Abstract
To further understand how the nematode Caenorhabditis elegans defends itself against pathogen attack, we analyzed enhanced pathogen resistance (epr) mutants obtained from a forward genetic screen. We also examined several well-characterized sterile mutants that exhibit an Epr phenotype. We found that sterility and pathogen resistance are highly correlated and that resistance in both epr and sterile mutants is dependent on DAF-16 activity. Our data indicate that a DAF-16-dependent signaling pathway distinct from previously described pathways is involved in the activation of genes that confer resistance to bacterial pathogens. The timing of DAF-16-dependent gene activation in sterile mutants coincides with the onset of embryonic development in wild-type animals, suggesting that signals from developing embryos normally downregulate the immune response.
ONE of the most important traits for natural selection is the maximal production of progeny. However, reproduction comes at the expense of decreased survival of the parent. A negative relationship between reproduction and longevity has been reported across a diverse array of organisms including plants, insects, and mammals (Fowler and Partridge 1989; Westendorp and Kirkwood 1998; Hautekeete et al. 2001). Several studies have linked successful reproduction and immunocompetence (McKean and Nunney 2001; Fedorka et al. 2004) and reviewed by Klein and Nelson (1999). Because reproduction utilizes resources necessary for somatic cell maintenance, growth, and survival, it appears likely that expression of immunity and reproduction must compete for energy allocation. This trade-off between increased reproduction and decreased survival rate may maximize survival of offspring depending on the availability of food and the presence of pathogens.
Both vertebrates and invertebrates rely on innate immunity as the first line of defense against pathogen attack. In plants, insects, and vertebrates, a wealth of data show that this ancient mechanism relies in part on the recognition of pathogen-associated molecular patterns (PAMPs) by defined genomically encoded receptors and subsequent expression of antimicrobial effector molecules (Tosi 2005; Akira et al. 2006; Ryan et al. 2007). Invertebrates and plants appear to depend solely on innate immunity because they lack an adaptive immune system that somatically generates an array of immune receptors that are selected and amplified in immune cells. Work carried out during the past 15 years shows that innate immune signaling pathways are at least partially conserved between vertebrate and invertebrates, including the innate immune response pathways of the model nematode Caenorhabditis elegans (reviewed by Ausubel 2005). Mounting evidence suggests that C. elegans is a facile model to study both the evolutionary origins of innate immunity as well as the resistance mechanisms by which hosts combat pathogen attack (reviewed by Kurz and Ewbank 2003; Millet and Ewbank 2004; Schulenburg et al. 2004; Gravato-Nobre and Hodgkin 2005; Kim and Ausubel 2005).
A wide variety of human pathogens, including the Gram-negative bacterial pathogen Pseudomonas aeruginosa and the Gram-positive bacterial pathogen Staphylococcus aureus, infect and kill C. elegans (Tan et al. 1999a; Sifri et al. 2003). Because C. elegans is normally propagated on bacterial lawns in the laboratory, the nematodes can be easily infected by simply transferring them from their normal laboratory food, Escherichia coli strain OP50, to a lawn of the pathogen of choice. Infection with live but not heat-killed pathogens leads to premature death of the worms and in many cases it has been demonstrated that many of the same bacterial virulence factors are required for nematode killing and for maximum virulence in mammalian hosts (Mahajan-Miklos et al. 1999; Tan et al. 1999b; Aballay et al. 2000; Sifri et al. 2003).
Both forward and reverse genetic approaches have been used to identify signaling pathways involved in activating innate immune responses. For example, a forward genetic screen for immunocompromised mutants identified a p38 MAPK signaling cascade (Kim et al. 2002) that functions to promote immunity in C. elegans. A candidate gene approach revealed that derepression of the FOXO/forkhead-like transcription factor DAF-16, by loss-of-function mutations in the upstream components of the insulin/IGF-1 pathway, confers resistance to a variety of pathogens (Garsin et al. 2003). When an insulin-like ligand binds to DAF-2, the only insulin/IGF-1 receptor in C. elegans, it activates a conserved phospho-relay cascade via AGE-1, a phosphatidylinositol 3-kinase [PI(3)K] (Morris et al. 1996), leading to phosphorylation of DAF-16, which blocks its translocation to the nucleus (reviewed by Nelson and Padgett 2003; Mukhopadhyay et al. 2006). Disruption of the DAF-2 signaling cascade by mutation of daf-2 or age-1, for example, promotes DAF-16 accumulation in the nucleus (Henderson and Johnson 2001; Lee et al. 2001; Lin et al. 2001), concomitant entry into the dauer developmental pathway (Gottlieb and Ruvkun 1994), and activation of a variety of stress-related responses leading to increased longevity (Murakami and Johnson 1996; Henderson and Johnson 2001). Temperature-sensitive loss-of-function mutations in daf-2 or age-1 increase longevity up to twofold when feeding on nonpathogenic E. coli, which is completely suppressed by loss-of-function mutations in daf-16 (Kenyon et al. 1993; Lin et al. 1997).
Since pathogen resistance is DAF-16-dependent in daf-2 and age-1 mutant animals, it appears likely that the genes regulated downstream of DAF-16 confer the pathogen resistance phenotype. Genomewide transcriptional analyses have shown that DAF-16 is predicted to transcriptionally target >500 genes whose functions include antioxidant, metabolic, antimicrobial, and stress responses in animals with reduced expression of DAF-2 (McKean and Nunney 2001; McElwee et al. 2003). Murphy et al. (2003) further demonstrated that the oxidative stress genes mtl-1 and sod-3 and the antimicrobial genes dod-6 and lys-7 play an important functional role in the increased life span of daf-2 animals. It appears that a variety of DAF-16-dependent genes play an important role in conferring enhanced immunity. SOD-3 is not only expressed in intestinal tissue, a site of infection, after exposure to the Gram-positive bacterial pathogen Enterococcus faecalis, but is also required for resistance to E. faecalis (Chavez et al. 2007). Similarly, lys-7 is induced by Microbacterium nematophilum, a nematode-specific Gram-positive bacterial pathogen, as well as the Gram-negative bacterial pathogen Serratia marcescens and plays a functional role in conferring resistance to M. nematophilum (Mallo et al. 2002; O'Rourke et al. 2006). Additionally, dod-6 encodes a ShK-toxin-like domain that is implicated in immunity (O'Rourke et al. 2006; Shapira et al. 2006; Troemel et al. 2006).
Several lines of evidence suggest that DAF-16 can be activated (dephosphorylated) independently of the DAF-2 signaling pathway (Hamilton et al. 2005; Hansen et al. 2005). Elimination of germ line stem cells by laser ablation or by mutation of glp-1 or mes-1 results in DAF-16 translocation to the nucleus (Lin et al. 2001) and DAF-16-dependent increased longevity (Hsin and Kenyon 1999; Arantes-Oliveira et al. 2002; Libina et al. 2003). Moreover, removal of the germ line precursor cells in daf-2 mutants further increased their longevity (Hsin and Kenyon 1999; Arantes-Oliveira et al. 2003). The ankyrin-repeat-containing protein KRI-1, acting upstream of DAF-16, is required for increased longevity in glp-1 animals (Berman and Kenyon 2006). In contrast, kri-1 does not suppress the enhanced longevity phenotype of daf-2 mutants, suggesting that DAF-2 and KRI-1 act in parallel to activate DAF-16 (Berman and Kenyon 2006). Whether these different signaling inputs regulate distinctive sets of DAF-16-dependent genes is not known.
Do all C. elegans mutants that exhibit an enhanced pathogen resistance phenotype depend on DAF-16 activity? Male C. elegans exhibit pathogen resistance toward the fungal pathogen Cryptococcus neoformans, and resistance is partly suppressed by a daf-16 mutation (van den Berg et al. 2006). The sterile mutant fer-1 is at least partially resistant to P. aeruginosa (Tan et al. 1999a) and Salmonella enterica (Aballay et al. 2000); however, it has not been demonstrated whether pathogen resistance depends on DAF-16 activity, requires other cellular-based defense mechanisms, or is simply explained by the lack of pathogen-induced matricide caused by internal hatching of embryos. It is thought that the resistance of sterile mutants to pathogens may be related to the observation that pathogen-mediated killing often coincides with retention of eggs in C. elegans hermaphrodites, resulting in internal hatching of the eggs and matricidal death (Aballay et al. 2000; O'Quinn et al. 2001; Sifri et al. 2003). Internal hatching of retained embryos results in a “bagging” phenotype because the hermaphrodite corpses fill up with hatchlings. Importantly, however, because sterile and male animals are still killed by pathogens (Tan et al. 1999a; Aballay et al. 2000; Kim et al. 2002), it appears that killing is not due solely to bagging.
