Abstract
Ataxia telangiectasia mutated (ATM) is a phosphatidyl-3-kinase-related protein kinase that functions as a central regulator of the DNA damage response in eukaryotic cells. In humans, mutations in ATM cause the devastating neurodegenerative disease ataxia telangiectasia. Previously, we characterized the homolog of ATM (AtmA) in the filamentous fungus Aspergillus nidulans. In addition to its expected role in the DNA damage response, we found that AtmA is also required for polarized hyphal growth. Here, we extended these studies by investigating which components of the DNA damage response pathway are interacting with AtmA. The AtmAATM loss of function caused synthetic lethality when combined with mutation in UvsBATR. Our results suggest that AtmA and UvsB are interacting and they are probably partially redundant in terms of DNA damage sensing and/or repairing and polar growth. We identified and inactivated A. nidulans chkACHK1 and chkBCHK2 genes. These genes are also redundantly involved in A. nidulans DNA damage response. We constructed several combinations of double mutants for ΔatmA, ΔuvsB, ΔchkA, and ΔchkB. We observed a complex genetic relationship with these mutations during the DNA replication checkpoint and DNA damage response. Finally, we observed epistatic and synergistic interactions between AtmA, and bimEAPC1, ankAWEE1 and the cdc2-related kinase npkA, at S-phase checkpoint and in response to DNA-damaging agents.
ATAXIA telangiectasia mutated/ataxia telangiectasia and Rad3 related (ATM/ATR) are members of the family of phosphoinositide 3-kinase-related kinases (PIKKs) and key regulators of the DNA damage response (Caspari and Carr 1999; Shiloh and Kastan 2001; Shiloh 2006; Hurley and Bunz 2007). The ATM and ATR protein kinases share many biochemical and functional similarities. Both activate signal transduction pathways that ultimately interface with the Cdk/cyclin machinery (Abraham 2001; Shiloh 2006). They trigger responses that promote the maintenance of genome integrity by phosphorylating multiple target proteins, such as p53, c-Abl, Chk2, and Brca1 (Baskaran et al. 1997; Lavin and Shiloh 1997; Shafman et al. 1997; Banin et al. 1998; Canman and Lim 1998; Cortez et al. 2001; McKinnon 2004). In mammalian cells, ATM responds to the presence of double-strand breaks (DSBs). ATR also responds to DSBs, but more slowly than ATM (Shiloh 2006). In addition, the ATR pathway can respond to agents that interfere with the function of replication forks, such as hydroxyurea (HU), ultraviolet (UV) light, and DNA-alkylating agents such as methyl methanesulfonate (MMS) (Nyberg et al. 2002; Osborn et al. 2002).
Mutations in the ATM (ataxia telangiectasia mutated) gene give rise to ataxia telangiectasia (AT) (Savitsky et al. 1995; Shiloh 2006). This autosomal recessive disease is characterized by progressive cerebellar ataxia, immunodeficiency, premature aging, gonadal dysgenesis, extreme radiosensitivity, and susceptibility to infections and tumors (McKinnon 2004). Studies have shown that ATM is present predominantly within the nucleus of cultured human cells with a small fraction present in the cytoplasm (Lakin et al. 1996; Brown et al. 1997; Watters et al. 1997). In response to DSBs, ATM is autophosphorylated at Ser-1981, which leads to the dissociation of inactive multimeric ATM to initiate ATM signaling (Bakkenist and Kastan 2003). ATM works in concert with other factors, among them the MRE11, RAD50, and NBS1 (MRN) complex (for a review, see Van Den Bosch et al. 2003). Activated ATM can directly associate with the MRN complex, and this interaction can control signaling by influencing the ATM substrate choice (Lee and Paull 2004; Paull and Lee 2005). Besides its nuclear role in DNA damage surveillance, in the cytoplasm ATM localizes to vesicles and interacts with β-adaptin, one of the components of the AP-2 adaptor complex, which is involved in clathrin-mediated endocytosis of receptors (Lim et al. 1998).
The filamentous fungus Aspergillus nidulans possesses a sophisticated DNA damage response that ensures the maintenance of genome integrity (Goldman et al. 2002; Goldman and Kafer 2004). The ATR homolog UvsBATR is a central component of this response, where it controls the activation of multiple checkpoints, regulates damage-induced gene expression, and also promotes DNA repair (De Souza et al. 1999; Hofmann and Harris 2000). UvsBATR displays an extensive web of genetic interactions with other proteins involved in the DNA damage response, including the Mre11 complex (ScaANBS1, MreAMRE11, and SldIRAD50), the cdc2-related kinase NpkA, and SepBCTF4 (Fagundes et al. 2004, 2005; Gygax et al. 2005; Malavazi et al. 2005). Recently, we characterized the homolog of ATM (AtmA) in A. nidulans (Malavazi et al. 2006, 2007). Here, we investigate genetic interactions of the A. nidulans atmAATM homolog with different components of the DNA damage response pathway. We demonstrated that the atmAATM loss of function causes synthetic lethality when combined with mutation in uvsBATR. Furthermore, we identified the A. nidulans CHK1 and CHK2 homologs and showed that these genes are redundantly involved in the DNA damage response. In addition, we characterized genetic interactions between atmA and bimEAPC1, ankAWEE1, and the cdc2-related kinase npkA, at S-phase checkpoint and in response to DNA-damaging agents.
MATERIALS AND METHODS
Strains and media methods:
A. nidulans strains used in this work are described in Table 1. The media used were of two basic types, i.e., complete and minimal. The complete media comprised the following three variants: 2% glucose, 0.5% yeast extract, 2% agar, and trace elements (YAG); YAG supplemented with 1.2 g/liter (each) of uracil and uridine (YUU); and liquid YG or YG + UU medium with the same composition (but without agar). The minimal media were a modified minimal medium (MM) of 1% glucose, original high-nitrate salts, trace elements, and 2% agar, pH 6.5. Expression of atmA under the control of the alcA promoter was regulated by repression on glucose (4%), derepression on glycerol (2%), and induction on threonine (100 mm) or ethanol (2%). Therefore, MM-G was identical to MM, except that glycerol and ethanol (2%) or threonine (100 mm) were used, instead of glucose as the sole carbon source. Trace elements, vitamins, and nitrate salts were included as described by Kafer (1977). Standard genetic techniques for A. nidulans were used for all strain constructions (Kafer 1977).
TABLE 1.
A. nidulans strains used in this work
| Strains | Genotypes | References |
|---|---|---|
| A4 | Glasgow wild type (veA+) | FGSCa |
| A776 | pabaA1; acrA1; bimE7; riboB2 chaA1 | FGSC |
| ASH270 | pyrG89; pabaA1; yA2; uvsB110 | Hoffman and Harris (2000) |
| AAH14 | pyrG89; pabaA1; yA2; argB; ΔuvsB∷argB | Hoffman and Harris (2000) |
| APK35 | chaA1; pabaA1; ankA | Kraus and Harris (2001) |
| GR5 | pyroA4 pyrG89, wA3 | FGSC A773 |
| IM25 | pyrG89; yA2; argB2; sldI∷pyrG | Malavazi et al. (2005) |
| IM69 | pyroA4 pyrG89, wA3; ΔatmA∷pyrG | Malavazi et al. (2006) |
| IM69-1 | pyroA4 pyrG89, wA3; alcA∷gfp∷atmA∷pyr4 | This work |
| IM69-228 | pyrG89; wA3; bimE7; ΔatmA∷pyrG | This work |
| IM69-25 | pyrG89 yA2; sldI∷pyrG; ΔatmA | This work |
| IM69-270.1 | wA3; alcA∷gfp∷atmA∷pyr4; uvsB101 | This work |
| IM69-3 | pyrG89 yA2; ΔatmA∷pyrG; ΔnpkA∷argB | This work |
| IM69-RE | wA3; mreA∷pyr4; ΔatmA | This work |
| JFL100 | pyroA4 pyrG89 yA2; argB2; ΔchkA∷pyrG; ΔchkB∷pyro | This work |
| JFL3 | pyroA4 pyrG89; chaA1 argB2; ΔchkA∷pyrG | This work |
| JFL3-14 | pyrG89 yA2; argB2; ΔchkA∷pyrG; ΔuvsB∷argB | This work |
| JFL3-69 | pyroA4 pyrG89; chaA1; ΔchkA∷pyrG; ΔatmA | This work |
| JFL4 | pyroA4 pyrG89; wA3; ΔchkB∷pyro | This work |
| JFL4-14 | pyroA4 pyrG89; yA2; argB2; ΔchkB∷pyrG; ΔuvsB∷argB | This work |
| JFL4-69 | pyroA4 pyrG89; wA3; ΔchkB∷pyro; ΔatmA∷pyrG | This work |
| MV3 | pyrG89 yA2; argB2; ΔnpkA∷argB | Fagundes et al. (2004) |
| TMRE | pyrG89 yA2; argB2; mreA∷pyr4 | Semighini et al. (2002) |
| TNO2A3 | pyroA4 pyrG89; chaA1; ΔnKuA∷argB | Nayak et al. (2006) |
| UI224 | pyrG89 yA2; argB2 | FGSC |
Fungal Genetics Stock Center (http://www.fgsc.net).
For the UV light viability assays, conidiospores (dormant in a quiescent G1 state; Bergen and Morris 1983) were suspended in 0.2% Tween-20 and plated out on YUU plates (∼100 conidia/plate). The plates were then immediately irradiated with UV light using a UV Stratalinker 1800 (Stratagene, La Jolla, CA) and incubated at 30° for 48 hr to determine UV light sensitivity of nondividing cells. To determine UV light survival of dividing cells, conidiospores on YUU plates were first allowed to germinate for 4.5 hr at 30°. By this time the germinated conidia had entered the cell cycle and were about to undergo the first mitosis. These germlings were UV irradiated on the plates and then similarly incubated at 30° for 48 hr. Viability was determined as the percentage of colonies on treated plates compared to untreated controls.
To establish the polarization kinetics, conidia were incubated in YG + 50 mm HU for 5 hr at 37°. Germlings were released from cell-cycle arrest by three sequential washes in prewarmed YG, followed by transfer to a new petri dish containing fresh prewarmed YG. Following release, samples were taken at 30-min intervals over the next 150 min. A conidium was counted as polarized if it possessed a germ tube readily detectable as small protuberances on the spherical shape of conidium surface. For the septation experiments, asexual spores were inoculated on coverslips in the presence or the absence of DNA-damaging agent 4-nitroquinoline 1-oxide (4-NQO). After 8–12 hr incubation at 30°, the percentage of germlings possessing septum was scored.
