Abstract
Objective
To describe the frequency, context and type of oncologists’ recommendations to patients that they participate in a clinical trial and to analyze the relationship between recommendations and patients’ decisions to participate.
Methods
Data included 38 video recorded outpatient interactions during which 15 oncologists invited 38 patients to participate in clinical trials. We described the frequency, context, and type of oncologists’ recommendations and analyzed the relationship between these factors and patient decisions to participate and socio-demographic characteristics.
Results
Sixty-eight percent (n = 26) of the 38 interactions included an explicit recommendation. Most recommendations were unprompted by patients and/or companions and were tailored to individual patients. A significant relationship was found between recommendations and patients’ decisions to participate. Positive trends were found between receiving a recommendation and being female and having higher education.
Conclusion
Oncologists routinely make recommendations to patients during the presentation of clinical trials. These recommendations may influence patients’ decisions and may occur more frequently with some demographic groups.
Practice Implications
Oncologists should be aware of the potential influence of their recommendations when discussing clinical trials with patients.
Keywords: physician-patient communication, clinical trials, recruitment, cancer
1. Introduction
Researchers and clinicians generally agree on several points regarding patient participation in clinical trials: First, clinical trials are the primary mechanism for evaluating the safety and efficacy of new cancer treatments and improving the standard of care [1]; second, meaningful informed consent is a precondition for the ethical involvement of humans in clinical research [1–3]; and third, physician communication during clinical encounters is an important factor in patients’ decisions about whether to participate in a clinical trial [4–6].
However, one aspect of physician communication and clinical trials has been the subject of debate. At issue is a key controversy regarding informed consent—whether clinicians should make explicit recommendations to patients about whether to accept the offer to participate in a clinical trial. Some ethicists and scholars have suggested that clinician recommendations (e.g., “I think this study would be good for you,”) may unduly influence patients, potentially inhibiting patients’ right to make autonomous decisions [7, 8], violating the principle of equipoise [9], and contributing to therapeutic misconception [10–15]. Others argue that true equipoise rarely exists [16] and therefore that physicians have a duty to provide their recommendations so that patients can consider this information as they make informed treatment decisions [17, 18]. In fact, some advocates of recommendations suggest the failure of clinicians to advise patients of their preference violates their duty to recommend the best treatment [16] and amounts to coercion [19]. Accordingly, the potential conflict of interest between the dual roles of clinician and investigator can be managed by carefully educating patients on how research differs from usual patient care [8, 11, 20, 21].
Research indicates that oncologists’ recommendations may indeed influence patients’ decision to participate in a clinical trial [5, 14, 15, 22]. However, existing empirical research to inform this debate is lacking. That is, descriptive data documenting oncologists’ practice of recommending clinical trials is limited to the frequency with which oncologists actively encourage patients to take part in trials or suggest participation as an acceptable option during the consent process [17, 23]. More detailed information is needed regarding the nature of oncologists’ recommendations and the extent to which oncologists’ recommendations are related to patient decisions to participate in the recommended trial.
The purpose of this study was, therefore, to provide evidence of oncologists’ practice by describing the frequency, context, and type of oncologists’ recommendations that occurred during outpatient interactions in which patients were presented with the opportunity to participate in a clinical trial. (Frequency refers to the proportion of interactions in which the oncologist explicitly recommended participation; context refers to whether the observed recommendations occurred in response to a direct request for a recommendation from a patient or their companion; and type refers to whether recommendations were individualized to the patient or general in nature.) Additionally, this study sought to extend current research by examining the relationship between observed oncologist recommendations and (a) patient self-reported decisions regarding participation in the trial and (b) patient socio-demographic characteristics.