In this report, we investigate DAF-16-dependent defenses against bacterial infection. A forward genetic screen for S. aureus-resistant mutants resulted in the isolation of six mutants, all of which exhibited DAF-16-dependent resistance. Two of the genes corresponding to these mutants were mapped and cloned; one corresponded to AGE-1, a component of the insulin signaling pathway, and the other to INX-14, a gap junction protein involved in oocyte maturation that is not a component of the insulin signaling pathway. All six mutants exhibited a small brood size, suggesting a correlation between sterility and pathogen resistance. Reverse genetic analysis showed that sterile mutants in general were resistant to P. aeruginosa in a DAF-16-dependent manner. Our results suggest that activation of DAF-16 in sterile mutants is distinct from DAF-2 and the germ line/stem cell-based regulation of DAF-16. In addition, the timing of the DAF-16-dependent defense response coincides with the onset of embryonic development. These results suggest the existence of a previously unknown pathway that modulates DAF-16 activity and can lead to pathogen resistance in sterile mutants.
MATERIALS AND METHODS
Bacterial strains:
Bacterial strains used in this study include E. coli strain OP50 and P. aeruginosa strain PA14 cultured at 37° in Luria–Bertani (LB) broth and S. aureus strain NCTC8325 cultured in tryptic soy (TS) broth containing 5 μg/ml nalidixic acid (Sigma, St. Louis). E. coli strain HT115 carrying the RNAi vector L4440 or L4440-derived plasmids engineered to express double-stranded RNA (dsRNA) targeting the C. elegans genes were obtained courtesy of the Ahringer laboratory (Kamath et al. 2003) and maintained in LB broth containing 50 μg/ml ampicillin and 15 μg/ml tetracycline.
C. elegans strains:
All strains were cultured on nematode growth media (NGM) supplemented with E. coli OP50 as a food source as described by Brenner (1974) and maintained at 15°, unless otherwise noted. Strains used in this study include DR1572 [daf-2(e1368)III], GR1329 [daf-16(mgDf47)I], GR1309 [daf-2(e1368);daf-16(mgDf47)], CB61 [dpy-5(e61)I], CB4037 [glp-1(e2141)III], CB4108 [fog-2(q71)V], CB4856 (wild-type Hawaiian isolate), CF512 [fer-15(b26)II;fem-1(hc17)IV], JK816 [fem-3(q20)IV], TJ1052 [age-1(hx546)II], TJ356: N2; zls356 [pG30 (DAF-16∷GFP)IV], VC832 [tag-296(ok1189)I/hT2[bli-4(e937) let-?(q782) qIs48](I;III)], and N2 (wild-type Bristol isolate). FX02864 [inx-14(tm2864/+)I] and FX02593 [inx-14(tm2593/+)I] were generated by the National Bioresource Project (http://www.nbrp.jp/index.jsp) and subsequently balanced with hT2[bli-4(e937) let-?(q782) qIs48](I;III)] to create AU0210 [tm2864/hT2] and AU0211 [tm2593/hT2], respectively.
The following double mutant strains were constructed for this study using standard genetic techniques: AU0144 [daf-2(e1368);glp-1(e2141)III], AU0145 [daf-2(e1368)III;fem-3(q20)IV], AU0146[daf-2(e1368)III;fog-2(q71)V], AU0147 [daf-16(mgDF47)I;glp-1(e2141)III], AU0148 [daf-16(mgDF47)I;fem-3(q20)IV], and AU0166 [daf-16(mgDF47)I;fog-2(q71)V].
Genetic screen for C. elegans mutants with enhanced resistance to pathogens:
N2 hermaphrodites in the L4 stage (P0 generation) were mutagenized by a 6-hr exposure to 25 mm ethyl methanesulfonate (EMS) (Sigma). Progeny (the F1 generation) were collected, allowed to mature to gravid adults, and their embryos harvested using hypochlorite treatment (Epstein 1995). Embryos were allowed to hatch in M9 buffer overnight, resulting in a synchronized culture of F2 animals arrested at the L1 larval stage, which were then transferred to 10 cm NGM plates spread with E. coli OP50, and incubated for ∼55 hr at 15° until the animals reached the L4 larval stage. Worms were then eluted from the growth plates in M9 and washed three times in M9 containing ampicillin (100 μg/ml) to kill the E. coli. Washed worms were transferred to 10 cm TS agar plates spread with S. aureus and incubated at 25°. Control wild-type N2 animals were treated identically (with the exception of EMS treatment) in parallel to mutagenized animals. After 48 hr, a time at which all animals on the control plates were dead, surviving nematodes were manually transferred from the killing plates to NGM plates containing E. coli OP50 to recover putative mutants. Animals that produced offspring were retested in the S. aureus killing assay to confirm their pathogen resistance phenotype. Selected mutants were backcrossed at least three times (ag17 was backcrossed five times) to the parental wild-type N2 worms. For each round of backcrossing, ∼20 F2 animals were singled and the progeny (F3 generation) tested for resistance using the S. aureus killing assay. Lines exhibiting a strong resistance phenotype were selected for at least three additional rounds of backcrossing.
SNP-based mapping of ag12:
To identify the chromosomal location of the genetic lesions responsible for the resistance phenotype in the ag12 mutant, a single nucleotide polymorphism (SNP)-based mapping strategy similar to the method described by Davis et al. (2005) was employed using the C. elegans Hawaiian strain CB4856. C. elegans Hawaiian males were mated to ag12 hermaphrodites and the resulting F2 population was tested for their resistance against S. aureus. Resistant animals were then genotyped using three defined SNPs per chromosome for all six chromosomes and the linkage patterns analyzed. ag12 showed strong linkage to the central region of chromosome II, where age-1 is located.
Mapping of ag17:
Using a SNP-based mapping strategy described above, the F2 population generated from a cross between ag17 and CB4856 was tested for its resistance against P. aeruginosa and both wild-type and enhanced resistance animals were genotyped with SNPs. P. aeruginosa was used for mapping because CB4856 showed a slightly enhanced resistance toward S. aureus compared to wild-type N2. A large number of F2 worms were generated by multiple rounds of crosses and were examined individually for both their genotypes and phenotypes. ag17 showed strong linkage to the central region of chromosome I within a 0.25-MU interval bounded by SNP markers F21C3 and H15M21. Of 61 genes that were found within this region according to WormBase (Harris et al. 2003), 50 RNAi clones were available in the Ahringer library (Kamath et al. 2003) and were analyzed for their role in pathogen resistance. Nine RNAi clones (F21C3.5, F52A8.5, F07A5.1, T28F4.1, C26C6.1, C26C6.2, C26C6.5, T25G3.3, and D2030.3) caused pathogen resistance phenotype in wild-type animals; however, four of the nine RNAi clones also exhibited obvious anatomical defects and were not analyzed further. The five candidate genes were sequenced as described below.
Complementation analysis:
Male age-1(hx546) nematodes were mated with ag12 or wild-type hermaphrodites, and male ag12 or wild-type nematodes were mated with age-1(hx546) hermaphrodites. The F1 progeny resulting from each cross were tested for their resistance phenotype using the S. aureus killing assay.
Similarly, complementation analyses were used to confirm that the mutation in ag17 was in inx-14 by mating male animals of ag17 with AU0210 or AU0211. The F1 progeny resulting from the crosses were tested for their resistance to P. aeruginosa.
To complement the inx-14 mutation in ag17, the cosmid F07A5, obtained from Sanger Institute (Cambridge, UK), or a PCR product containing a full-length copy of inx-14 (5–10 ng/μl) was injected into ag17 or wild-type animals as described by Mello and Fire (1995). The primers inx-14_1A (5′-GTCTGTCACTCTCTAACTATCTACAC-3′) and inx-14_4R (5′-GAAGACGACATCTCCGAGTTG-3′) were used to amplify a 9.9-kb PCR product containing inx-14 from C. elegans N2 genomic DNA. The co-injection marker pTG96 encoding sur-5∷GFP (Yochem et al. 1998) (10 ng/μl) was used as a control.