To evaluate the statistical differences among the strains used for growth in the presence of DNA-damaging agents, mitosis, and UV light assays, we used one-way ANOVA and the Student–Newman–Keuls multicomparison test as a post hoc test. The data shown are the average of three independent experiments and means ± standard deviations are shown in the tables and figures. Analyses were performed using the software package Sigma Stat 3.1 (Systat) and the statistical significance was set at α = 0.05. In the epistasis grouping, P-values >0.05 and <0.05 were used to discriminate between epistatic and synergistic interactions, respectively.
Mitosis assay:
Conidiospores were inoculated on coverslips in YG + UU medium containing 0, 6, or 100 mm of HU. After 5–7 hr incubation at 37°, coverslips with adherent germlings were transferred to fixative solution and stained with 4′,6-diamidino-2-phenyylindole (DAPI) as described below. The number of nuclei was assessed and germlings that had two or more nuclei after the HU incubation were scored as having a nonfunctional checkpoint response in comparison to the wild-type strain.
Staining and microscopy:
For germling nuclear and septum staining, conidia were inoculated on coverslips. After incubation at the appropriate conditions for each experiment, coverslips with adherent germlings were transferred to fixative solution (3.7% formaldehyde, 50 mm sodium phosphate buffer pH 7.0, 0.2% Triton X-100) for 30 min at room temperature. Then, they were briefly rinsed with PBS buffer (140 mm NaCl, 2 mm KCl, 10 mm NaHPO4, 1.8 mm KH2PO4, pH 7.4) and incubated for 5 min in a solution with 100 ng/ml of DAPI (Sigma Chemical, St. Louis) and/or 100 ng/ml of calcofluor (fluorescent brightener, Sigma Chemical). After incubation with the dyes, they were washed with PBS buffer for 10 min at room temperature and then rinsed in distilled water, mounted, and visualized in an epifluorescence microscope. Slides were viewed using a Carl Zeiss (Oberkochen, Germany) microscope, using a 100× magnification oil immersion objective lens (EC Plan-Neofluar, NA 1.3) equipped with a 100-W HBO mercury lamp epifluorescence module. Phase contrast for the brightfield images and fluorescent images was captured with a AxioCan camera (Carl Zeiss), processed using the AxioVision software version 3.1, and saved as tiff files. Further processing was performed using Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA). The living cells were visualized at room temperature in a confocal microscope. For cell imaging of atmAATM protein fused to GFP, conidiospores were grown in glass-bottom dishes (Mattek, Ashland, MA) in 2 ml of MM-G and/or ethanol plus supplements for 15 hr at 30°. Germlings were fixed for 10 min in a fixative solution containing 1× PBS, 5% DMSO, 3.7% formaldehyde, and 10% methanol. The nucleus was DAPI stained as described above. Images were analyzed using the Leica TCS SP5 spectral laser scanning confocal microscope (Leica Microsystems, Heidelberg, Germany) (Laboratory of Confocal Microscopy, FMRP, University of São Paulo, São Paulo, Brazil), using a 63× magnification water immersion objective lens, using laser line 488 nm for GFP and 405 nm for DAPI. Images were captured by direct acquisition with the Z step ranging from 0.5 to 2 μm with the Leica LAS AF software (Leica Microsystems) and additional processing was carried out using Adobe Photoshop 7.0 (Adobe Systems).
RNA isolation:
A total of 1.0 × 107 conidia/ml were used to inoculate 50 ml of liquid cultures that were incubated in a reciprocal shaker at 37° for 16 hr. When necessary, mycelia were aseptically transferred to fresh YG medium in the presence or the absence of DNA-damaging agent indicated for each experiment. RNA extraction, RNAse free DNAse treatment, and real-time RT–PCR experiments were performed as previously described (Semighini et al. 2002). Table 2 describes the primers and Lux fluorescent probes (Invitrogen, San Diego) used in this work.
TABLE 2.
Primers and probes used in this work
| Name | Sequence |
|---|---|
| CHK1_1 | 5′-TAATGCAGAAGGACAACCG-3′ |
| CHK1_2 | 5′-CTCAGACAGAATGTTCTTCATTCTTCTTGGGC-3′ |
| CHK1_3 | 5′-TGAGGCGAATTCCACCTGATTCTTGATCTTCC-3′ |
| CHK1_4 | 5′-TGGTATGCATCTTCTGCC-3′ |
| CHK1PYRAF | 5′-AGAATGAAGAACATTCTGTTCGAGAGGAGGC-3′ |
| CHK1PYRAF | 5′-AAGAATCAGGTGGAATTCGCCTCAAACAATGC-3′ |
| CHK1_An672RL | 5′-CGGAAGCTATCCCAAGGTGTATTTC[FAM]G-3′ |
| CHK1_672RL/65FU | 5′-AGACATCTGGTCGTGTGGCATT-3′ |
| CHK2_1 | 5′-CGACATATTTTGCGCTCCA-3′ |
| CHK2_2 | 5′-GTAATCCAGCATCTGATGTCCTCCTTTCGTAAATTGCCCGT-3′ |
| CHK2_3 | 5′-GAATTCTGAAAAGCTGATTGTGATTGAAGATCAGGGCCCATTC-3′ |
| CHK2_4 | 5′-TGGCTGTACATCTTGGGAAA-3′ |
| CHK2PYRO-5′ | 5′-ACGGGCAATTTACGAAAGGAGGACATCAGATGCTGGATTAC-3′ |
| CHK2PYRO-3′ | 5′-GAATGGGCCCTGATCTTCAATCACAATCAGCTTTTCAGAATTC-3′ |
| An_CHK2_820RL | 5′-CGGCCAAGACTCGATACTGCTGC[FAM]G-3′ |
| An_CHK2_820RL768FU | 5′-TGACGAGGTGACCGTATTGGAC-3′ |
DNA manipulations, chkA and chkB deletion strains isolation, and construction of an alcA∷gfp∷atmA strain:
These are described in supplemental Materials and Methods at http://www.genetics.org/supplemental/.
RESULTS
The atmAATM loss of function causes synthetic lethality when combined with mutation in uvsBATR:
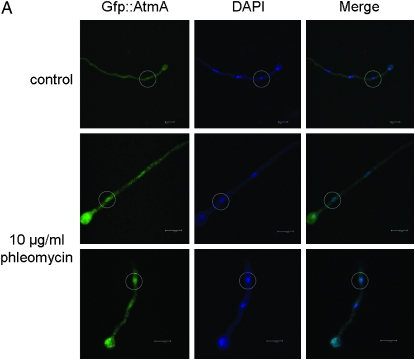
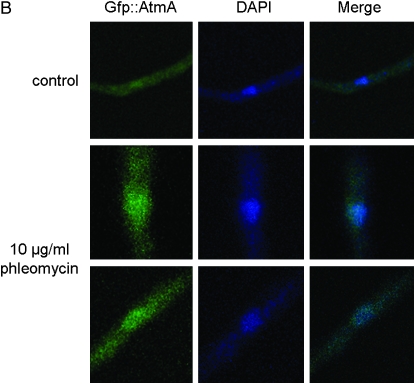
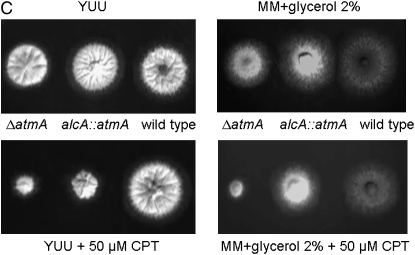
To investigate possible interactions between the atmAATM and uvsBATR genes, we tried to construct double mutants either by crossing single mutants or by deleting both genes by DNA-mediated transformation. After several trials, we were unable to observe any segregant or transformant carrying both mutations. These results suggested that the loss of function of both genes causes synthetic lethality. Thus, we decided to construct a conditional double mutant with alcA∷gfp∷atmA uvsB101. As first step, we validate the alcA∷atmA construction in the wild-type strain (Figure 1). The alcA promoter is induced to high levels by glycerol, ethanol, and l-threonine and repressed by glucose (Flipphi et al. 2002). The construction (p)alcA∷gfp∷atmA1kb was transformed into the wild-type strain and several transformants were obtained in which the plasmid had integrated homologously (as verified by PCR) and produced a functional Gfp∷AtmA when derepressed with glycerol (Figure 1A). Figure 1, A and B, shows wild-type hyphae that were grown in the presence of glycerol and exposed for 2 hr to 10 μg/ml of phleomycin (PHLEO). The Gfp∷AtmA was detected along the hyphae and barely detected in the nuclei of untreated wild-type hyphae; however, it was clearly and most abundantly present within nuclei of hyphae exposed to PHLEO (Figure 1, A and B). When the wild-type strain harboring the alcA∷gfp∷atmA construct was grown in the presence of either glucose or glycerol as single carbon sources and 50 μm of camptothecin (CPT), this strain was sensitive and resistant to CPT, respectively (Figure 1C; compare with the growth and inhibition of the wild-type and ΔatmA mutant strains in the presence of CPT in both carbon sources). When grown on glucose as a single carbon source, the alcA∷atmA strain behaves identically to the ΔatmA strain in terms of drug sensitivity (data not shown). We have observed the same results for two independent transformants obtained for each treatment (data not shown). These results validated our alcA∷atmA construction and showed that apparently AtmA has increased localization to nuclei in response to DNA damage (i.e., DSBs).
Figure 1.—
AtmA localizes to nuclei in response to DNA damage. (A) Conidiospores from the alcA∷gfp∷atmA strain were grown in MM-G and ethanol plus supplements for 15 hr at 30°. In one of the treatments, 10 μg/ml phleomycin was added for 2 hr at 30°. Bar, 5 μm. (B) Higher magnification of the nuclei surrounded by a dashed white circle in A. (C) Growth phenotypes of the wild-type, ΔatmA, and alcA∷gfp∷atmA strains grown in YUU (4% glucose) or in MM + G or in both media in the presence of 50 μm CPT.