2. Methods
2.1 Setting and participants
Data for this study were taken from an archive of oncologist-patient/companion interactions video recorded between April 2002 and March 2006 in multidisciplinary outpatient clinics at two comprehensive cancer centers. Interactions were video recorded as part of a larger study on strategic oncologist-patient/companion interactions and treatment decision-making [5, 24]. Approval by the Human Investigations Committee at each data collection site was obtained as part of the parent study. Participants for the larger study included patients (and companions, if present) who were: (a) >18 years old; (b) able to speak and read English; and (c) visiting an oncologist who was participating in the research. After signing consent and HIPAA release forms, participants completed a self-report questionnaire about their personal history and demographic characteristics. Oncologist-participant interactions were video recorded using a remote-controlled digital video recording system with two shielded cameras placed in the consult room. Digital processing technology allowed simultaneous recording of the physician, patient, and companions via a split-screen format on a single monitor [25]. Patients participated in follow-up telephone interviews approximately two weeks later in which they were asked about their perceptions of the interaction with the oncologist and their decision to participate in the clinical trial that had been offered. For the current study, interactions were included if (a) the oncologist offered the patient the opportunity to participate in a Phase II or III clinical trial during the discussion, and (b) patients provided information about their treatment decision during the follow-up interview. Video recorded interactions of 38 oncologist-patient visits from the parent archive met the inclusion criteria for this study.
2.2 Procedures
Trained coders, including two first-year medical students, two research assistants, and one author (SE), observed and analyzed video recorded interactions in two stages. In the first stage, coders used Observer Video Pro 5.0® software (designed for observing and analyzing video recorded data) to identify oncologist recommendations in favor of the clinical trial and note whether the recommendation was provided in response to a patient or companion’s direct request for a recommendation (e.g., “How do you feel about the study?”) Recommendations were defined as “explicit verbal statements encouraging participation in the trial under discussion” (e.g., “I personally feel that somebody like you should consider going on the study quite strongly. I don’t see a downside to it”).
The second stage consisted of transcribing and classifying each recommendation. The first author transcribed verbatim each recommendation along with a patient or companion request for a recommendation, if present. Two members of the coding team read all transcripts of recommendations by interaction and classified each interaction into one of two categories: (1) those that included at least one recommendation targeted at the individual patient (e.g., “This is a good trial for your situation”) or (2) those that included recommendations endorsing the specific trial being offered or clinical trials in general, but not specific to the individual patient (e.g., “I think this is a good trial and we will learn a lot from it”). Coders worked independently; disagreements between coders were resolved by a third coder.
3. Results
3.1 Sample Characteristics
Table 1 summarizes socio-demographic characteristics of patients who completed demographic questionnaires (n = 37).
Table 1.
Patient Characteristics
| Completed demographic questionnaire | n = 37 |
|---|---|
| Mean age (years) | 55.19,
SD=10.6 |
| Sex:
Male Female |
20 (54%) 17 (46%) |
| Race/ethnicity:
White, Non-Hispanic Black Other |
27 (73%) 6 (16%) 4 (11%) |
| Marital Status
Married Other |
23 (62%) 14 (38%) |
| Education:
High school or less Technical or trade school Some college or greater |
13 (35%) 5 (14%) 19 (51%) |
| Brought at least one companion to the visit
Came alone |
28 (76%)
9 (24%) |
Although information regarding the type of visit (e.g., new or return) and cancer type is not available, patients were treated by 15 male oncologists who specialized in the following areas: gastrointestinal (n = 14 patients); multiple myeloma (n = 4), chemoprevention following head and neck cancer treatment (n = 4), thoracic (n = 11), gynecology (n = 1); 1 breast (n = 3); and prostate (n = 1). Each of the 15 oncologists treated, on average, 2.3 patients, although one oncologist accounted for 23.7% (n = 9) of the visits. Number of visits for the remaining 14 oncologists ranged from 1 to 4 with an average of 2.07 patient visits per oncologist.