RNA interference:
All the RNAi clones used in this study were from the Ahringer library (Kamath et al. 2003), except for RNAi clones corresponding to fem-3 and fog-2 (Rual et al. 2004) and daf-2 (Dillin et al. 2002). All of the RNAi constructs that contributed to data presented in figures were verified by DNA sequence analysis. Each RNAi clone in E. coli strain HT115 was grown overnight at 37° in LB with 50 μg/ml ampicillin and 15 μg/ml tetracycline and seeded onto RNAi agar plates containing 10 μg/ml carbenicillin and 5 mm isopropylthiogalactosidase. RNAi clones were incubated overnight at room temperature to induce dsRNA expression. Two gravid adult epr mutants were transferred to each plate in at least triplicate and incubated at 15° to allow them to lay eggs. When sterile mutants were fed with RNAi clones, they were raised at the permissive temperature and then allowed to lay eggs at 15° for ∼48 hr or at 20° for ∼16 hr then moved to 25°. After feeding on RNAi plates, L4-stage animals were transferred for pathogen infection assays. Worms grown under the same conditions on E. coli HT115 expressing dsRNA targeting unc-22 were included as a positive control to confirm the efficacy of RNAi. Animals grown on E. coli harboring the empty L4440 vector were used as a negative control (Fire et al. 1998).
Sequencing of the age-1 and inx-14 genes:
From the genomic DNA isolated from adult wild-type C. elegans or ag12 animals, the age-1 gene was amplified using the primers age-1F (5′-ATGCATGTTAACATTTTACATC-3′) and age-1R (5′-TCAGTAGTGTTTGACTGC-3′). From the resulting PCR product, each exon of the age-1 PCR product in the wild-type and mutant genomic DNA was sequenced in both the forward and reverse direction and analyzed using the Vector NTI Suite 7 software package (InforMax, Bethesda, MD). The sequences obtained from wild-type and ag12 DNA were compared to the published sequence in WormBase (Harris et al. 2003). Sequence alignments of homologous Ras-binding domains (RBDs) from other PI3 kinases were obtained from the Conserved Domain Database (Marchler-Bauer et al. 2005).
A similar method was used to sequence the 5 candidate genes (C26C6.1, C26C6.2, F07A5.1, F52A8.5, and T28F4.1) corresponding to ag17 that had been identified by RNAi analysis in both wild type and ag17. The primers used to amplify the region where the mutation was found were inx-14_4F (5′-GATATAAATTGAATGACACTGAT-3′) and INX-14_8R (5′-CTTTGTGAAATTATGGTGTACTG-3′).
C. elegans growth conditions for pathogen infection assays and for quantitative RT–PCR:
For pathogenicity assays, epr mutants were maintained at 15°, unless otherwise noted, until the L4 larval stage. Temperature-sensitive sterile mutants were allowed to lay eggs and grow at 20° for ∼18 hr and were then shifted to 25° until the L4 larval stage. daf-2 animals were allowed to grow until the L3 larval stage at 20° and were then shifted to 25°. For qRT–PCR, the growth stage of animals was synchronized by hypochlorite treatment (Epstein 1995). About 3000 animals arrested at the L1 larval stage were plated on 10-cm NGM plates seeded with OP50 and grown at 15° for 48 hr then moved to 25° for 24 hr to the young adult stage before harvesting for RNA extraction. For RNA extraction from adults, animals were prepared and grown until the L4 larval stage as described above, then transferred onto fresh 10-cm NGM plates seeded with OP50 for another 26 hr at 25° before harvesting. For RNA extraction from 4-day-old adults, animals were transferred onto fresh 10-cm NGM plates seeded with OP50 twice a day for four consecutive days after the L4 larval stage at 25° before harvesting. These transfers were done to separate adults from their progeny. Temperature-sensitive sterile mutants were monitored to ensure complete sterility when used for either infection assays or qRT–PCR.
Pathogen infection assays:
C. elegans killing assays were performed as previously described for S. aureus strain NCTC8325 (Sifri et al. 2003) and P. aeruginosa strain PA14 (Tan et al. 1999a). Briefly, a saturated culture of S. aureus was diluted 1:5 in TS broth containing 5 μg/ml nalidixic acid. Ten microliters of diluted culture was plated on TS agar plates containing 5 μg/ml nalidixic acid, and incubated for 3 hr at 37°. Five microliters of saturated cultures of P. aeruginosa PA14 in LB was plated on modified NGM plates (Tan et al. 1999a) and incubated for 24 hr at 37° followed by another incubation at room temperature for ∼24 hr. For the pathogen assays in the presence of 5-fluorodeoxyuridine (FUDR), FUDR was added to the edge of the lawn to give a final concentration of 0.1 mg/ml and dried before the animals were placed on the plates. Killing assays were carried out in triplicate and performed by manually transferring ∼30 L4-staged animals from E. coli OP50 plates to pathogen plates (t = 0). Worm mortality was monitored over time, and nematodes were considered dead when they failed to respond to head tapping with a platinum wire. For pathogen assays involving FUDR pretreatment, animals were transferred at the L4 larval stage to plates containing OP50 with 0.1 mg/ml FUDR for 24 hr at 25°. Subsequently, pathogen infections were performed as described above without FUDR. For the pathogen assays that involved mated fer-15;fem-1 animals, fer-15;fem-1 was grown at the nonpermissive temperature until the L4 larval stage and then mated with wild-type male animals at 25° for 24 hr on OP50. Subsequently, mated fer-15;fem-1 animals were transferred onto plates containing P. aeruginosa and tested for survival.
Longevity assays:
Longevity assays were performed as previously described (Wolkow et al. 2000). About 30 L4 hermaphrodites that had been raised at 15° were transferred to each of three NGM plates containing 0.1 mg/ml FUDR, seeded with E. coli OP50, and incubated at 25°. The life span was defined as the length of time from when animals were put down on the plates at the L4 larval stage (t = 0) until they were scored as dead.
Brood size measurements:
Animals were grown at 15° until the L4 larval stage then singled onto plates seeded with OP50 and incubated at 25° to allow them to lay eggs. Adult animals were transferred once a day for 3 consecutive days to fresh plates. The total number of progeny that grew up from a single animal was counted. At least eight animals were used for the analysis for each strain.
DAF-16 nuclear localization assays:
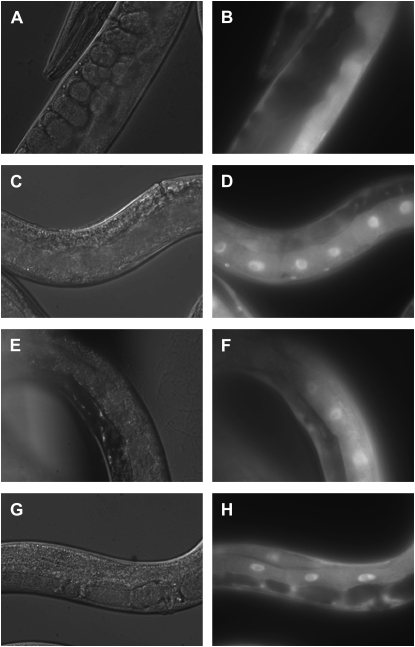
A synchronous culture of TJ356 animals expressing DAF-16∷GFP was seeded onto relevant RNAi plates at 20° for one or two generations as necessary to induce sterility. inx-8 RNAi caused complete sterility in animals in the first generation. fem-3, glp-1, inx-9, and inx-14 RNAi caused complete sterility in the majority of animals in the second generation. fem-1, fer-1, and fog-2 RNAi caused complete sterility only in a small number of animals. On day 1 of adulthood, DAF-16 nuclear translocation in intestinal cells was analyzed using an Axioplan 2 fluorescent microscope and processed using Openlab 4.0.3 (Improvision, Coventry, UK) and Photoshop CS2 9.0.2 (Adobe, San Jose, CA) software. Except for the animals treated with control RNAi, only completely sterile animals were analyzed as shown in Figure 7 and supplemental Figure S3 at http://www.genetics.org/supplemental/.