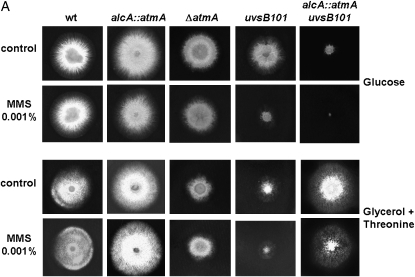
As next step, the ASH270 (uvsB101) strain was crossed with IM69-1 [(p)alcA∷gfp∷AtmA] and we have identified several segregants [(p)alcA∷gfp∷AtmA uvsB101] that produced Gfp∷AtmA when induced with glycerol (data not shown). We have grown the wild-type, alcA∷gfp∷atmA, ΔatmA, uvsB101, and one of these segregant alcA∷gfp∷atmA uvsB101 mutant strains in the presence of glucose, glycerol, or glycerol plus threonine as single carbon sources. As predicted, the combination of atmA mRNA repression with the mutation uvsB101 caused synthetic lethality (Figure 2A, first lane). The same results were observed when a double mutant alcA∷gfp∷atmA ΔuvsBATR was constructed (data not shown). These strains were also incubated in the presence of different concentrations or dosages of several DNA-damaging agents (such as CPT, bleomycin (BLEO), 4-NQO, MMS, and UV light) and the atmA gene was either repressed or overexpressed. As expected, when the atmA gene is repressed, the double-inactivation mutant is highly sensitive to these DNA-damaging agents. However, interestingly, when atmA is overexpressed, it confers resistance to 0.001% MMS (Figure 2A). An increase in survival was also observed when the double mutant was grown in the presence of different MMS concentrations (supplemental Figure S3 at http://www.genetics.org/supplemental/). This effect was not observed for the other DNA-damaging agents tested (data not shown).
Figure 2.—
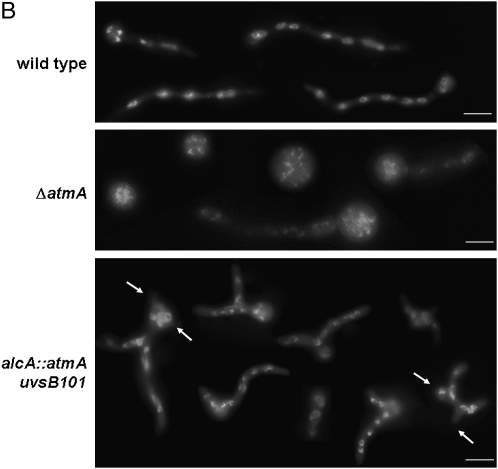
(A) Growth phenotypes of the wild-type, alcA∷gfp∷atmA, ΔatmA, uvsB101, and alcA∷gfp∷atmA uvsB101 mutant strains grown in the presence of either YUU (4% glucose) or MM + 2% glycerol + 100 mm threonine with or without 0.001% MMS. (B) Conidia from the same strains from A were grown for 8 hr at 37° in YUU + 4% glucose and DAPI stained. Arrows indicate multiple polar axes. Bar, 10 μm.
We compared the nuclear division kinetics (number of nuclei per cell compartment) and the polar growth of the wild-type, alcA∷gfp∷atmA, uvsB101, and ΔatmA mutant strains (Figure 2B). As previously shown by Malavazi et al. (2006, 2007), the ΔatmA mutant strain has an accelerated rate of proliferation, i.e., increased nuclear kinetics, when compared to the wild-type strain (Figure 2B, first and second lanes). The double mutant showed an increased number of nuclei that was comparable to the ΔatmA mutant strain (Figure 2B, third lane). Interestingly, the double-mutant strain displayed germlings with more polarity axes than the wild-type and the ΔatmA mutant strains (Figure 2B, third lane). Taken together, these results suggest that atmA and uvsB are interacting and they are probably partially redundant in terms of DNA damage sensing and/or repairing and polar growth. Interestingly, the data also suggest that uvsB101 may be partially suppressing the ΔatmA polarity defects (see Figure 2B) and that, at least with respect to polarity defects, the two kinases are not merely redundant.
Genetic interactions of AtmAATM, UvsBATR, and ChkACHK1 and ChkBCHK2:
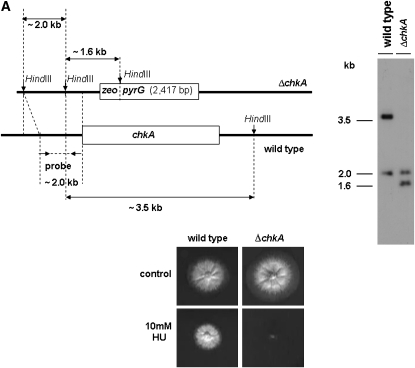
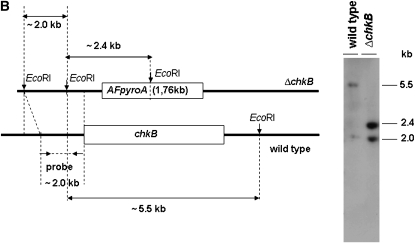
Chk1 and Chk2 are serine/threonine kinases that are required for cell-cycle arrest in response to DNA damage. As downstream kinases, they are phosphorylated by ATM/ATR-dependent processes and may additionally undergo autophosphorylation that potentiates phosphorylation of downstream targets (Nyberg et al. 2002). A BLASTp search of the A. nidulans genome database (http://www.broad.mit.edu/annotation/fungi/aspergillus/) using human CHK1 and CHK2 as queries revealed two single open reading frames with significant similarity. The potential homologs, AN5494.3 (chkA) and AN4279.3 (chkB), are predicted 534- and 648-amino-acid proteins that possessed homology to the Homo sapiens CHK1 and CHK2 (e-value 7e-46 and 2e-63; 47 and 55% similarity and 30 and 36% identity), respectively. A. nidulans ChkA and ChkB also showed high identity with its Neurospora crassa homologs NCU08346.1 (e-value 2e-155; 63% similarity and 49% identity) and pdr-4, recently shown as a regulatory link between the circadian and cell cycles (e-value 0.0; 67% similarity and 52% identity; Pregueiro et al. 2006). The serine/threonine active-site signatures for ChkA and ChkB are located at residues 395–407 and 139–151 for ChkA and ChkB, respectively. There is a single forkhead-associated (FHA) domain in the ChkB located at residues 186–238. The FHA domain is a putative nuclear signaling domain found in a variety of otherwise unrelated proteins; this domain comprises ∼55–75 amino acids and contains three highly conserved blocks separated by divergent spacer regions (PROSITE entry PS50006, www.expasy.org). To characterize the function of ChkA and ChkB, ORF deletions were generated using a fusion PCR-based approach (see materials and methods). Protoplasts of A. nidulans strain TNO2A3 were transformed using the fusion PCR products, and several transformants were obtained by their ability to grow in YAG. Allelic replacement of chkA and chkB was verified in several transformants as confirmed by the analysis of Southern blots (data not shown). One of these transformants for each gene was crossed with the wild-type UI224 strain to eliminate the ΔnkuA mutation; accordingly the deletion strains are wild type for these loci. These allelic replacements of chkA and chkB were also confirmed by the analysis of Southern blots (Figure 3), thereby generating the ΔchkA and ΔchkB strains named JFL3 and JFL4, respectively.
Figure 3.—
Schematic illustration for the chkA/chkB deletion strategy. (A) Genomic DNA from both wild-type and ΔchkA strains was isolated and cleaved with the enzyme HindIII; a 2.0-kb 5′-upstream chkA DNA fragment was used as a hybridization probe. This fragment recognizes two DNA bands (∼3.5 and 2.0 kb) in the wild-type strain and two DNA bands (∼1.6 and 2.0 kb) in the ΔchkA mutant strain as shown in the Southern blot analysis. Growth phenotypes of the wild-type and chkA deletion mutant strains grown either in YUU or in YUU + 10 mm HU for 48 hr at 37° are shown. (B) Genomic DNAs from both wild-type and ΔchkB strains were isolated and cleaved with the enzyme EcoRI; a 2.0-kb 5′-upstream chkB DNA fragment was used as a hybridization probe. This fragment recognizes two DNA bands (∼2.0 and 5.5 kb) in the wild-type strain and two DNA bands (∼2.0 and 2.4 kb) in the ΔchkB mutant strain as shown in the Southern blot analysis.
Two approaches were used as initial tests of ChkA and ChkB function in the DNA damage response: we examined (i) the growth of the ΔchkA and ΔchkB strains in the presence of DNA-damaging agents and (ii) how the mRNA expression of these genes is affected by these agents by using real-time RT–PCR. In the first test, the ΔchkA strain was more sensitive only to HU while the ΔchkB strain showed a comparable sensitivity to the wild-type strain in 4-NQO, BLEO, CPT, HU, and MMS (Figure 3A and data not shown). All these phenotypes were identical in media either supplemented with UU or not.
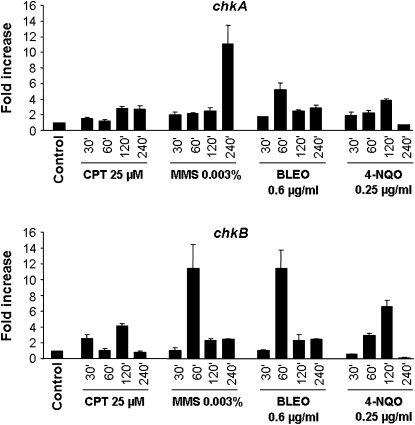
To accomplish the second test, A. nidulans wild type was grown in the absence of any drug and transferred to a specific concentration of a DNA-damaging agent for 30, 60, 120, and 240 min, and then RNA was isolated and analyzed for the expression of these genes (Figure 4). The chkA gene expression was increased 2–3 times after 30–240 min growth in the presence of CPT, ∼11 times after 240 min growth in the presence of MMS, 2–5 times after 30–240 min of growth in the presence of BLEO, and 2–4 times after 30–120 min growth in the presence of 4-NQO. The chkB gene expression was increased 3 and 4 times after 30 and 120 min growth in the presence of CPT; ∼11 and 2 times after 60, 120, and 240 min growth in the presence of MMS, respectively; 13 and 2 times after 60–120 min of growth in the presence of BLEO; and 3–6 times after 60 and 120 min growth in the presence of 4-NQO. Thus, these results indicate that chkA and chkB genes transcriptionally respond to DNA-damaging agents.