3.2 Frequency, context, and type oncologist recommendations
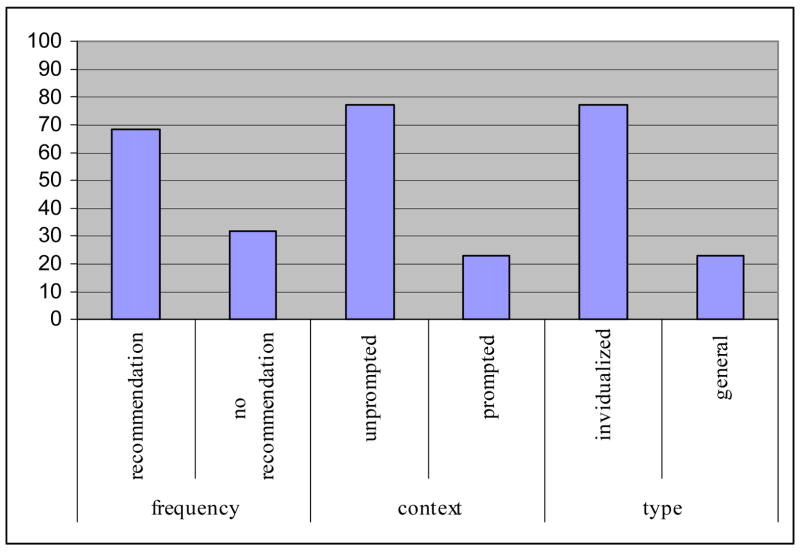
Figure 1 represents the frequency, context, and type of recommendations as defined above.
Figure 1.
Frequency (presence), context (prompted by patient/companion request) and type (individualized or general) of oncologist recommendations
Regarding frequency, 68 % (n = 26) of the 38 interactions were observed to include at least one explicit oncologist recommendation in favor of trial participation. Among the 15 oncologists, 73% (n = 11) made an explicit recommendation in at least one interaction. Regarding consistency of making recommendations, 60% (n = 9) of oncologists made recommendations to all their patients; 27% (n = 4) did not make recommendations to any of their patients, and 13% (n = 2) did not show a consistent pattern of recommendations to patients. (The oncologist accounting for 23.7% of the patient visits was one of the physicians who made recommendations to some patients and not to others).
Regarding context, among the 26 interactions with a recommendation, recommendations in 77% (n = 20) of the interactions were unprompted by a patient or companion request, while the remaining 23% (n = 6) included a recommendation that was directly preceded by a patient or companion request. Requests consisted of statements such as: “So my best path would be what?” or “If this were your sister or your wife, what would you do?”
Regarding type, 77% (n = 20) of the 26 interactions in which recommendations were made included at least one recommendation targeted specifically at the individual patient. Examples of individualized recommendations were: “The nice thing about the study that makes it perfect for you is that it has drugs that are active against all of the possible sites where your cancer may have come from,” or “I want you to go on the study because I think it’s giving you the chance, the opportunity to try a drug that is not yet available on the market, that may have benefits over and above chemotherapy.” Recommendations in the remaining interactions (n = 6, 23.1%) made reference only to patients in general (e.g., “I think this is a well-designed sort of exciting trial” and “But compared to the usual chemotherapy, I think this is a reasonable thing to try”).
3.3 Relationship between recommendations and decision to participate
Table 2 displays the relationship between oncologist recommendation and patient decisions regarding participation as reported in the telephone follow-up interview.
Table 2.
Oncologist recommendation and patient decision to participate in the trial
| Decided to participate
(n = 28) |
Decided not to participate or undecided
(n = 10) |
|
|---|---|---|
| Recommendation | 22 | 4 |
| No recommendation | 6 | 6 |
p = .045 (Fisher’s Exact Test)
A Fisher’s Exact Test showed a significant relationship (p = .045) between oncologists’ recommendations and patients’ decisions to participate. Among the 26 patients who received a recommendation, 85% (n = 22) decided to participate in the trial. In comparison, among the 12 patients who did not receive a recommendation, 50% (n = 6) decided to participate.
Table 3 shows the relationship between the context of oncologists’ recommendations (i.e., prompted or unprompted) and patients’ decision to participate. Similarly, Table 4 shows the relationship between the type of oncologist recommendation (i.e., individualized to the patient or general). Fisher’s Exact Tests showed no significant relationships between context or type of recommendation and patients’ decisions.