Figure 7.—
DAF-16∷GFP is localized to the nucleus in animals treated with RNAi clones that cause sterility. Shown are representative images of DAF-16∷GFP protein in intestinal cells of TJ356 animals fed with various RNAi clones including L4440 vector control (A and B), glp-1 (C and D), inx-14 (E and F), or fog-2 (G and H). Differential interference contrast images are in A, C, E, and G and GFP images are in B, D, F, and H. Only fully sterile animals were analyzed for DAF-16∷GFP.
RNA extraction and qRT–PCR:
Both RNA extraction and qRT–PCR were performed as described (Troemel et al. 2006). Briefly, animals were harvested from OP50 plates by washing once with M9 buffer and then total RNA was extracted using TRI Reagent (Molecular Research Center, http://mrcgene.com) according to the manufacturer's protocol. The RNA was reverse transcribed to make cDNA using the Retroscript kit (Ambion, Austin, TX), and the resulting cDNA was subjected to qRT–PCR analysis using SYBR green detection on an iCycler machine (Bio-Rad, http://bio-rad.com). Primers for qRT–PCR for sod-3, mtl-1, and lys-7 have been described (Troemel et al. 2006). Other primers were manually designed, and their specificity was tested against the cDNA and their efficiency was calculated using a serially diluted cDNA template. snb-1 was used as a control gene because expression of snb-1 was very similar among wild-type and different mutant backgrounds used in this study. All the values were normalized to snb-1, and then each value was expressed in fold change compared to that of wild type. Fold change was calculated according to the Pfaffl method (Pfaffl 2001). Primer sequences are listed in supplemental Table 2 at http://www.genetics.org/supplemental/).
Statistical analysis:
Each survival and longevity curve is based on data from 60 to 100 animals. Curves were analyzed by calculating the TD50 (the time for 50% of the nematodes to die) using a nonlinear regression analysis (GraphPad Prism, version 4.0). The significance of the differences between different survival assays was assessed using a log-rank test (GraphPad Prism, version 4.0).
RESULTS
A forward genetic screen for mutants with enhanced resistance to pathogens:
Mutations in the daf-2 or age-1 genes in the C. elegans insulin-like signaling pathway confer DAF-16-dependent resistance to both Gram-negative and Gram-positive bacterial pathogens (Garsin et al. 2003), demonstrating that the immune response of C. elegans can be genetically enhanced. To identify additional genes that when mutated result in enhanced resistance to killing by bacterial pathogens, we undertook a forward genetic screen for mutants with an enhanced pathogen resistance (Epr) phenotype.
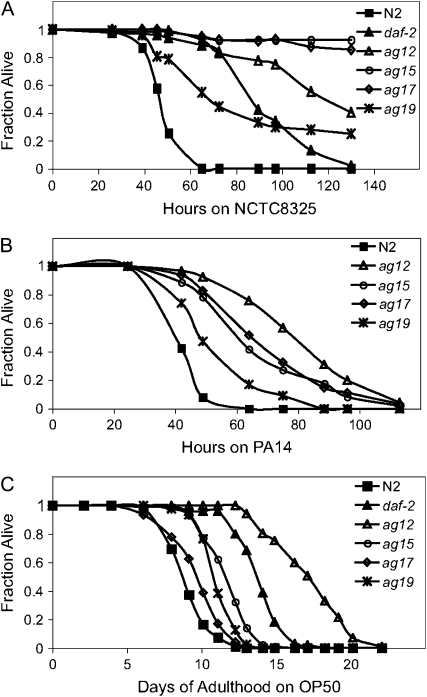
EMS-mutagenized L4 larval stage N2 animals were transferred from their normal laboratory food E. coli strain OP50 to the Gram-positive human pathogen, S. aureus strain NCTC8325. This strain of S. aureus kills essentially 100% of C. elegans wild-type animals within 48 hr and prevents progeny from maturing past the L1 larval stage (Sifri et al. 2003), thereby facilitating the identification of pathogen-resistant mutants without developing progeny obscuring the assay. From 33,000 genomes screened, 319 putative epr mutants were identified in the primary screen on the basis that they appeared healthy after 48 hr of exposure to S. aureus. Of these 319 putative mutants, 110 produced broods when transferred to E. coli. After retesting, 39 of the 110 fertile putative epr mutants reproducibly exhibited enhanced resistance to S. aureus. Among these 39 mutants, 6 mutants with the strongest resistance to S. aureus were further characterized. When these 6 mutants (ag12, ag13, ag14, ag15, ag17, and ag19) were mated with each other in all combinations, the pathogen resistance phenotype was complemented in all cases (data not shown), indicating that the 6 mutations fell into six different complementation groups. These 6 mutants exhibited various degrees of resistance to S. aureus after being backcrossed at least three times to wild-type animals. The resistance phenotypes of ag12, ag15, ag17, and ag19 are shown in Figure 1A and supplemental Table 1 at http://www.genetics.org/supplemental/). The resistance phenotypes of ag13 and ag14 are shown in supplemental Figure S1A.
Figure 1.—
C. elegans epr mutants exhibit enhanced resistance to both S. aureus and P. aeruginosa and various degrees of enhanced longevity on E. coli. L4 larval-stage epr mutants, ag12, ag15, ag17, and ag19, were transferred from E. coli OP50 plates onto plates containing S. aureus NCTC8325 (A), P. aeruginosa PA14 (B), and OP50 (C) and monitored for their survival over time at 25°. The time at which 50% of the worms had died (TD50) for this assay for each epr mutant is shown in supplemental Table 1. daf-2(e1368) was included as positive controls (A and C). The experiment was repeated at least three times with similar results.
All six mutants also exhibited enhanced resistance to the Gram-negative bacterial pathogen P. aeruginosa (Figure 1B; supplemental Figure S1C and supplemental Table 1 at http://www.genetics.org/supplemental/). Unless otherwise noted, further characterization of the epr mutants was carried out using P. aeruginosa because it elicits less matricidal death (bagging) than S. aureus and because P. aeruginosa is still infectious in the presence of FUDR, unlike S. aureus (J. Irazoqui, personal communication).
Mutants in the daf-2 insulin signaling pathway exhibit both enhanced longevity and enhanced pathogen resistance, both of which can be completely suppressed by a mutation in daf-16 (Garsin et al. 2003; Kenyon et al. 1993). To determine whether any of the six pathogen-resistant mutants carried defects in the insulin signaling pathway components, we examined their longevity on E. coli OP50 (Figure 1C; supplemental Figure S1B and supplemental Table 1) and determined whether DAF-16 was required for their enhanced resistance to P. aeruginosa (Figure 2, A and B, and supplemental Figure S1C). One mutant, ag12, exhibited significantly increased longevity on OP50 (Figure 1C and supplemental Table 1). daf-16 RNAi completely suppressed the enhanced pathogen resistance phenotype of ag12 (Figure 2A). ag12 also exhibited a dauer-constitutive phenotype at 25° (data not shown). All three of these phenotypes are characteristic of mutations in the daf-2 signaling pathway, suggesting that ag12 might encode a component of the daf-2 signaling pathway. There was a modest increase in longevity of two other epr mutants, ag15 and ag19, on OP50. In contrast, there was no increase in longevity of ag14 (supplemental Figure S1B). The longevity on OP50 of the remaining two mutants, ag13 and ag17, while statistically greater than wild type, was only marginally increased (Figure 1C and supplemental Figure S1B). Furthermore, none of these latter five mutants became dauer at 25° (data not shown), suggesting that they did not encode components of the daf-2 signaling pathway. Unexpectedly however, the enhanced resistance to P. aeruginosa in all of these latter five mutants was suppressed by daf-16 RNAi (Figure 2, A and B, and supplemental Figure S1C).