Figure 4.—
Fold increase in chkA/chkB RNA levels in response to DNA-damaging agents. Mycelia were grown either in the absence of any drug or in the presence of 25 μm CPT, 0.003% MMS, 0.6 μg/ml BLEO, or 0.25 μg/ml 4-NQO for 30, 60, 120, and 240 min. Real-time RT–PCR was the method used to quantify the mRNA. The measured quantity of the chkA/chkB mRNA in each of the treated samples was normalized using the CT values obtained for the tubC RNA amplifications run in the same plate. The relative quantitation of chkA/chkB and tubulin gene expression was determined by a standard curve (i.e., CT values plotted against the logarithm of the DNA copy number). Results of four sets of experiments were combined for each determination; means ± standard deviations are shown. The values represent the number of times the genes are expressed compared to the wild-type control grown without any drug (represented absolutely as 1.00).
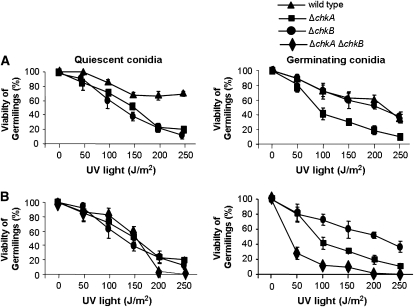
We also constructed a double-mutant ΔchkA ΔchkB and investigated its sensitivity to DNA-damaging agents. We have observed that both ΔchkA and ΔchkB mutants have increased sensitivity to UV light as quiescent conidia (Figure 5A, left; P ≤ 0.03). However, only the ΔchkA mutant strain has increased sensitivity to UV light as germinating conidia (Figure 5A, right; P < 0.001). Accordingly, the double-mutant ΔchkA ΔchkB showed comparable sensitivity to UV light as quiescent conidia, suggesting that ΔchkA was epistatic to ΔchkB (Figure 5B, left). Interestingly, the double-mutant ΔchkA ΔchkB was more sensitive to UV light as germinating conidia than the ΔchkA and ΔchkB mutant strains (P ≤ 0.02). The double-mutant ΔchkA ΔchkB showed the same sensitivity to 4-NQO, MMS, and CPT as the wild-type strain (data not shown) and it was as sensitive to HU as was ΔchkA (Figure 6A).
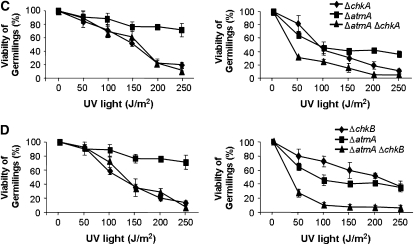
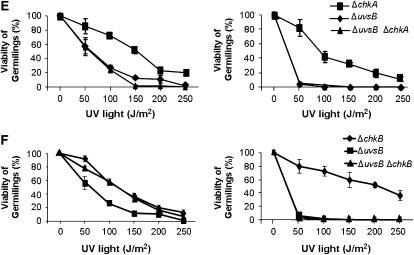
Figure 5.—
The double-mutant ΔchkA ΔchkB showed increased sensitivity to UV light. Viability of the wild-type, ΔchkA, ΔchkB (A), ΔchkA ΔchkB (B), ΔatmA ΔchkA (C), ΔatmA ΔchkB (D), ΔuvsB ΔchkA (E), and ΔuvsB ΔchkB (F) germlings was scored after exposure to UV light of quiescent and germinating conidiospores. Viability was determined as the percentage of colonies on treated plates compared to untreated controls. The results were expressed by the average of three independent experiments and means ± standard deviations are shown. The ΔchkA and ΔchkA ΔchkB strains were significantly different from the wild-type and ΔchkB strains in germinating conidiospores, P < 0.02.
Figure 6.—
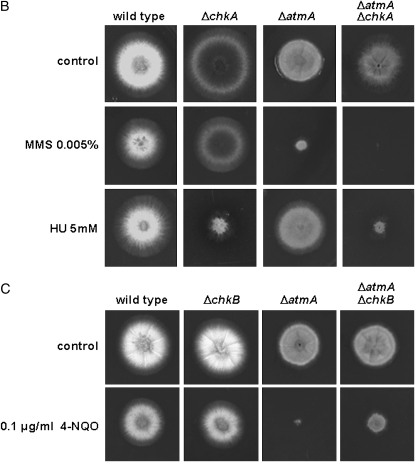
Growth phenotypes of the wild type, ΔchkA, ΔchkB, and ΔchkA ΔchkB mutant strains grown in YUU with or without 2.5 and 5.0 mm HU (A); of ΔchkA, ΔatmA, and ΔatmA ΔchkA grown in YUU with or without 0.005% MMS and 5.0 mm HU (B); and of ΔchkB, ΔatmA, and ΔatmA ΔchkB grown in YUU with or without 0.1 μg/ml 4-NQO (C).
We were not able to observe any defect in the sexual cycle in both A. nidulans chkA and chkB inactivation strains (data not shown). However, the mRNA accumulation of both genes was increased during the sexual cycle (supplemental Figure S2 at http://www.genetics.org/supplemental/).
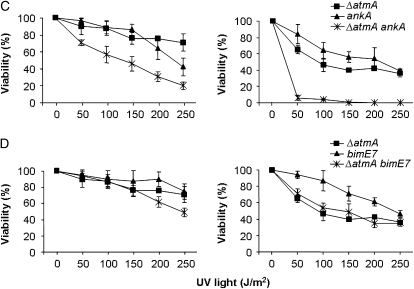
We also constructed double mutants ΔatmAATM with ΔchkA and ΔchkB and ΔuvsBATR with ΔchkA and ΔchkB. The double-mutant ΔatmAATM ΔchkA showed an increased sensitivity to MMS and HU (Figure 6B). The ΔchkB mutation partially suppressed the ΔatmAATM sensitivity to 0.1 μg/ml of 4-NQO (Figure 6C). The ΔatmA and ΔchkA mutations are in epistasis during exposure to UV light of quiescent conidia of single and double mutants at 50–150 J/m2 (Figure 5C, left, P > 0.05). The ΔatmA and ΔchkA mutations are synergistic during UV light exposure of germinating single and double mutants (Figure 5C, right, P < 0.05). The ΔatmA and ΔchkB mutations are not interacting when quiescent germlings are exposed to UV light (Figure 5D, left); however, there is a synergistic interaction when ΔatmA ΔchkB mutant germinating conidia are exposed to UV light (Figure 5D, right, P < 0.001). We have observed that there is no interaction between ΔuvsB and ΔchkA when quiescent and germinating conidia from the single and double mutants were exposed to UV light (Figure 5E); and also there is no interaction between ΔuvsB and ΔchkB when germinating conidia from the single and double mutants were exposed to UV light (Figure 5F, right). However, the ΔchkB mutation is suppressing the ΔuvsB sensitivity to UV light (Figure 5F, left).
An important survival function when DNA damage occurs is the inhibition of the cell cycle through the activation of cell-cycle checkpoints. HU is an inhibitor of ribonucleoside diphosphate reductase, the rate-limiting enzyme in deoxyribonucleotide (dNTP) biosynthesis. Depletion of dNTPs activates the DNA replication checkpoint, which slows progression through S phase (Desany et al. 1998). Furthermore, initiation of DNA replication in the presence of high levels of HU causes DSBs (Merrill and Holm 1999). HU is an effective inhibitor of DNA synthesis in A. nidulans (Bergen and Morris 1983). We examined the ability of the ΔatmA, ΔuvsB, ΔchkA, and ΔchkB single- and double-mutant strains to replicate DNA during a transient period of growth in the presence of HU. A mitosis assay was used to verify if DNA replication checkpoint response is impaired in mutant strains (Fagundes et al. 2004). This assay monitors mitosis in mutant and wild-type strains incubated in 0, 6, or 100 mm of HU for 5–7 hr. The number of nuclei was assessed by DAPI staining, and if germlings had two or more nuclei after the HU incubation, they were scored as defective in mitotic arrest (Table 3). This assay measures the state of the replication checkpoint response. The atmA and uvsB inactivation strains had significant defects in the mitosis assay at 6 mm and 100 mm (Table 3). Interestingly, the ΔchkA and ΔchkB mutant strains had an intact replication checkpoint at both 6 and 100 mm, but the double-mutant strain ΔchkA ΔchkB had defects only at 6 mm (Table 3). Curiously, the ΔchkA and ΔchkB mutations suppressed both ΔatmA and ΔuvsB defects in the intra-S-phase checkpoint.
TABLE 3.
Mitosis assay from A. nidulans wild-type and mutant strains
| HU (mm)
|
|||
|---|---|---|---|
| Strains | 0 | 6 | 100 |
| GR5 (wild type) | 62.60 ± 2.88 | 10.40 ± 2.19 | 1.00 ± 0.00 |
| IM69 (Δatm∷pyrG) | 67.70 ± 2.50 | 31.33 ± 4.04a | 3.67 ± 0.58a |
| AAH14 (ΔuvsB) | 62.67 ± 5.86 | 15.33 ± 2.52a | 12.67 ± 3.21a |
| JFL3 (ΔchkA) | 58.33 ± 7.37 | 6.33 ± 1.53bcd | 0.00 ± 0.00 |
| JFL4 (ΔchkB) | 60.50 ± 5.92 | 10.00 ± 3.92bcd | 0.00 ± 0.00 |
| JFL100 (ΔchkA ΔchkB) | 60.00 ± 5.00 | 19.00 ± 1.00a | 1.00 ± 0.00 |
| JFL3-14 (ΔchkA ΔuvsB) | 56.00 ± 1.73 | 7.67 ± 2.08c | 0.00 ± 0.00 |
| JFL3-69(ΔchkA ΔatmA) | 58.33 ± 2.89 | 9.00 ± 1.00b | 0.00 ± 0.00 |
| JFL4-14 (ΔchkB ΔuvsB) | 51.67 ± 2.89 | 8.33 ± 1.53c | 0.00 ± 0.00 |
| JFL4-69 (ΔchkB ΔatmA) | 64.33 ± 8.14 | 10.00 ± 0.00b | 1.00 ± 0.00 |
Significantly different from the wild-type strain (P < 0.001).