Table 3.
Prompted recommendation and patient decision to participate in the trial
| Decided to participate
(n =22) |
Decided not to participate or undecided
(n = 4) |
|
|---|---|---|
| Prompted Recommendation | 6 | 0 |
| Unprompted recommendation | 16 | 4 |
p = .54 (Fisher’s Exact Test)
Table 4.
Individualized recommendation and patient decision to participate in the trial
| Decided to participate
(n = 22) |
Decided not to participate or undecided
(n = 4) |
|
|---|---|---|
| Individualized | 17 | 3 |
| General | 5 | 1 |
p = 1.0 (Fisher’s Exact Test)
3.4 Relationship between recommendations and patient characteristics
Table 5 displays the relationship between oncologists’ recommendations and patients’ socio-demographic characteristics.
Table 5.
Oncologist recommendation and patient socio-demographic characteristics
| Recommendation
(n = 25) |
No recommendation
(n = 12) |
|
|---|---|---|
| Mean age (years) | 56.16, SD = 9.97 | 53.17, SD = 12.01 |
| Sex
Male Female |
11 14 |
9 3 |
| Race/Ethnicity
White, non-Hispanic Black Other |
19 5 1 |
8 2 2 |
| Marital Status
Married Other |
15 10 |
8 4 |
| Education
≤High school ≥More than high school |
6 19 |
7 5 |
| Companion in interaction
Came alone |
19
6 |
9
3 |
Fisher’s Exact Tests showed trend relationships between being offered a treatment recommendation and patient gender (p = .09) and between treatment recommendation and patient education (p = .07). A post-hoc analysis of the data showed that women and people with higher education received more recommendations than men and those with lower education, respectively. Among the 26 patients who received a recommendation, 56% were women and 44% men, and 24% had a high school education or less and 76% had more than a high school education. Interestingly, levels of education across men and women were similar (65% of men and 65% women reported education beyond high school). A Fisher’s Exact Test of the relationship between gender and education was not significant, suggesting that the influence of gender and education on treatment recommendations are independent effects. Fisher’s Exact Tests and a t-test showed no significant relationships between race, marital status, or age and receiving a treatment recommendation from the oncologist.
4. Discussion and conclusion
4.1. Discussion
Explicitly recommending participation in a clinical trial to an individual patient has been described by some authors as a controversial practice because of the potential to unduly influence, and thereby inhibit, patients’ ability to make an informed, autonomous decision [8, 26]. The purpose of this study was to contribute empirical data to inform the debate by describing the frequency, context, and type of oncologists’ recommendations during offers of clinical trials and analyzing the extent to which recommendations are related to both patients’ decisions to participate and to patients’ socio-demographic characteristics.
Results showed that oncologists made explicit recommendations to patients during most discussions in which clinical trials were offered. These results are consistent with previous research showing that oncologists routinely engage in the practice of making recommendations to patients as a part of clinical trial offers [15, 17, 22, 23]. For example, Jenkins et al. [23] analyzed the content of 82 audio-recorded interactions in which oncologists attempted to obtain consent for a randomized controlled trial from patients and found that 29% (n = 24) of patients were actively encouraged to take part in the trial. Similarly, Brown et al. investigated the extent to which 10 Australian oncologists adhered to a set of ethical strategies for obtaining informed consent (developed by the authors) in 59 consultations in which oncologists sought informed consent from patients eligible for a phase II or III clinical trial. Results showed that 44% of the oncologists explicitly stated that standard treatment would be acceptable, and 56% explicitly stated that the clinical trial would be an acceptable treatment option [17, 19]. Our findings and previous research suggest that many oncologists hold similar beliefs to those who argue that physician recommendations are based on preliminary evidence, training, and clinical experience, and that clinicians’ duty is not only to educate their patients about the experimental nature of clinical trials but also to offer their opinion as an expert [11, 18, 19, 26].