Figure 2.—
epr mutants require DAF-16 for pathogen resistance. epr mutants, ag12 and ag15 (A), ag17 and ag19 (B), were fed with either a vector control or daf-16 RNAi and then transferred to P. aeruginosa PA14 plates. The differences in survival kinetics between wild-type N2 and epr mutants (ag12, ag15, ag17, and ag19) treated with the control RNAi were significant (P < 0.0001). The differences in survival kinetics between the epr mutants treated with control RNAi and daf-16 RNAi were also significant (P < 0.0001). Experiments with ag12 and ag17 were repeated three times and experiments with ag15 and ag19 were repeated twice with similar results.
ag12 has a mutation in age-1:
Using a SNP-based mapping strategy (Davis et al. 2005), we mapped ag12 to the central region of chromosome II (see materials and methods for details). The insulin signaling pathway gene, age-1, is located in this region (Morris et al. 1996) and the age-1(hx546) mutation failed to complement ag12 for S. aureus susceptibility and longevity on OP50 (data not shown). Sequencing of PCR products corresponding to the exons of the age-1 gene in ag12 genomic DNA revealed a single point mutation in exon 3 resulting in a cytosine to thymine transition (see materials and methods for details). This mutation results in the substitution of a highly conserved leucine residue with phenylalanine in the RBD of the AGE-1 protein (Figure 3). Thus, consistent with its dauer and longevity phenotypes, ag12 appears to be defective in a component of the daf-2 signaling pathway, namely the PI3 kinase, age-1.
Figure 3.—
ag12 harbors a point mutation in the RBD of the PI(3)K AGE-1. Protein alignment of the RBDs of PI(3)Ks among different species including C. elegans, which is shown at bottom. The conserved leucine residue, which is mutated to phenylalanine in ag12, is indicated by an arrow.
ag17 has a mutation in inx-14:
Because five of the epr mutants exhibited substantially shorter longevity on OP50 than daf-2 or age-1 mutants (Figure 1C) and because they did not become dauer at elevated temperatures, it did not seem likely that they carried mutations in components of the DAF-2 insulin signaling pathway. However, similar to daf-2 and age-1 animals, these five epr mutants exhibited enhanced resistance to P. aeruginosa in a DAF-16-dependent manner (Figure 2, A and B, and supplemental Figure S1C), suggesting additional mechanisms to activate DAF-16 to exert a pathogen resistance phenotype in addition to the canonical DAF-2 insulin signaling pathway. To further investigate this possibility, we chose ag17 for additional analysis because its longevity on OP50 was only marginally increased compared to that of wild type (Figure 1C) and because it exhibited a very high level of resistance to S. aureus-mediated killing (Figure 1A).
As described in detail in materials and methods, SNP-based mapping showed strong linkage of ag17 to the central region of chromosome I within a 0.25-MU interval bounded by SNP markers F21C3 and H15M21 (data not shown). This map position was confirmed by showing that ag17 is tightly linked to dpy-5, which also maps to the central region of chromosome I [in a cross between ag17 and the semidominant dpy-5(e61) mutant, 100% of F2 Dpy animals had progeny with wild-type resistance, while 90% of F2 non-Dpy had progeny with enhanced resistance to pathogens; data not shown].
Perusal of WormBase (http://www.wormbase.org/ release WS176, June 19, 2007) showed 61 predicted genes in the 0.25-MU interval on chromosome I where ag17 maps. A combination of RNAi feeding analysis and sequencing of five candidate genes identified a single point mutation in exon 5 of the inx-14 gene in ag17 resulting in a guanine-to-adenine transition. INX-14 is a predicted member of the innexin family of proteins, which have been shown to form gap junctions that are important for cell–cell communication (reviewed by Phelan (2005). INX-14 in particular is thought to form sheath/oocyte gap junctions involved in oocyte maturation and fertilization (Whitten and Miller 2007). The identified mutation in ag17 causes an arginine-to-histidine substitution in inx-14 near the end of the predicted third transmembrane domain (Bairoch and Apweiler 2000).
To confirm that the arginine-to-histidine mutation in inx-14 was responsible for the enhanced pathogen phenotype of ag17, we obtained two additional alleles of inx-14, tm2864 and tm2593, from the National Bioresource Project (http://www.shigen.nig.ac.jp) for complementation analysis. Because both the tm2864 and tm2593 alleles cause sterility when homozygous, the mutations were first balanced with hT2 marked with GFP and then crossed into ag17. F1 cross progeny that inherited the hT2 balancer chromosome did not show the pathogen resistance phenotype of ag17 (data not shown). In contrast, F1 progeny lacking the balancer and therefore heterozygous for ag17 and an inx-14 deletion were resistant to pathogens (data not shown), indicating that inx-14 failed to complement ag17 and suggesting that the mutation in ag17 is an allele of inx-14. These trans-heterozygotes exhibited complete sterility and a stronger Epr phenotype relative to ag17 homozygotes (data not shown), suggesting that the mutation in ag17 is most likely not null.
We also attempted to rescue the phenotype of ag17 with a wild-type genomic copy of inx-14. Extrachromosomal expression of inx-14 in transgenic animals carrying either the cosmid F07A5 or a PCR product corresponding to the full-length inx-14 gene resulted in partial complementation of the pathogen resistance phenotype of ag17 (supplemental Figure S2 and data not shown). The fact that the pathogen resistance of ag17 was only partially complemented in these experiments is most likely due to the fact that INX-14 is expressed in the germ line (Govindan et al. 2006) and transgenes are generally expressed at low levels in the germ line (Dernburg et al. 2000). Nevertheless, the data presented in this section support the conclusion that the pathogen-resistant phenotype of ag17 is due to a mutation in inx-14. From here on, ag17 is referred to as inx-14(ag17).
Twenty-five genes make up the innexin family of proteins in C. elegans (Starich et al. 2001). To determine whether any other innexin proteins are also involved in pathogen resistance, we carried out RNAi feeding analysis of all of the innexin genes except for inx-2, inx-21, and inx-22 (for which RNAi constructs were not available in the Ahringer RNAi library). Among the 22 genes examined, inx-8, inx-9, and inx-14 RNAi clones caused resistance to P. aeruginosa (data not shown). We also observed that these three RNAi clones resulted in sterility (data not shown). In this study we use the term “sterility” to refer to the phenotype of animals that do not produce viable progeny, without implying a molecular mechanism leading to no brood or a small brood size.
Sterility is a common phenotype of pathogen resistance mutants:
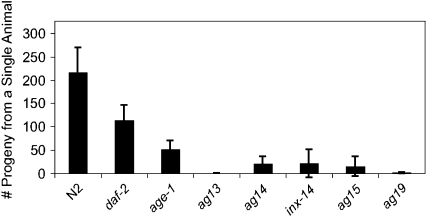
The screen for epr mutants identified inx-14(ag17), which is not a component of the canonical insulin signaling pathway, although its pathogen resistance depends on DAF-16 (Figure 2B). Importantly, inx-14(ag17) exhibited a partially penetrant sterility phenotype leading to a smaller brood size at 25°, which is the temperature at which the pathogen infection assays were performed. Because INX-14 is required to regulate oocyte maturation and sperm recruitment to the spermatheca, the site of fertilization (Whitten and Miller 2007), fertilization may be disrupted in inx-14 mutants. Moreover, in the process of mapping inx-14(ag17), an unexpectedly high number of RNAi clones were found to cause pathogen resistance (9 of 50) and these mostly coincided with sterility (8 of 9). Finally, as reported above, RNAi against inx-8 and inx-9 in addition to inx-14 resulted in both resistance to P. aeruginosa and in sterility. To examine whether the other epr mutants also have a temperature-sensitive sterility phenotype, we compared the brood size of each mutant to that of wild-type animals at 25°. Similar to inx-14(ag17), the other five epr mutants also exhibited a much smaller brood size than wild-type animals (Figure 4).
Figure 4.—
epr mutants have a small brood size at 25°. N2, daf-2(e1368), age-1(ag12), ag13, ag14, inx-14(ag17), ag15, and ag19 were cultured at 15° and then transferred at the L4 larval stage to 25°. The total number of progeny that hatched from a single animal was counted. The average of more than eight animals for each genotype is shown. Error bars represent standard deviation.