Significantly different from the IM69 strain (P < 0.001).
Significantly different from the AAH14 strain (P < 0.05).
Significantly different from the JFL100 strain (P < 0.004).
We also evaluated the polar growth of the ChkA and ChkB inactivation mutants by following the same methodology described above, i.e., the HU-block-release assay. As was previously seen (Malavazi et al. 2006, 2007, and this work), the ΔatmA mutant strain showed decreased polar growth when compared to the wild-type strain (Table 4). The ΔchkA and ΔchkB mutant strains have not displayed polar growth defects while the double-mutant ΔchkA ΔchkB had decreased polar growth. Interestingly, all the double mutants with ΔchkA and ΔchkB, and with ΔatmA and ΔuvsB, showed to different extents intensification of the polar growth defect (Table 4). Notably, the most pronounced effect was observed for the double-mutant ΔatmA ΔchkB (3% of polar growth at 150 min after HU release; Table 4, last row). Taken together, these data indicate that ChkA and ChkB are redundantly involved in the DNA damage response in A. nidulans.
TABLE 4.
Polar growth upon HU-block-release experiments
| Strains | 0 min | 30 min | 60 min | 90 min | 120 min | 150 min |
|---|---|---|---|---|---|---|
| Wild type | 2.66 ± 0.57 | 7.30 ± 1.50 | 20.66 ± 1.15 | 35.00 ± 3.00 | 41.70 ± 1.20 | 56.00 ± 1.00 |
| ΔuvsB | 4.33 ± 1.15 | 10.00 ± 2.00 | 14.66 ± 4.16 | 22.00 ± 6.24 | 33.30 ± 5.68e | 42.33 ± 4.04e |
| ΔatmA | 0.00 ± 0.00a | 1.00 ± 1.00a | 4.66 ± 0.57a | 14.33 ± 0.57ac | 22.75 ± 2.63acd | 27.66 ± 2.08acd |
| ΔchkA | 3.33 ± 0.57 | 9.33 ± 3.21 | 16.00 ± 5.09b | 33.50 ± 5.44b | 56.30 ± 5.18bd | 77.50 ± 3.16bd |
| ΔchkB | 1.25 ± 0.50 | 2.75 ± 0.50a | 18.00 ± 2.16b | 25.00 ± 3.91abc | 39.75 ± 5.67bce | 51.25 ± 6.65bce |
| ΔchkA; ΔchkB | 2.66 ± 1.52 | 4.66 ± 1.52 | 9.33 ± 3.21a | 14.66 ± 3.05a | 22.00 ± 1.00a | 32.00 ± 6.08a |
| ΔchkA; ΔuvsB | 3.66 ± 0.57 | 8.66 ± 1.52 | 12.33 ± 2.30a | 17.33 ± 3.21a | 27.60 ± 2.08a | 36.66 ± 3.50a |
| ΔchkA; ΔatmA | 0.50 ± 0.70 | 0.00 ± 0.00a | 4.00 ± 1.41a | 8.00 ± 1.41a | 7.50 ± 0.70a | 11.00 ± 1.41a |
| ΔchkB; ΔuvsB | 1.50 ± 1.90 | 3.75 ± 2.12a | 12.50 ± 1.73a | 15.75 ± 1.70a | 19.25 ± 0.95a | 24.80 ± 6.65a |
| ΔchkB; ΔatmA | 0.40 ± 0.54a | 0.8 ± 1.09a | 1.0 ± 1.14a | 1.80 ± 0.83a | 2.20 ± 1.09a | 3.00 ± 1.00a |
Significantly different from the wild-type strain.
Significantly different from the ΔchkA ΔchkB strain.
Significantly different from the ΔchkB ΔatmA strain.
Significantly different from the ΔchkA ΔatmA strain.
Significantly different from the ΔchkB ΔuvsB strain.
Genetic interactions of the AtmAATM with other components of the DNA damage response:
We investigated possible genetic interactions between atmA and several genes with known roles in the regulation of the A. nidulans DNA damage response, including npkA, ankAWEE1, bimEAPC1, sldIRAD50, and mreAMRE11. Accordingly, we constructed the following double mutants: ΔatmA ΔnpkA (strain IM69-3), ΔatmA ankA (strain IM69-35), ΔatmA bimE7 (strain IM69-228), and ΔatmA mreA∷pyr4 (strain IM69-RE).
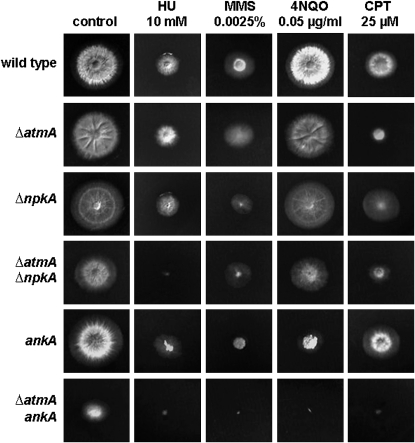
The double-mutant strain ΔatmA ΔnpkA was more sensitive to HU and 4-NQO while ΔatmA ankA was more sensitive to MMS, 4-NQO, and CPT than the corresponding parental strains (Figure 7 and supplemental Figure S1 at http://www.genetics.org/supplemental/). Our results suggest that AtmA functions in a different pathway from either NpkA or AnkA for the repair of DNA damage caused by these agents. By contrast, AtmA appears to display epistatic interactions with BimE and MreA under all tested conditions. Interestingly, the double-mutant ΔatmA ankA has a reduced growth rate in the absence of any DNA-damaging agent and poorly conidiates when compared to parental strains (Figure 7, supplemental Figure S1, and data not shown), providing more evidence for a genetic interaction between these two mutations.
Figure 7.—
Growth phenotypes of the wild-type, ΔatmA, ΔnpkA, ΔatmA ΔnpkA, ankA, and ΔatmA ankA strains. Strains GR5 (wild type), IM69 (ΔatmA), MV3 (ΔnpkA), IM69-3 (ΔatmA ΔnpkA), APK35 (ankA), and IM69-35 (ΔatmA ankA) were grown for 72 hr at 37° in YUU medium in the presence or absence of 10 mm HU, 0.0025% MMS, 0.05 μg/ml 4-NQO, and 25 μm CPT.
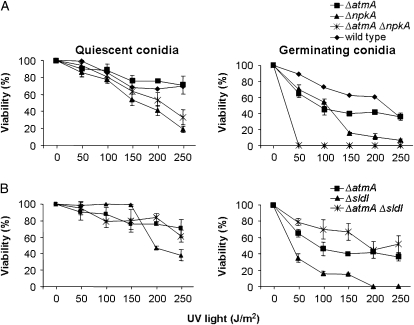
We checked UV sensitivities of nondividing (quiescent) and dividing (germinating) double mutants with the aim of identifying possible interactions between these genes. Germinating conidia from the double ΔatmA ΔnpkA strain were more sensitive to UV light than the corresponding parental strains (Figure 8A; P < 0.001). Notably, the sldIRAD50∷pyrG mutation suppresses the UV sensitivity of quiescent (200 and 250 J/m2) and germinating ΔatmA conidia (Figure 8B), thereby suggesting that AtmA and SldI may act in parallel to mediate checkpoint responses. The double-mutant ΔatmA ankA was also more sensitive when UV irradiation was applied to quiescent and germinating conidia than the corresponding parental strains (Figure 8C; P < 0.001). These results suggest that atmA and ankA function in parallel UV repair pathways for both quiescent and germinating conidia, whereas atmA and npkA function in parallel pathways only when germinating conidia are exposed to UV light. By contrast, epistasis was observed between ΔatmA and bimE7 when quiescent conidia were exposed to 50–150 J/m2 of UV light (Figure 8D). We were unable to test the UV-light sensitivity of the double-mutant ΔatmA mreAMRE11 because this strain cannot conidiate (data not shown).
Figure 8.—
Sensitivity to UV light. Quiescent and germinating conidiospores from wild type, ΔatmA, ΔnpkA, and ΔatmA ΔnpkA (A); ΔatmA, sldIRAD50, and ΔatmA sldIRAD50 (B); ΔatmA, ankA, and ΔatmA ankA (C); and ΔatmA, bimE7, and ΔatmA bimE7 (D) were exposed to UV light, and viability was scored after exposure to this DNA-damaging agent. Viability was determined as in Figure 5. The results were expressed by the average of four independent experiments and means ± standard deviations are shown.
We also tested if the DNA replication checkpoint response was intact in these double-mutant strains (Table 5). As previously reported, the bimE7, ΔnpkA, sldIRAD50∷pyrG, and ankA mutants have impaired checkpoint response at 6 mm (Fagundes et al. 2004; Malavazi et al. 2005). During the mitosis assay at 100 mm of HU, there was a synergistic interaction between ankA and ΔatmA (Table 5). There was a suppression of the DNA replication checkpoint in the ΔatmA mutation by ΔsldIRAD50 (at both 6 and 100 mm of HU), ΔnpkA (at 6 mm HU), and bimE7 (at 6 mm HU) mutations (Table 5). Accordingly, we were also not able to perform the mitosis assay for the ΔatmA mreAMRE11 mutant due to the conidiation defect. These data indicate that atmA has a complex genetic relationship with these genes during the DNA replication checkpoint and support the notion that there are genetic interactions between atmAATM and ankAWEE1, npkA, and bimEAPC1 during the DNA damage response in A. nidulans.
TABLE 5.
Mitosis assay from A. nidulans wild-type and mutant strains
| HU (mm)
|
|||
|---|---|---|---|
| Strains | 0 | 6 | 100 |
| GR5 (wild type) | 62.60 ± 2.9 | 10.40 ± 2.19 | 1.00 ± 0.00 |
| IM69 (Δatm) | 67.70 ± 2.50 | 31.33 ± 4.04a | 3.67 ± 0.58a |
| A776 (bimE7) | 39.33 ± 2.52 | 21.67 ± 3.06abc | 0.00 ± 0.00bc |
| MV3 (Δnpka) | 67.00 ± 3.00 | 27.33 ± 3.21ad | 2.67 ± 1.15 |
| IM25 (sldI∷pyrG) | 61.00 ± 2.16 | 23.50 ± 3.11abe | 3.00 ± 0.82 |
| APK35 (ankA) | 78.50 ± 4.51 | 31.50 ± 2.08af | 1.25 ± 0.50bf |
| IM69-25 (Δatm sldI∷pyrG) | 61.50 ± 0.71 | 9.50 ± 3.54b | 0.00 ± 0.00b |
| IM69-3 (Δatm Δnpka) | 64.25 ± 4.35 | 7.25 ± 3.20b | 0.0 ± 0.00b |
| IM69-228 (Δatm bimE7) | 56.67 ± 3.51 | 9.00 ± 1.00b | 4.00 ± 0.00a |
| IM69-35 (Δatm ankA) | 69.00 ± 7.07 | 41.20 ± 4.09a | 13.00 ± 4.24ab |
Significantly different from the wild-type strain (P < 0.001).