Further, our findings demonstrated that most recommendations were not directly prompted by patients’ (or companions’) requests for the oncologist’s opinion and that recommendations generally referred directly to the individual patient rather than to a population of patients. For example, one oncologist stated, “I think it’s a good idea for you to have further treatment and I think this trial is worthwhile participating in.” Another spoke for his colleagues in stating, “This morning we talked about your case and it was decided that we should recommend to you a treatment protocol that involves hormones or hormones plus chemotherapy.” Given their personalized nature, it is possible that individualized recommendations carry more persuasive force than general statements endorsing clinical trial participation.
Although our study did not specifically investigate the impact of individual versus general recommendations, results of the study did show a significant relationship between oncologists’ recommendations and patients’ decisions to participate in clinical trials. This finding is consistent with research on social influence, which indicates that people are likely to comply with requests of authority figures [27]. In fact, when patients in the current study were asked during a follow-up phone interview about factors that played a role in treatment decisions, 45% said that physician recommendations were a major factor, and another 35% said that physician recommendations were somewhat of a factor. This finding also supports previous studies demonstrating that physician behavior influences patient decisions regarding clinical trials [5, 6, 14, 15, 22]. Daugherty et al [15] found that many patients who participated in Phase I clinical trials were motivated by trust in their oncologist and in the institution. Similarly, Tu et al. [22] found “recommendation by a trusted oncologist” to be one of the most frequently cited factors in facilitating trial participation among Chinese-American female cancer patients. Given that findings from this study empirically demonstrate a pervasive practice of making recommendations, further research is needed to investigate whether there is a causal relationship between recommendations, and particularly individualized versus general recommendations, and decisions to participate.
Finally, our findings show a trend for oncologists to be more likely to make recommendations to women and to those with higher education. One caveat is that the trend toward a gender bias may have been an indirect effect of having more breast and gynecologic studies open for accrual during the period of our data collection. Nevertheless, increasing participation by cancer patients representative of the diverse American population has been identified as a national research goal, in light of evidence demonstrating the under-representation of specific populations in cancer clinical trials [4, 28–31]. This study was limited to a small and rather homogeneous sample, but even evidence of a trend toward recommending trials to some patient subgroups and not others warrants extending this research to a more diverse population.
4. 2 Conclusion
Although the sample size is somewhat small, this study offers the strength of two research methodologies (observational and self-report) to contribute empirical data to inform ethical and practical considerations of oncologists’ communication behavior during the presentation of clinical trials. Findings from this study demonstrate that, at least in the outpatient clinics of comprehensive cancer centers, oncologists frequently recommend participation as part of the process of presenting clinical trials to their patients, and that their recommendations are frequently tailored to individual patients. Results demonstrate that oncologists’ recommendations may indeed influence patients’ treatment decisions, and further, may occur more frequently with specific populations. Although practice may not drive policy, an understanding of the persuasive power of physicians heightens the need for careful and informed debate among ethicists, clinicians, medical scientists, and patients. Further research with larger and more diverse samples will likely permit analysis of recommendations in a variety of settings, by type and stage of cancer and by type of clinical trial in an effort to better understand both oncologists’ practice and the implications of recommending trials to patients.
4.3 Practice Implications
Despite their eagerness to improve cancer care through recruiting patients to clinical trials, oncologists must be cognizant of the potential influence of their recommendations on patient decisions to participate. This and future research on the communication practice of discussing clinical trials brings us closer to our goal of increasing the participation of patients in oncology clinical trials, while protecting patients by upholding the ethical informed consent process that is so critical to the participation of humans in research.
Acknowledgments
This research was supported by National Cancer Institute research grant (T. Albrecht, PI RO1CA075003-03).