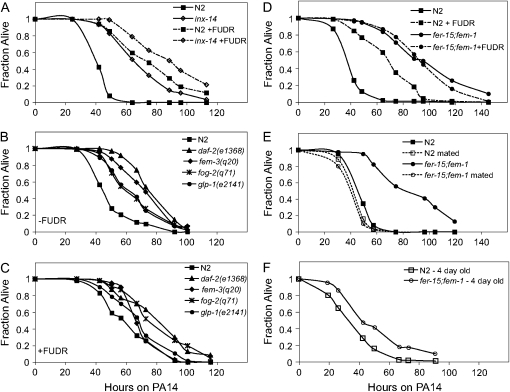
To test the hypothesis that partially sterile mutants are pathogen resistant because of a lower frequency of internal progeny hatching, we examined inx-14(ag17) pathogen resistance in the presence of FUDR, which prevents the production of viable eggs. Overall, both wild-type and inx-14(ag17) animals died more slowly when feeding on P. aeruginosa in the presence of FUDR; however, inx-14(ag17) animals were still more resistant to P. aeruginosa than wild-type animals on FUDR (Figure 5A). Similarly, when daf-2(e1368) was compared to wild type in the presence of FUDR, daf-2(e1368) exhibited enhanced resistance to P. aeruginosa (Figure 5C). These data suggest that the DAF-16-dependent enhanced pathogen resistance phenotype of daf-2(e1368) and inx-14(ag17) is not simply a consequence of a reduced level of matricidal hatching of progeny.
Figure 5.—
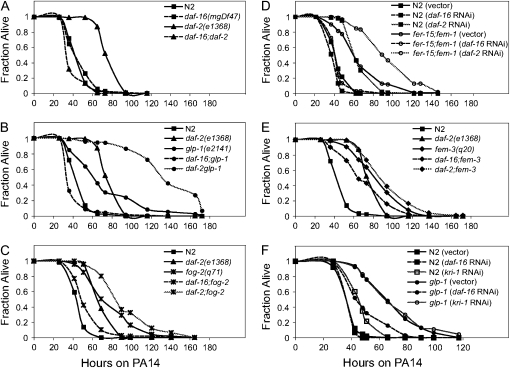
inx-14(ag17) mutants and sterile mutants are more resistant than wild-type animals in the presence and absence of FUDR (A–D). N2 and inx-14(ag17) animals were transferred at the L4 larval stage onto plates preseeded with P. aeruginosa PA14 with and without FUDR (A). inx-14(ag17) exhibited enhanced pathogen resistance compared to wild type in the absence of FUDR (P < 0.0001) and in the presence of FUDR (P < 0.005). glp-1(e2141), fog-2(q71), and fem-3(q20) were grown at the nonpermissive temperature of 25° and then transferred onto plates preseeded with PA14 in the absence (B) or presence (C) of FUDR. Compared to wild-type N2, daf-2(e1368), glp-1(e2141), fog-2(q71), and fem-3(q20) exhibited significantly enhanced pathogen resistance in the absence (P < 0.0001) or presence of FUDR (P < 0.05). N2 and fer-15;fem-1 were pretreated or untreated with FUDR prior to infection with PA14 in the absence of FUDR (D). FUDR-pretreated fer-15;fem-1 exhibited enhanced pathogen resistance (P < 0.0001) compared to FUDR-pretreated wild-type N2. N2 and fer-15;fem-1 were mated with wild-type males prior to infection with PA14 (E). Mated fer-15;fem-1 animals no longer exhibited enhanced pathogen resistance (P = 0.2961) compared to mated N2. Four-day-old adults of N2 and fer-15;fem-1 were infected with PA14 (F). The difference in pathogen resistance phenotype between N2 and fer-15;fem-1 was much smaller in 4-day-old adults than in 1-day-old adults (E). Similar results were observed in two independent experiments for inx-14(ag17) and three independent experiments for the other sterile mutants.
Sterility activates a signaling pathway(s) to enhance resistance to P. aeruginosa infection through DAF-16 activity:
All of the epr mutants that were examined in detail exhibited partially sterile phenotypes as well as DAF-16-dependent pathogen resistance. Therefore, we examined a variety of previously characterized sterile mutants to determine whether they also exhibit Epr phenotypes that could be suppressed by a daf-16 mutation.
glp-1 is a sterile mutant that lacks mitotic germ-line stem cells (and thus the entire germ line) and exhibits DAF-16-dependent increased longevity on OP50 (Arantes-Oliveira et al. 2002). In comparison, fog-2, fer-15;fem-1, and fem-3 are sterile mutants that have mitotic germ cells, but they differentiate into only oocytes (fog-2), oocytes with dysfunctional sperm (fer-15;fem-1), or only sperm (fem-3gf) (and thus produce unfertilized embryos) (Barton et al. 1987; Schedl and Kimble 1988; McCarroll et al. 2004). fem-3, fog-2, and glp-1 animals all showed enhanced pathogen resistance either in the absence (Figure 5B) or presence (Figure 5C) of FUDR compared to wild-type animals.
One problem in interpreting the results of pathogen-mediated killing assays carried out in the presence of FUDR is that FUDR could be affecting the virulence of the pathogen as well as the fertility of C. elegans. To circumvent this problem, we exposed animals to FUDR at the L4 larval stage for 24 hr and then transferred the animals to lawns of P. aeruginosa PA14. This FUDR pretreatment was sufficient to maintain the sterility of wild-type animals throughout the course of the infection assay (data not shown). Wild-type animals pretreated with FUDR survived longer on P. aeruginosa compared to wild-type animals without the FUDR pretreatment (Figure 5D), exhibiting similar killing kinetics as animals treated continuously with FUDR (Figure 5, A and C). This result indicated that FUDR affected the sterility of C. elegans rather than the virulence of P. aeruginosa. Moreover, fer-15;fem-1 animals pretreated with FUDR were significantly more resistant to P. aeruginosa than wild-type animals pretreated with FUDR (Figure 5D). This result demonstrates that fer-15;fem-1 animals, like the other sterile mutants examined in Figure 5, B and C, are more resistant to killing than wild-type animals even in the absence of bagging.
Finally, as shown in Figure 6, removal of DAF-16 in sterile mutants, either with RNAi or by mutation, resulted in a striking suppression of the Epr phenotype. This suppression was complete for glp-1, fer-15;fem-1, and fog-2 mutants (Figure 6, B–D) and partial for fem-3gf (Figure 6E). The effects of DAF-16 appeared to be specific to the Epr phenotype, as elimination of DAF-16 function did not suppress the sterility of any of the mutants tested (data not shown).
Figure 6.—
daf-16 is required for the pathogen resistance of sterile mutants. The survival kinetics of N2 were compared to those of daf-16 and daf-2 upon P. aeruginosa PA14 infection (A). Similarly, the survival kinetics of the following sterile mutants were compared to their respective daf-16 and daf-2 mutants; glp-1 to daf-16;glp-1 and daf-2 glp-1 (B), fog-2 to daf-16;fog-2 and daf-2;fog-2 (C), fer-15;fem-1 with control RNAi to fer-15;fem-1 with daf-16 RNAi and with daf-2 RNAi (D), and fem-3 to daf-16;fem-3 and daf-2;fem-3 (E). In wild-type N2, a daf-16(mgDf47) mutation caused no significant change in pathogen resistance (P = 0.6281), whereas a daf-2(e1368) mutation enhanced the pathogen resistance significantly (P < 0.0001). In glp-1(e2141), fog-2(q71), fer-15;fem-1, and fem-3(q20) animals, either the daf-16(mgDf47) mutation or daf-16 RNAi suppressed the pathogen resistance phenotype of the respective sterile mutants (P < 0.0001). daf-2(e1368) or daf-2 RNAi significantly increased the pathogen resistance phenotype of the respective sterile mutants (P < 0.05). Similar results were observed in three independent experiments with glp-1(e2141), fer-15;fem-1, and fem-3(q20) and two independent experiments with fog-2(q71). N2 and glp-1(e2141) animals were fed either with vector control RNAi, daf-16 RNAi, or kri-1 RNAi and then tested for PA14 resistance (F). The differences between glp-1(e2141) treated with control RNAi and with daf-16 RNAi were significant (P < 0.0001). The differences between glp-1(e2141) treated with control RNAi and with kri-1 RNAi were not significant (P = 0.3990). kri-1 RNAi in glp-1 animals appeared to be effective because 100% of glp-1 animals (n = 94) treated with kri-1 RNAi appeared paler and smaller compared to the glp-1 animals treated with the control RNAi, as previously reported (Berman and Kenyon 2006). This experiment was repeated three times with similar results.