Significantly different from the IM69 strain (P < 0.03).
Significantly different from the IM69-228 strain (P < 0.001).
Significantly different from the IM69-3 strain (P < 0.001).
Significantly different from the IM69-25 strain (P < 0.001).
Significantly different from the IM69-35 strain (P < 0.001).
DISCUSSION
The atmAATM loss of function causes synthetic lethality when combined with mutation in uvsBATR:
ATM and ATR are paralogous PIKKs that orchestrate the DNA damage response in eukaryotic cells (Shiloh and Kastan 2001; McKinnon 2004). These kinases possess both overlapping and distinct roles in the regulation of this response. ATM and ATR respond to different types of signals and they were assigned to independent pathways that were parallel in structure, but with distinct inputs and outputs (Hurley and Bunz 2007). Recently, it was shown that ATR responds to DSBs and that this response is ATM dependent (Adams et al. 2006; Cuadrado et al. 2006; Jazayeri et al. 2006; Myers and Cortez 2006). Stiff et al. (2006) showed that UV light and HU also activate ATM and that this activation is ATR dependent. Thus, it seems these two regulatory kinases can have some degree of communication as if they belong to an integrated molecular circuit that can process diverse types of signals linking DNA replication with the DNA damage response pathway (Hurley and Bunz 2007). In A. nidulans, we have previously shown that UvsBATR regulates the DNA damage checkpoint response, inhibits septation in predivisional hyphae that have experienced DNA damage, and controls both the expression and the localization of proteins involved in the processing and repair of DNA damage (Harris and Kraus 1998; Hofmann and Harris 2000; Fagundes et al. 2005). Recently, we characterized the homolog of ATM (AtmA) in A. nidulans (Malavazi et al. 2006, 2007). In addition to its expected role in the DNA damage response, we found that AtmA is also required for polarized hyphal growth. Here, we present an analysis of the genetic interactions of different components of the A. nidulans DNA damage response pathway with the atmAATM.
In Saccharomyces cerevisiae, the double-mutant tel1 mec1 (the ATM and ATR homologs, respectively) is viable (Morrow et al. 1995). Furthermore, Tel1p appears to have a role in the repair of DNA damage that is functionally redundant with that of Mec1p because tel1 mec1 double-mutant strains are more sensitive to DNA-damaging agents than are mec1 strains (Morrow et al. 1995; Sanchez et al. 1996; Usui et al. 2001) and an extra copy of TEL1 partially suppresses the DNA damage sensitivity of mec1 strains (Morrow et al. 1995). Differently from S. cerevisiae, we have shown that the double A. nidulans inactivation mutant atmA uvsB is nonviable. Here, we provided several examples that strongly suggest these genes are interacting and are probably partially redundant in terms of DNA damage sensing and/or repairing and polar growth. We were not able to introduce either a copy of the atmA gene into a uvsB mutant or a copy of uvsB into a atmA mutant (data not shown). However, surprisingly, when atmA is overexpressed in the uvsB101 background, the strain becomes more resistant only to 0.001% MMS. We are currently investigating the possible causes for this phenomenon.
ATM function has never been linked to the establishment or the maintenance of cell polarity in yeast cells. However, recently Enserink et al. (2006) and Shi et al. (2007) showed that Rad53CHK2 was involved in polar growth in S. cerevisiae and Candida albicans, respectively. We have also observed a role in these morphogenetic events for C. albicans Mec1 but not for Atm inactivation strains (I. Malavazi and G. H. Goldman, unpublished results). We were not able to observe any polar growth defect in A. nidulans uvsBATR, chkACHK1, and chkBCHK2 inactivation strains. However, every double mutant constructed with ΔatmA and ΔchkB displayed delayed polar growth, suggesting that these two genes are important for coordinating morphogenetic events with DNA replication and DNA damage response during growth. But, we cannot eliminate the possibility that endogenous DNA damage response exacerbates the polarization defects of AtmA mutants. Interestingly, the double-mutant ΔatmA ΔuvsB germlings have an increased number of polarity axes. These results once more suggest a redundant role for AtmA and UvsB during early morphogenetic events.
Molecular characterization of the chkA and chkB genes:
ATM and ATR are central to entire DNA damage response and directly regulate at least two effector kinases, Chk1 and Chk2 that are responsible for relaying the checkpoint signals (Bartek and Lukas 2003; Ahn et al. 2004; Yinhuai and Sanchez 2004). These kinases are structurally unrelated serine/threonine kinases activated in response to several DNA-damaging insults that regulate fundamental cellular functions such as DNA replication and cell-cycle progression, chromatin restructuring, and apoptosis (Bartek and Lukas 2003). We have characterized the A. nidulans chkACHK1 and chkBCHK2 homologs. The A. nidulans ChkA is the first CHK1 homolog characterized in filamentous fungi. Both A. nidulans inactivation strains are viable. Mammalian Chk2 shares many of the Chk1 substrates and seems to function to support the role of Chk1 in the response to genotoxic stress in addition to its role in the regulation of apoptosis in response to ionizing radiation (IR) (Chen and Sanchez 2004). Yeast Chk1 responds to DNA damage that occurs in late S or G2 but is inert to damage that occurs in S phase or replication blocks; in contrast, mammalian Chk1 has much broader roles in the response to all forms of genotoxic stress (Chen and Sanchez 2004).
Our results suggest that A. nidulans ChkA and ChkB have conserved and specific biological roles during the DNA damage response. Furthermore, they display an intense level of redundancy; i.e., when both genes were inactivated, the double mutant showed increased defects or epistatic relationship during the DNA damage response depending on its triggering agent. Actually, the model proposed by Bartek and Lukas (2003, p. 426) that the “‘biological mission’ (of Chk1 and Chk2) is one of mutual complementation and intimate cooperation, a partnership where Chk1 operates as workhorse, while Chk2 contributes decisively yet only under circumstances that cause DSBs,” is applicable to the A. nidulans ChkA and ChkB genes. However, we were not able to observe an involvement of ChkB in DSBs, except for its mRNA accumulation when A. nidulans is challenged with BLEO and CPT. Additionally, the fact that the S-phase replication checkpoint is intact at 100 mm HU suggests that possibly a third kinase or a different relay pathway is involved in the S-phase checkpoints. There are still a large number of issues to be addressed before a complete view of the A. nidulans ChkA and ChkB functions in the DNA damage response can be understood.
It has been proposed that Chk1 is primarily regulated by ATR in response to replication blocks and UV treatment, while Chk2 is regulated by ATM in response to IR-induced DSBs. This view needs to be modified if we consider recent studies showing that Chk1 is activated by the ATM pathway (Gatei et al. 2003) and the phosphorylation of Chk2 in response to UV light and HU does not require ATM (Matsuoka et al. 2000; Wang et al. 2006). In response to DNA damage or replication stress, Chk1 is phosphorylated and activated by ATR and/or ATM in yeasts, Xenopus, and mammals (Chen and Sanchez 2004). To investigate the genetic relationship among AtmA and UvsB, and ChkA and ChkB, we constructed several double-inactivation mutants and observed genetic interactions among them when these strains were tested in the presence of genotoxins and/or UV light. Surprisingly, we determined that the presence of either a ΔchkA or a ΔchkB mutation was enough to suppress the intra-S-phase DNA replication checkpoint in the ΔatmA or ΔuvsB mutations. Suppression of a null allele is expected to be due to downstream mutations that activate the pathway independent of the original (suppressed) gene product (Prelich 1999). Thus, it is possible that ChkA and ChkB are positioned downstream of AtmAATM and UvsBATR in the intra-S-phase checkpoint pathway, like in other organisms. However, the results imply that atmA and uvsB negatively control chkA and chkB for the intra-S checkpoint (Figure 9). Our results revealed a very complex web of interactions in A. nidulans among the apical protein kinases AtmAATM and UvsBATR and the signal relay protein kinases ChkACHK1 and ChkBCHK2. It seems these proteins display very redundant and complementary functions during the A. nidulans DNA damage response.
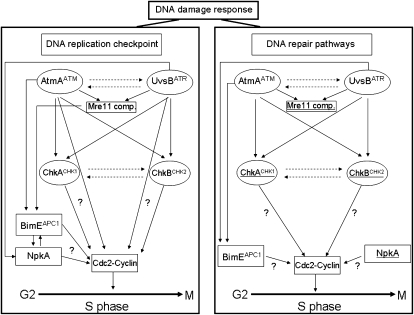
Figure 9.—
The diagram is based on data from this article, Ye et al. (1996), Fagundes et al. (2004, 2005), and Malavazi et al. (2005). The DNA damage response was divided into DNA replication checkpoint (DRC, left) and DNA repair pathways (DRP, right). As in other eukaryotic cells, the DNA damage checkpoint in A. nidulans functions via phosphorylation of the NimXCdc2 Y15 (Ye et al. 1997). Ye et al. (1996) have shown the existence of two S-phase checkpoint regulatory systems that control initiation in mitosis. One, which involves Y15 phosphorylation of both NimXCdc2 and BimE, prevents initiation of mitosis when DNA replication is arrested and the other restrains mitosis via Y15 phosphorylation of NimXCdc2 when DNA replication is slowed. Our work showed that AtmA and UvsB as well as ChkA and ChkB played redundant roles during both DRC and DRP (dashed lines). Other interactions can also be observed during DRC. BimE interacts with AtmA and the Mre11 complex (Mre11 comp.) as well as with NpkA, a Cdc2-related kinase that plays a role in cell-cycle progression during S phase in A. nidulans (Fagundes et al. 2004). NimXCdc2 (AnkA) is interacting with AtmA in the DRC but not in the same pathway. The underlined genes had increased mRNA accumulation in the DRP (right). In the DRP, AtmA interacts with the Mre11 complex and BimE interacts with both AtmA and UvsB. NpkA and AnkACdc2 are interacting with AtmA in the DRP but not in the same pathway.
