi: 10.1074/jbc.M504583200. [DOI] [PubMed] [Google Scholar]
- Hart TC, Pallos D, Bowden DW, Bolyard J, Pettenati MJ, Cortelli JR. Genetic linkage of hereditary gingival fibromatosis to chromosome 2p21. Am J Hum Genet. 1998;62:876–883. doi: 10.1086/301797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart TC, Pallos D, Bozzo L, Almeida OP, Marazita ML, O'Connell JR, et al. Evidence of genetic heterogeneity for hereditary gingival fibromatosis. J Dent Res. 2000;79:1758–1764. doi: 10.1177/00220345000790100501. [DOI] [PubMed] [Google Scholar]
- Hart TC, Zhang Y, Gorry MC, Hart PS, Cooper M, Marazita ML, et al. A mutation in the SOS1 gene causes hereditary gingival fibromatosis type 1. Am J Hum Genet. 2002;70:943–954. doi: 10.1086/339689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou LT. Synthesis of collagen and fibronectin in fibroblasts derived from healthy and hyperplastic gingivae. J Formos Med Assoc. 1993;92:367–372. [PubMed] [Google Scholar]
- Igarashi M, Irwin CR, Locke M, Mackenzie IC. Construction of large area organotypical cultures of oral mucosa and skin. J Oral Pathol Med. 2003;32:422–430. doi: 10.1034/j.1600-0714.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- Johnson BD, el-Guindy M, Ammons WF, Narayanan AS, Page RC. A defect in fibroblasts from an unidentified syndrome with gingival hyperplasia as the predominant feature. J Periodontal Res. 1986;21:403–413. doi: 10.1111/j.1600-0765.1986.tb01474.x. [DOI] [PubMed] [Google Scholar]
- Jorgenson RJ, Cocker ME. Variation in the inheritance and expression of gingival fibromatosis. J Periodontol. 1974;45:472–477. doi: 10.1902/jop.1974.45.7.472. [DOI] [PubMed] [Google Scholar]
- Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Martelli-Junior H, Lemos DP, Silva CO, Graner E, Coletta RD. Hereditary gingival fibromatosis: report of a five-generation family using cellular proliferation analysis. J Periodontol. 2005;76:2299–2305. doi: 10.1902/jop.2005.76.12.2299. [DOI] [PubMed] [Google Scholar]
- Martin KH, Slack JK, Boerner SA, Martin CC, Parsons JT. Integrin connections map: to infinity and beyond. Science. 2002;296:1652–1653. doi: 10.1126/science.296.5573.1652. [DOI] [PubMed] [Google Scholar]
- OMIM Online Mendelian Inheritance in Man. http://www.ncbi.nlm.nih.gov/Omim/ (for fibrous enlargement of maxillary and mandibular keratinized gingiva [MIM 135300]; Son of sevenless-1[182530]
- Raeste AM, Collan Y, Kilpinen E. Hereditary fibrous hyperplasia of the gingiva with varying penetrance and expressivity. Scand J Dent Res. 1978;86:357–365. doi: 10.1111/j.1600-0722.1978.tb00638.x. [DOI] [PubMed] [Google Scholar]
- Saygun I, Ozdemir A, Gunhan O, Aydintug YS, Karslioglu Y. Hereditary gingival fibromatosis and expression of Ki-67 antigen: a case report. J Periodontol. 2003;74:873–878. doi: 10.1902/jop.2003.74.6.873. [DOI] [PubMed] [Google Scholar]
- Séguier S, Godeau G, Brousse N. Collagen fibers and inflammatory cells in healthy and diseased human gingival tissues: a comparative and quantitative study by immunohistochemistry and automated image analysis. J Periodontol. 2000;71:1079–1085. doi: 10.1902/jop.2000.71.7.1079. [DOI] [PubMed] [Google Scholar]
- Shirasuna K, Okura M, Watatani K, Hayashido Y, Saka M, Matsuya TT. Abnormal cellular property of fibroblasts from congenital gingival fibromatosis. J Oral Pathol. 1988;17:381–385. doi: 10.1111/j.1600-0714.1988.tb01301.x. [DOI] [PubMed] [Google Scholar]
- Sibilia MA, Fleischmann A, Behrens A, Stingl L, Carroll J, Watt FM, et al. The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development. Cell. 2000;102:211–220. doi: 10.1016/s0092-8674(00)00026-x. [DOI] [PubMed] [Google Scholar]
- Tipton DA, Howell KJ, Dabbous MK. Increased proliferation, collagen, and fibronectin production by hereditary gingival fibromatosis fibroblasts. J Periodontol. 1997;68:524–530. doi: 10.1902/jop.1997.68.6.524. [DOI] [PubMed] [Google Scholar]
- Tipton DA, Woodard ES, 3rd, Baber MA, Dabbous MKh. Role of the c-myc proto-oncogene in the proliferation of hereditary gingival fibromatosis fibroblasts. J Periodontol. 2004;75:360–369. doi: 10.1902/jop.2004.75.3.360. [DOI] [PubMed] [Google Scholar]
- Witkop CJ., Jr Heterogeneity in gingival fibromatosis. Birth Defects Orig Artic Ser. 1971;7:210–221. [PubMed] [Google Scholar]
- Xiao S, Wang X, Qu B, Yang M, Liu G, Bu L, et al. Refinement of the locus for autosomal dominant hereditary gingival fibromatosis (GINGF) to a 3.8-cM region on 2p21. Genomics. 2000;68:247–252. doi: 10.1006/geno.2000.6285. [DOI] [PubMed] [Google Scholar]
- Xiao S, Bu L, Zhu L, Zheng G, Yang M, Qian M, et al. A new locus for hereditary gingival fibromatosis (GINGF2) maps to 5q13-q22. Genomics. 2001;74:180–185. doi: 10.1006/geno.2001.6542. [DOI] [PubMed] [Google Scholar]
- Ye X, Shi L, Cheng Y, Peng Q, Huang S, Liu J, et al. A novel locus for autosomal dominant hereditary gingival fibromatosis, GINGF3, maps to chromosome 2p22.3-p23.3. Clin Genet. 2005;68:239–244. doi: 10.1111/j.1399-0004.2005.00488.x. [DOI] [PubMed] [Google Scholar]
- Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J Cell Sci. 2001;114(Pt 20):3583–3590. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]