The authors would like to thank the following individuals for their assistance in the development and reporting of this research: Melissa Hendriks, Michelle Figueroa, Louis A. Penner, Sonica Rehan, and Jatin Rana.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Society of Clinical Oncology policy statement: oversight of clinical research. J Clin Oncol. 2003:2377–86. doi: 10.1200/JCO.2003.04.026. [DOI] [PubMed] [Google Scholar]
- 2.Beauchamp TL, Childress JF. Principles of Biomedical Ethics. New York: Oxford University Press; 2001. [Google Scholar]
- 3.Albrecht TL, Franks MM, Ruckdeschel JC. Communication and informed consent. Curr Opin Oncol. 2005;17(4):336–9. doi: 10.1097/01.cco.0000166654.23169.a2. [DOI] [PubMed] [Google Scholar]
- 4.Tournoux C, Katsahian S, Chevret S, Levy V. Factors influencing inclusion of patients with malignancies in clinical trials. Cancer. 2006;106(2):258–70. doi: 10.1002/cncr.21613. [DOI] [PubMed] [Google Scholar]
- 5.Albrecht TL, Blanchard C, Ruckdeschel JC, Coovert M, Strongbow R. Strategic physician communication and oncology clinical trials. J Clin Oncol. 1999;17(10):3324–32. doi: 10.1200/JCO.1999.17.10.3324. [DOI] [PubMed] [Google Scholar]
- 6.Siminoff LA, Zhang A, Colabianchi N, Sturm CM, Shen Q. Factors that predict the referral of breast cancer patients onto clinical trials by their surgeons and medical oncologists. J Clin Oncol. 2000;18(6):1203–11. doi: 10.1200/JCO.2000.18.6.1203. [DOI] [PubMed] [Google Scholar]
- 7.Fried C. Medical Experimentation: Personal Integrity and Social Policy. Amsterdam: North Holland; 1974. [Google Scholar]
- 8.Miller FG, Brody H. A critique of clinical equipoise. Therapeutic misconception in the ethics of clinical trials. Hastings Cent Rep. 2003;33(3):19–28. [PubMed] [Google Scholar]
- 9.Djulbegovic B. Acknowledgment of uncertainty: a fundamental means to ensure scientific and ethical validity in clinical research. Curr Oncol Rep. 2001;3(5):389–95. doi: 10.1007/s11912-001-0024-5. [DOI] [PubMed] [Google Scholar]
- 10.Lidz CW, Appelbaum PS, Grisso T, Renaud M. Therapeutic misconception and the appreciation of risks in clinical trials. Soc Sci Med. 2004;58(9):1689–97. doi: 10.1016/S0277-9536(03)00338-1. [DOI] [PubMed] [Google Scholar]
- 11.Brody H, Miller FG. The clinician-investigator: unavoidable but manageable tension. Kennedy Inst Ethics J. 2003;13(4):329–46. doi: 10.1353/ken.2004.0003. [DOI] [PubMed] [Google Scholar]
- 12.Daugherty CK. Impact of therapeutic research on informed consent and the ethics of clinical trials: a medical oncology perspective. J Clin Oncol. 1999;17(5):1601–17. doi: 10.1200/JCO.1999.17.5.1601. [DOI] [PubMed] [Google Scholar]
- 13.Appelbaum PS, Roth LH, Lidz CW, Benson P, Winslade W. False hopes and best data: consent to research and the therapeutic misconception. Hastings Cent Rep. 1987;17(2):20–4. [PubMed] [Google Scholar]
- 14.Nurgat ZA, Craig W, Campbell NC, Bissett JD, Cassidy J, Nicolson MC. Patient motivations surrounding participation in phase I and phase II clinical trials of cancer chemotherapy. Br J Cancer. 2005;92(6):1001–5. doi: 10.1038/sj.bjc.6602423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daugherty C, Ratain MJ, Grochowski E, Stocking C, Kodish E, Mick R, et al. Perceptions of cancer patients and their physicians involved in phase I trials. J Clin Oncol. 1995;13(5):1062–72. doi: 10.1200/JCO.1995.13.5.1062. [DOI] [PubMed] [Google Scholar]
- 16.Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987;317(3):141–5. doi: 10.1056/NEJM198707163170304. [DOI] [PubMed] [Google Scholar]