Genetic interactions of the AtmAATM with other components of the DNA damage response:
We investigated the possible genetic interactions between atmA and three other genes, bimEAPC1 (that encodes a subunit of the anaphase-promoting complex), the kinase ankAWEE1, and the S-phase cdc-2-related kinase npkA, by constructing double mutants ΔatmA bimEAPC1, ΔatmA ankA, and ΔatmA ΔnpkA, respectively. In most of the investigated phenotypes, we observed consistent epistatic relationship between ΔatmA and bimE and synergistic interactions between atmA and npkA, and ankA mutations. Figure 9 summarizes these interactions. At this moment, we can only speculate on the molecular basis of these interactions. The interaction between ΔatmA and bimE could reveal a relationship between DSBs and the mitotic spindle checkpoint. Lobachev et al. (2004) have shown that the Mre11 complex is required in vivo to hold the two ends of a broken chromosome together throughout DSB repair. The same authors have shown that a mutation in the Rad50 Zn-hook structure resulted in DNA ends that are dispersed in ∼10–20% of cells. The Mre11 complex holds broken ends together and counteracts mitotic spindle forces that can be destructive to damaged chromosomes (Lobachev et al. 2004). Malavazi et al. (2005) have shown epistatic and synergistic interactions between sldIRAD50 and bimEAPC1 at S-phase checkpoints and in response to HU and UV light. During mitosis in undamaged wild-type cells, the sister chromatids are separated after proteolytic cleavage of cohesin by the pulling forces of the centromere-attached mitotic spindle (Uhlmann 2004). The cohesion of the sister chromatids is established by the cohesion protein complex during DNA replication and persists until chromosome segregation (Haering and Nasmyth 2003). The spindle checkpoint ensures the high-fidelity transmission by the controlled proteolytic cleavage of a subunit of the cohesion complex, Scc1, by separase, destroying the cohesion between the sister chromatids and triggering the onset of anaphase (for a review, see Yu 2002). The separase proteolytic activity is blocked by securin, and the APC in association with one of its substrate cofactors tags the securin proteins with polyubiquitin chains (Peters 2002). Finally, degradation of the ubiquitinated securin activates the separase (Yu 2002). Tritarelli et al. (2004) showed that ATM is involved in p53 localization to the centrosomes in mitosis and that the ATM-dependent association of p53 to centrosomes is strictly linked to the postmitotic checkpoint response to spindle disruption. Thus, it is possible that the observed interaction between the atmA-inactivated strain and the bimE7 mutant reflects an interaction between this protein kinase and the microtubule-mediated forces through the spindle checkpoint that would affect the transition from DSBs to chromosome breaks.
Cell-cycle progression is coupled with the sequential activation of cyclin-dependent kinases (CDKs) (Pines 1999). ATM and ATR also activate CHK1 and CHK2, which negatively regulate CDK1 and CDK2 kinases, via the Cdc25 phosphatase family, thus enforcing the cell-cycle arrest signal (Nyberg et al. 2002). The synergistic interactions between atmA and npkA, and ankA mutations could reflect the fact that critical molecular events involved in the cell-cycle checkpoint upon DNA damage are disregulated and thus cannot inhibit the advance of the cell cycle. Recently, Maude and Enders (2005) reported that inhibitors of Cdk1/2 resulted in disparate effects on the Chk1 and Chk2 pathways. Chk1 expression is reduced, and this effect contributed to a broad DNA damage response marked by activation of ATM and Chk2. These authors support a model where Cdk inhibitors produce DNA damage and sensitize cells to DNA-damaging agents. Thus, these results could explain the synergistic effects observed in the double-mutant ΔatmA ankA. It is interesting to emphasize the synergistic interaction with the npkA deletion mutant. NpkA appears to be a component of the ATM/ATR signaling pathway because (i) npkA gene expression is dependent on uvsBATR in the presence of camptothecin, (ii) the npkA deletion can partially suppress the HU sensitivity of the uvsBATR and uvsDATRIP mutants, and (iii) the double-mutant ΔnpkA ankA is more sensitive to genotoxins that interfere with the function of replication forks (Fagundes et al. 2004). The synergistic effects observed between ΔatmA and ΔnpkA indicates that NpkA plays a broader role in A. nidulans DNA damage response, interacting not only with uvsBATR but also with atmA.
Acknowledgments
We thank José Luiz Cappelaro for technical assistance in some of the A. nidulans crosses, the two anonymous reviewers for their suggestions, and the Laboratory of Confocal Microscopy, Faculdade de Medicina de Ribeirão Preto, University of São Paulo, Brazil, for the use of the confocal microscope. This research was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo, Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil, and the John Simon Guggenheim Memorial Foundation.
References
- Abraham, R. T., 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15 2177–2196. [DOI] [PubMed] [Google Scholar]
- Adams, K. E., A. L. Medhurst, D. A. Dart and N. D. Lakin, 2006. Recruitment of ATR to sites of ionising radiation-induced DNA damage requires ATM and components of the MRN protein complex. Oncogene 29 3894–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, J., M. Urist and C. Prives, 2004. The Chk2 protein kinase. DNA Repair 3 1039–1047. [DOI] [PubMed] [Google Scholar]
- Bakkenist, C. J., and M. B. Kastan, 2003. DNA damage activates ATM through intermolecular authophosphorylation and dimer dissociation. Nature 421 499–506. [DOI] [PubMed] [Google Scholar]
- Banin, S., L. Moyal, S. Shieh, Y. Taya, C. W. Anderson et al., 1998. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281 1674–1677. [DOI] [PubMed] [Google Scholar]
- Bartek, J., and J. Lukas, 2003. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3 421–429. [DOI] [PubMed] [Google Scholar]
- Baskaran, R., L. D. Wood, L. L. Whitaker, C. E. Canman, S. E. Morgan et al., 1997. Ataxia telangiectasia mutant protein activates c-Abl tyrosine kinase in response to ionizing radiation. Nature 387 516–519. [DOI] [PubMed] [Google Scholar]
- Bergen, L. G., and N. R. Morris, 1983. Kinetics of the nuclear division cycle of Aspergillus nidulans. J. Bacteriol. 156 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, K. D., Y. Ziv, S. N. Sadanandan, L. Chessa, F. S. Collins et al., 1997. The ataxia-telangiectasia gene product, a constitutively expressed nuclear protein that is not up-regulated following genome damage. Proc. Natl. Acad. Sci. USA 94 1840–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canman, C. E., and D. S. Lim, 1998. The role of ATM in DNA damage responses and cancer. Oncogene 17 3301–3308. [DOI] [PubMed] [Google Scholar]
- Caspari, T., and A. M. Carr, 1999. DNA structure checkpoint pathways in Schizosaccharomyces pombe. Biochimie 81 173–181. [DOI] [PubMed] [Google Scholar]
- Chen, Y., and Y. Sanchez, 2004. Chk1 in the DNA damage response: conserved roles from yeasts to mammals. DNA Repair 3 1025–1032. [DOI] [PubMed] [Google Scholar]
- Cortez, D., S. Guntuku, J. Qin and S. J. Elledge, 2001. ATR and ATRIP partners in checkpoint signalling. Science 294 1713–1716. [DOI] [PubMed] [Google Scholar]
- Cuadrado, M., B. M. Martinez-Pastor, L. I. Murga, P. Toledo, E. Gutierrez-Martinez et al., 2006. ATM regulates ATR chromatin loading in response to DNA double-strand breaks. J. Exp. Med. 203 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desany, B. A., A. A. Alcasabas, J. B. Bachant and S. J. Elledge, 1998. Recovery from DNA replicational stress is the essential function of the S phase checkpoint pathway. Genes Dev. 12 2956–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza, C. P., X. S. Ye and S. A. Osmani, 1999. Checkpoint defects leading to premature mitosis also cause endoreplication of DNA in Aspergillus nidulans. Mol. Biol. Cell 10 3661–3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink, J. M., M. B. Smolka, H. Zhou and R. D. Kolodner, 2006. Checkpoint proteins control morphogenetic events during DNA replication stress in Saccharomyces cerevisiae. J. Cell Biol. 175 729–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes, M. R. Z. K., J. F. Lima, M. S. Savoldi, I. Malavazi, R. E. Larson et al., 2004. The Aspergillus nidulans npkA gene encodes a Cdc2-related kinase that genetically interacts with the UvsBATR kinase. Genetics 167 1629–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes, M. Z. K., C. P. Semighini, I. Malavazi, M. S. Savoldi, J. F. de Lima et al., 2005. The Aspergillus nidulans uvsBATR and scaANBS1 genes show genetic interactions during recovery from replication stress and DNA damage. Eukaryot. Cell 4 1239–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flipphi, M., J. Kocialkowska and B. Felenbok, 2002. Characteristics of physiological inducers of the ethanol utilization (alc) pathway in Aspergillus nidulans. Biochem. J. 15 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatei, M., K. Sloper, C. Sorensen, R. Syljuasen, J. Falck et al., 2003. Ataxia-telangiectasia-mutated (ATM) and NBS1-dependent phosphorylation of Chk1 on Ser-317 in response to ionising radiation. J. Biol. Chem. 278 14806–14811. [DOI] [PubMed] [Google Scholar]
- Goldman, G. H., and E. Kafer, 2004. Aspergillus nidulans as a model system to characterize the DNA damage response in eukaryotes. Fungal Genet. Biol. 41 428–442. [DOI] [PubMed] [Google Scholar]