The results in this section show that the pathogen resistance of sterile mutants appears to rely on DAF-16 and is not simply a consequence of reduced internal hatching of progeny. These results are similar to the effects of the germ line on life span; removal of the germ line increases life span in a DAF-16-dependent manner. However, unlike the effects on life span, which are only observed in animals lacking germ-line stem cells, the effects on pathogen resistance are also observed in sterile animals that have stem cells, such as fog-2 mutants. The main difference between these sterile animals and wild-type animals is the presence of developing embryos.
Sterility causes DAF-16 nuclear translocation:
The results described in the previous section demonstrated that a variety of sterile mutants exhibit a DAF-16-dependent Epr phenotype. These findings prompted us to determine whether DAF-16 is translocated into the nucleus in these sterile mutants. Previously, ablation of germ cells (Lin et al. 2001) or mutation of glp-1 (Berman and Kenyon 2006) had been shown to cause DAF-16 nuclear translocation. We used RNAi to examine DAF-16 nuclear translocation in a variety of sterile mutants, including those that contain germ cells. We performed feeding RNAi targeting fer-1, fem-1, fem-3, fog-2, glp-1, inx-8, inx-9, or inx-14 and then examined DAF-16 nuclear translocation in strain TJ356 (carrying a DAF-16∷GFP). As shown in Figure 7, all RNAi constructs tested caused DAF-16 nuclear translocation in sterile adult animals (Figure 7, supplemental Figure S3 at http://www.genetics.org/supplemental/), and data not shown).
KRI-1 is not required for pathogen resistance of glp-1 mutants:
Animals lacking germ-line stem cells such as glp-1 mutants exhibit DAF-16-dependent life-span extension when feeding on E. coli OP50 (Arantes-Oliveira et al. 2002). It is thought that germ-line cells signal through the KRI-1 protein to mediate DAF-16 nuclear translocation in intestinal cells (Berman and Kenyon 2006). The role of KRI-1 is specific to germ-line-mediated enhanced longevity since a mutation in kri-1 only suppresses the longevity phenotype of glp-1 but not of daf-2 mutants (Berman and Kenyon 2006). Our analysis of the pathogen resistance of sterile mutants suggested that KRI-1 is not involved in conferring resistance in glp-1 animals since all sterile mutants tested were pathogen resistant, not just those lacking germ-line stem cells. Consistent with this reasoning, we found that KRI-1 was not required for the enhanced resistance of glp-1 (Figure 6F) or fer-15;fem-1 (data not shown).
A daf-2 mutation can enhance the pathogen resistance of sterile animals:
To determine whether the enhanced pathogen resistance of glp-1, fog-2, fem-3, and fer-15;fem-1 mutants was additive with daf-2 pathogen resistance, we examined the pathogen resistance of double mutants that were both sterile and defective in daf-2. As shown in Figure 6, B–E, all double mutants exhibited more enhanced pathogen resistance than single mutants alone. These data are consistent with a model in which sterility and daf-2 independently upregulate pathogen resistance. However, since daf-2(e1368) is not a null mutation, it is formally possible that daf-2 could act in the same pathway as the sterile mutations to confer pathogen resistance. But the fact that the daf-2(e1368) mutation can enhance the resistance of fully sterile mutants confirms that daf-2 pathogen resistance is not just a consequence of its slightly smaller brood size (Figure 4).
DAF-16-dependent genes are upregulated in sterile adult animals:
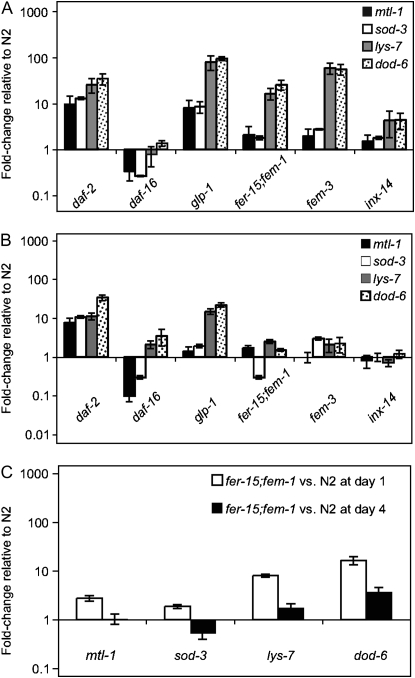
Previous transcriptional profiling studies identified a variety of genes that are expressed in a DAF-16-dependent manner in animals treated with daf-2 RNAi (Murphy et al. 2003) or in a daf-2 mutant (McElwee et al. 2003). We reasoned that if the pathogen resistance phenotype of most of the sterile mutants was a consequence of activated DAF-16, as suggested by the nuclear translocation of DAF-16 in the sterile mutants (Figure 7), then these sterile mutants should exhibit a transcriptional profile similar to that of daf-2 animals. To test this hypothesis, we examined the expression of four DAF-16-dependent genes by quantitative RT–PCR that had been identified as DAF-2/DAF-16 dependent in three published transcriptional profiling studies (McElwee et al. 2003; Murphy et al. 2003; Troemel et al. 2006). We selected these four DAF-16-dependent genes because of their robust regulation by DAF-16 in other studies (mtl-1 and sod-3) (Hsu et al. 2003; Wolff et al. 2006), and because they have been implicated in immunity (lys-7 and dod-6) (Mallo et al. 2002; Murphy et al. 2003; O'Rourke et al. 2006). The expression levels of these genes were examined in four different sterile mutant backgrounds: glp-1, fer-15;fem-1, fem-3, and inx-14(ag17), as well as daf-2 and daf-16 mutants.
We first compared expression of DAF-16-dependent genes in 1-day-old adults, in which wild-type animals contain many developing embryos. As expected, all the DAF-16-dependent genes tested were more highly expressed in daf-2 mutants (Figure 8A). These four genes were either expressed at lower levels or at not substantially different levels in daf-16 animals compared to wild-type animals (presumably because DAF-16 is not very active in well-fed wild-type animals) (Figure 8A). Similar to daf-2 mutants, all four DAF-16-dependent genes, mtl-1, sod-3, dod-6, and lys-7, were expressed more highly in glp-1 mutants compared to wild-type animals (Figure 8A). Furthermore, lys-7 and dod-6 were also expressed more highly in fer-15;fem-1 and fem-3 mutants compared to wild type (Figure 8A). lys-7 and dod-6 were also more highly expressed in inx-14(ag17) adult animals; however, the levels of expression were slightly lower compared to the other sterile mutants perhaps due to the observation that the inx-14(ag17) mutation causes variable sterility (Figure 8A). In contrast, the expression of two other DAF-16-dependent genes, mtl-1 and sod-3, was not substantially different in fer-15;fem-1, fem-3, or inx-14(ag17) mutants compared to wild-type animals (Figure 8A).
Figure 8.—
DAF-16-dependent genes are upregulated in adult sterile mutants. The expression of four DAF-16-dependent genes, mtl-1, sod-3, lys-7, and dod-6, in gravid adult animals (A) or young adult animals without embryos (B) was determined by qRT–PCR and compared to that of wild-type N2. Mutants analyzed were daf-2(e1368), daf-16(mgDf47), glp-1(e2141), fer-15;fem-1, fem-3(q20), and inx-14(ag17). DAF-16-dependent gene expression in 4-day-old fer-15;fem-1 adults was compared to 4-day-old wild-type N2 (C). Fold changes represent the average of at least three different biological samples. Error bars represent standard error of the mean.
To test whether the difference in DAF-16-dependent gene expression observed between sterile and fertile animals was due to the presence of developing embryos, we compared DAF-16-dependent gene expression in animals at an earlier stage. We analyzed animals slightly older than the L4 larval stage, when embryos have not yet developed in wild-type animals. Consistent with the hypothesis that developing embryos suppress the expression of DAF-16-dependent genes, none of the four DAF-16-dependent genes, mtl-1, sod-3, lys-7, or dod-6 were differentially expressed in the sterile mutants fer-15;fem-1, fem-3, or inx-14(ag17) compared to wild-type animals (Figure 8B). In glp-1 mutants, lys-7 and dod-6 were more highly expressed than wild-type animals (Figure 8B). All four genes were more highly expressed in daf-2 mutants and were either expressed less or not substantially differently in daf-16 mutants compared to wild type (Figure 8B). We also examined expression in postreproductive adults in which egg laying had ceased. In these older animals, there was less difference in DAF-16-dependent gene expression between the sterile mutant fer-15;fem-1 and wild-type animals compared to what was seen in 1-day-old adults (Figure 8C).