- 17.Brown RF, Butow PN, Ellis P, Boyle F, Tattersall MH. Seeking informed consent to cancer clinical trials: describing current practice. Soc Sci Med. 2004;58(12):2445–57. doi: 10.1016/j.socscimed.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Lilford RJ. Ethics of clinical trials from a bayesian and decision analytic perspective: whose equipoise is it anyway? BMJ. 2003;326(7396):980–1. doi: 10.1136/bmj.326.7396.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown RF, Butow PN, Butt DG, Moore AR, Tattersall MH. Developing ethical strategies to assist oncologists in seeking informed consent to cancer clinical trials. Soc Sci Med. 2004;58(2):379–90. doi: 10.1016/s0277-9536(03)00204-1. [DOI] [PubMed] [Google Scholar]
- 20.Miller FG, Rosenstein DL, DeRenzo EG. Professional integrity in clinical research. JAMA. 1998;280(16):1449–54. doi: 10.1001/jama.280.16.1449. [DOI] [PubMed] [Google Scholar]
- 21.Yanos PT, Ziedonis DM. The patient-oriented clinician-researcher: advantages and challenges of being a double agent. Psychiatr Serv. 2006;57(2):249–53. doi: 10.1176/appi.ps.57.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.]Tu SP, Chen H, Chen A, Lim J, May S, Drescher C. Clinical trials: understanding and perceptions of female Chinese-American cancer patients. Cancer. 2005;104(12 Suppl):2999–3005. doi: 10.1002/cncr.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins VA, Fallowfield LJ, Souhami A, Sawtell M. How do doctors explain randomised clinical trials to their patients? Eur J Cancer. 1999;35(8):1187–93. doi: 10.1016/s0959-8049(99)00116-1. [DOI] [PubMed] [Google Scholar]
- 24.Albrecht TL, Penner LA, Ruckdeschel JC. Understanding patient decisions about clinical trials and the associated communication process: a preliminary report. J Cancer Educ. 2003;18(4):210–4. doi: 10.1207/s15430154jce1804_8. [DOI] [PubMed] [Google Scholar]
- 25.Albrecht TL, Ruckdeschel JC, Ray FL, Pethe BJ, Riddle DL, Strohm J, et al. A portable, unobtrusive device for video recording clinical interactions. Behav Res Methods Instrum Comp. 2005;37(1):165–169. doi: 10.3758/bf03206411. [DOI] [PubMed] [Google Scholar]
- 26.Chen DT, Miller FG, Rosenstein DL. Clinical research and the physician-patient relationship. Ann Intern Med. 2003;138(8):669–72. doi: 10.7326/0003-4819-138-8-200304150-00015. [DOI] [PubMed] [Google Scholar]
- 27.Cialdini RB. Interpersonal Influence. In: Shavitt S, Brock T, editors. Persuasion. Boston: Allyn & Bacon; 1994. pp. 195–218. [Google Scholar]
- 28.Ford JG, Howerton MW, Bolen S, Gary TL, Lai GY, Tilburt J, et al. Knowledge and access to information on recruitment of underrepresented populations to cancer clinical trials. Evid Rep Technol Assess (Summ) 2005;(122):1–11. doi: 10.1037/e439572005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman LA, Hurd T, Leitch M, Kuerer HM, Diehl K, Lucci A, et al. A report on accrual rates for elderly and minority-ethnicity cancer patients to clinical trials of the American College of Surgeons Oncology Group. J Am Coll Surg. 2004;199(4):644–51. doi: 10.1016/j.jamcollsurg.2004.05.282. [DOI] [PubMed] [Google Scholar]
- 30.Lai GY, Gary TL, Tilburt J, Bolen S, Baffi C, Wilson RF, et al. Effectiveness of strategies to recruit underrepresented populations into cancer clinical trials. Clin Trials. 2006;3(2):133–41. doi: 10.1191/1740774506cn143oa. [DOI] [PubMed] [Google Scholar]
- 31.Du W, Gadgeel SM, Simon MS. Predictors of enrollment in lung cancer clinical trials. Cancer. 2005 doi: 10.1002/cncr.21638. [DOI] [PubMed] [Google Scholar]