- Goldman, G. H., S. L. McGuire and S. D. Harris, 2002. The DNA damage response in filamentous fungi. Fungal Genet. Biol. 35 183–195. [DOI] [PubMed] [Google Scholar]
- Gygax, S. E., C. P. Semighini, G. H. Goldman and S. D. Harris, 2005. BCTF4 is required for the formation of DNA damage-induced UvsCRAD51 foci in Aspergillus nidulans. Genetics 169 1391–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haering, C. H., and K. Nasmyth, 2003. Building and breaking bridges between sister chromatids. BioEssays 25 1178–1191. [DOI] [PubMed] [Google Scholar]
- Harris, S. D., and P. R. Kraus, 1998. Regulation of septum formation in Aspergillus nidulans by a DNA damage checkpoint pathway. Genetics 148 1055–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann, A. F., and S. D. Harris, 2000. The Aspergillus nidulans uvsB gene encodes an ATM-related kinase required for multiple facets of the DNA damage response. Genetics 154 1577–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley, P., and F. Bunz, 2007. ATM and ATR. Components of an integrated circuit. Cell Cycle 6 E1–E4. [DOI] [PubMed] [Google Scholar]
- Jazayeri, A., J. Falck, C. Lukas, J. Bartek, G. C. Smith et al., 2006. ATM and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat. Cell Biol. 8 37–45. [DOI] [PubMed] [Google Scholar]
- Kafer, E., 1977. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv. Genet. 19 33–131. [DOI] [PubMed] [Google Scholar]
- Kraus, P. R., and S. D. Harris, 2001. The Aspergillus nidulans snt genes are required for the regulation of septum formation and cell cycle checkpoints. Genetics 159 557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakin, N. D., P. Weber, T. Stankovic, S. T. Rottinghaus, A. M. Taylor et al., 1996. Analysis of the ATM protein in wild-type and ataxia telangiectasia cells. Oncogene 13 2707–2716. [PubMed] [Google Scholar]
- Lavin, M. F., and Y. Shiloh, 1997. The genetic defect in ataxia-telangiectasia. Annu. Rev. Immunol. 15 177–202. [DOI] [PubMed] [Google Scholar]
- Lee, J. H., and T. T. Paull, 2004. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science 304 93–96. [DOI] [PubMed] [Google Scholar]
- Lim, D. S., D. G. Kirsch, C. E. Canman, J. H. Ahn, Y. Ziv et al., 1998. ATM binds to β-adaptin in cytoplasmic vesicles. Proc. Natl. Acad. Sci. USA 95 10146–10151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobachev, K., E. Vitriol, J. Stemple, M. A. Resnick and K. Bloom, 2004. Chromosome fragmentation after induction of a double-strand break is an active process prevented by the RMX repair complex. Curr. Biol. 14 2107–2112. [DOI] [PubMed] [Google Scholar]
- Malavazi, I., J. F. de Lima, M. R. Z. K. Fagundes, V. P. Efimov, M. H. S. Goldman et al., 2005. The Aspergillus nidulans sldIRAD50 gene interacts with bimEAPC1, a homolog of an anaphase promoting complex subunit. Mol. Microbiol. 57 222–237. [DOI] [PubMed] [Google Scholar]
- Malavazi, I., C. P. Semighini, M. R. Kress, S. D. Harris and G. H. Goldman, 2006. Regulation of hyphal morphogenesis and the DNA damage response by the Aspergillus nidulans ATM homolog AtmA. Genetics 173 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavazi, I., M. Savoldi, M. E. da Silva Ferreira, F. M. Soriani, P. S. Bonato et al., 2007. Transcriptome analysis of the Aspergillus nidulans AtmA (ATM, ataxia-telangiectasia) null mutant. Mol. Microbiol. 66 74–99. [DOI] [PubMed] [Google Scholar]
- Matsuoka, S., M. Huang and S. J. Elledge, 2000. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 282 1893–1897. [DOI] [PubMed] [Google Scholar]
- Maude, S. L, and G. H. Enders, 2005. Cdk inhibition in human cells compromises chk1 function and activates a DNA damage response. Cancer Res. 65 780–786. [PubMed] [Google Scholar]
- McKinnon, P. J., 2004. ATM and ataxia telangiectasia. EMBO Rep. 5 772–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill, B. J., and C. Holm, 1999. A requirement for recombinational repair in Saccharomyces cerevisiae is caused by DNA replication defects of mec1 mutants. Genetics 153 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow, D. M., D. A. Tagle, Y. Shiloh, F. S. Collins and P. Hieter, 1995. TEL1, an S. cerevisiae homologue of the human gene mutated in ataxia telangiectasia, is functionally related to the yeast checkpoint gene MEC1. Cell 82 831–840. [DOI] [PubMed] [Google Scholar]
- Myers, J. S., and D. Cortez, 2006. Rapid activation of ATR by ioinising radiation requires ATM and Mre11. J. Biol. Chem. 281 9346–9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak, T., E. Szewczyk, C. E. Oakley, A. Osmani, L. Ukil et al., 2006. A versatile and efficient gene targeting system for Aspergillus nidulans. Genetics 172 1557–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg, K. A., R. J. Michelson, C. W. Putnam and T. A. Weinert, 2002. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 36 617–656. [DOI] [PubMed] [Google Scholar]
- Osborn, A. J., S. J. Elledge and L. Zou, 2002. Checking on the fork: the DNA-replication stress-response pathway. Trends Cell Biol. 12 509–516. [DOI] [PubMed] [Google Scholar]
- Paull, T. T., and J. H. Lee, 2005. The Mre11/Rad50/Nbs1 complex and its role as a DNA double-strand break sensor for ATM. Cell Cycle 6 737–740. [DOI] [PubMed] [Google Scholar]
- Peters, J. M., 2002. The anaphase promoting complex. Proteolysis in mitosis and beyond. Mol. Cell 9 931–943. [DOI] [PubMed] [Google Scholar]
- Pines, J., 1999. Four-dimensional control of the cell cycle. Nat. Cell Biol. 1 E73–E79. [DOI] [PubMed] [Google Scholar]
- Pregueiro, A. M., Q. Liu, C. L. Baker, J. C. Dunlap and J. J. Loros, 2006. The Neurospora checkpoint kinase 2: a regulatory link between the circadian and cell cycles. Science 313 644–649. [DOI] [PubMed] [Google Scholar]
- Prelich, G., 1999. Suppression mechanisms: themes from variations. Trends Genet. 15 261–266. [DOI] [PubMed] [Google Scholar]
- Sanchez, Y., B. A. Desany, W. L. Jones, Q. Liu, B. Wang et al., 1996. Regulation of RAD53 by the ATM-like kinases MEC1 and TEL1 in yeast cell cycle checkpoint pathways. Science 271 357–360. [DOI] [PubMed] [Google Scholar]
- Savitsky, K., A. Bar-Shira, S. Gilad, G. Rotman, Y. Ziv et al., 1995. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 268 1749–1753. [DOI] [PubMed] [Google Scholar]
- Semighini, C. P., M. Marins, M. H. S. Goldman and G. H. Goldman, 2002. Quantitative analysis of the relative transcript levels of ABC transporter Atr genes in Aspergillus nidulans by real-time reverse transcription-PCR assay. Appl. Environ. Microbiol. 68 1351–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafman, T., K. K. Khanna, P. Kedar, K. Spring, S. Kozlov et al., 1997. Interaction between ATM protein and c-Abl in response to DNA damage. Nature 387 520–523. [DOI] [PubMed] [Google Scholar]
- Shi, Q.-M., Y.-M. Wang, X.-D. Zheng, R. T. H. Lee and Y. Wang, 2007. Critical role of DNA checkpoints in mediating genotoxic-stress-induced filamentous growth. Mol. Biol. Cell 18 815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh, Y., 2006. The ATM-mediated DNA-damage response: taking shape. Trends Biochem. Sci. 31 402–410. [DOI] [PubMed] [Google Scholar]
- Shiloh, Y., and M. B. Kastan, 2001. ATM: genome stability, neuronal development, and cancer cross paths. Adv. Cancer Res. 83 209–254. [DOI] [PubMed] [Google Scholar]
- Stiff, T., S. A. Walker, K. Cerosaletti, A. A. Gooodarzi, E. Petermann et al., 2006. ATR-dependent phosphorylation and activation of ATM in response to UV treatment or replication fork stalling. EMBO J. 25 5775–5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritarelli, A., E. Oricchio, M. Ciciarello, R. Mangiacasale, A. Palena et al., 2004. p53 localization at centrosomes during mitosis and postmitotic checkpoint are ATM-dependent and require serine 15 phosphorylation. Mol. Biol. Cell 15 3751–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann, F., 2004. The mechanism of sister chromatid cohesion. Exp. Cell Res. 296 80–85. [DOI] [PubMed] [Google Scholar]
- Usui, T., H. Ogawa and J. H. J. Petrini, 2001. A DNA damage pathway controlled by Tel1 and the Mre11 complex. Mol. Cell 7 1255–1266. [DOI] [PubMed] [Google Scholar]
- Van den Bosch, M., R. T. Bree and N. F. Lowndes, 2003. The MRN complex: coordinating and mediating the response to broken chromosomes. EMBO Rep. 4 844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. Q., J. L. Redpath, S. T. Fan and E. J. Stanbridge, 2006. ATR dependent activation of Chk2. J. Cell Physiol. 208 613–619. [DOI] [PubMed] [Google Scholar]
- Watters, D., K. K. Khana, H. Beamish, G. Birrell, K. Spring et al., 1997. Cellular localisation of the ataxia-telangiectasia (ATM) gene product and discrimination between mutated and normal forms. Oncogene 14 1911–1921. [DOI] [PubMed] [Google Scholar]
- Yinhuai, C., and Y. Sanchez, 2004. Chk1 in the DNA damage response: conserved roles from yeasts to mammals. DNA Repair 3 1025–1032. [DOI] [PubMed] [Google Scholar]
- Ye, X. S., R. R. Fincher, A. Tang, K. O'Donnell and S. A. Osmani, 1996. Two S-phase checkpoint systems, one involving the function of both BIME and Tyr15 phosphorylation of p34Cdc2, inhibit NIMA and prevent premature mitosis. EMBO J. 15 3599–3610. [PMC free article] [PubMed] [Google Scholar]
- Ye, X. S., R. R. Fincher, A. Tang and S. A. Osmani, 1997. The G2/M DNA damage checkpoint inhibits mitosis through Tyr 15 phosphorylation of p34Cdc2 in Aspergillus nidulans. EMBO J. 16 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, H., 2002. Regulation of APC-Cdc20 by the spindle checkpoint. Curr. Opin. Cell Biol. 14 706–714. [DOI] [PubMed] [Google Scholar]