To further test the hypothesis that the presence of embryos might reduce the pathogen resistance of sterile animals, we forced fer-15;fem-1 to produce progeny by mating them with wild-type males prior to infection. fer-15;fem-1 mutants produce defective sperm at 25° but do produce functional oocytes (McCarroll et al. 2004). Thus fer-15;fem-1 is capable of producing progeny if functional sperm is provided. When fer-15;fem-1 “females” were mated with wild-type males, viable progeny were produced (data not shown), and the mated fer-15;fem-1 animals became as susceptible to P. aeruginosa as wild-type animals (Figure 5E). This result supports the hypothesis that developing embryos suppress immunity.
As a final test of whether the presence of embryos suppresses immunity, we analyzed the Epr phenotypes of wild-type and sterile animals in the postreproductive period. When 4-day-old adults were infected with P. aeruginosa, both wild-type and fer-15;fem-1 animals died faster than L4 larval-stage animals (Figure 5F). Moreover, the difference in Epr phenotype between fer-15;fem-1 and wild type was markedly less in the older animals compared to the 1-day-old adult animals (compare Figure 5, D and F).
DISCUSSION
Reproduction and immunity:
Here we provide genetic and phenotypic evidence that suggests that embryonic development causes immune suppression in C. elegans. The following observations support a model of embryo-mediated immunosupppression. First, animals without embryos exhibit DAF-16-dependent pathogen resistance that is not simply a consequence of matricidal internal embryo hatching in wild-type animals. Second, the enhanced pathogen resistance phenotype of sterile fer-15;fem-1 mutants is completely lost when fer-15;fem-1 is induced to make embryos by mating with wild-type animals. Third, DAF-16 is located in the nucleus in sterile animals, and DAF-16-dependent immune-related genes are expressed at higher levels in sterile animals relative to wild-type animals carrying embryos. Fourth, the enhanced pathogen resistance (and DAF-16-dependent gene activation) seen in sterile fer-15;fem-1 mutants compared to fertile wild-type animals is greatly reduced when comparing these strains during their postreproductive period. It is important to note that our data do not provide any information concerning the stage(s) of embryogenesis at which the putative immunoregulatory signals are generated. Indeed, it is possible that the signal is not generated by the embryo per se, but rather by adult tissues that are affected by the presence of embryos. Analysis of mutants defective in different stages of embryonic development may confirm the existence of a signal that is produced during embryogenesis to downregulate a DAF-16-dependent immune pathway.
The results reported here are most consistent with a model in which embryo-mediated immunosuppression involves the inhibition of DAF-16 activity. However, we cannot rule out the possibility that a DAF-16-independent mechanism targeting the same effector genes as DAF-16 could be involved in mediating pathogen resistance of sterile mutants. For example, removing DAF-16 left substantial resistance in the gain-of-function fem-3 mutant that was characterized. Alternatively, the pathogen resistance phenotype of sterile mutants could potentially be explained by a radical disruption of metabolic homeostasis as a consequence of sterility in which enhanced metabolic resources are recruited to immunity.
If pathogen resistance in sterile mutants is due solely to a lack of embryo-mediated immunosuppression, why are sterile animals more resistant than wild-type animals when progeny production is blocked by FUDR (Figure 5C)? In the absence of FUDR, eggs are fertilized and reach about the 100-cell stage of development, at which point they are laid (Bany et al. 2003). In contrast, FUDR blocks in the mid-proliferation stage of embryonic development (Stroeher et al. 1994). Under our FUDR assay conditions, eggs are fertilized, go through multiple rounds of cell division, and are laid; however, the cellular organization is disturbed inside the eggs, and they fail to hatch (data not shown). Thus, it is likely that FUDR does not completely block the generation of the putative signal during embryogenesis in wild-type animals to downregulate DAF-16 activation of defense response pathways and that any stage of embryo development from fertilization to egg laying could generate the signal leading to immune deficiency. Thus, instead of blocking the embryo-derived signal completely, FUDR may affect the level of the signal at least to some degree because wild-type animals survive longer on pathogens with FUDR than without FUDR. This difference in pathogen resistance between the wild-type animals in the presence and absence of FUDR cannot be explained solely as an effect of FUDR on the virulence of the pathogens (Figure 5D). Nevertheless, the results of experiments using FUDR highlight the requirement of normal embryonic development to downregulate immunity in adult animals.
Why does embryonic development decrease immunity in adult animals? To successfully generate offspring, an adult animal must store enough energy for both its own cellular maintenance and to foster embryonic development. The expression of defense-related antimicrobials is presumably energy intensive and competes with embryos for limited nutrients. Although it may be beneficial to turn down defense mechanisms to save energy for successful reproduction in the absence of pathogen attack, our data suggest that wild-type animals prioritize reproduction over immunity even in the presence of a lethal pathogen.
Screen for epr mutants highlights sterility and DAF-16-dependent pathogen resistance:
When we carried out a forward genetic screen to identify factors important for pathogen resistance, the 6 Epr mutants that were characterized in depth had fertility defects. Importantly, however, our characterization of sterile mutants indicates that lack of internal progeny hatching is not the sole mechanism by which animals become resistant to bacterial pathogens like P. aeruginosa. This is in agreement with previous findings that showed that the inability to bag does not fully account for the resistance of C. elegans male animals to the fungal pathogen C. neoformans (van den Berg et al. 2006). In fact, we found that most sterile mutants had pathogen sensitivity similar to wild-type animals when DAF-16 was removed, despite the fact that they were still completely sterile and did not die from internal progeny hatching.
In addition to being partially sterile, all six epr mutants we characterized exhibited DAF-16-dependent resistance, suggesting that a major resistance mechanism in C. elegans is likely to be governed by DAF-16. The epr mutant screen identified a new allele of age-1, a component of the DAF-2 insulin-like signaling pathway. AGE-1 is a class IA phosphoinositide-3-OH kinase (PI3K) most similar to the mammalian PI3K p110 form (Morris et al. 1996). Mutations in the C. elegans AGE-1 RBD have not been previously reported. Our data suggest that the RBD in AGE-1 has a role in both longevity and pathogen resistance. Our screen also identified a new allele of inx-14, which is not directly related to DAF-2 signaling. The inx-14(ag17) allele that we identified causes a very modest increase in longevity and incomplete sterility, but in agreement with previous results (Hamilton et al. 2005), our data show that INX-14 functions upstream of DAF-16.
A new mode of DAF-16 activation:
DAF-16 was translocated to the nucleus in the animals induced to become sterile by RNAi knockdown of eight of eight genes examined. This observation is consistent with the observed DAF-16-dependent pathogen resistance of the sterile mutants we examined. Furthermore, our observations that a daf-2 mutation or daf-2 RNAi enhanced the resistance of glp-1, fog-2, fer-15;fem-1, and fem-3 animals (Figure 6, B–E) suggest that the DAF-16-dependent signaling pathway leading to pathogen resistance in these sterile mutants works in parallel with the DAF-2 pathway. Finally, whether the germ-line stem cells were absent (glp-1) or not (fer-15;fem-1), these sterile mutants exhibited enhanced pathogen resistance that was DAF-16 dependent and KRI-1 independent. Even though our epistasis experiments were not carried out with null alleles of daf-2 or kri-1, these data suggest the existence of a signaling pathway that is distinct from the KRI-1 and DAF-2 pathways that functions to promote resistance in a DAF-16-dependent manner. Hereafter, the proposed third pathway is referred as the “defense response pathway.”
If the three pathways, DAF-2, KRI-1, and the defense response pathway, act independently upstream of DAF-16 to exert pathogen resistance, why are glp-1 animals, which have both KRI-1 and the defense response pathways activated, not more pathogen resistant than the sterile mutants that only have the defense response pathway activated (Figure 5B)? One possibility is that the KRI-1 pathway may be responsible for regulating genes that function in longevity and the defense response pathway is dedicated to regulating genes to enhance pathogen resistance.
DAF-16-dependent pathogen response:
Previous transcriptional profiling analyses of P. aeruginosa-infected worms indicated that DAF-16 is not required for the induction of certain classes of antimicrobial genes, including those regulated by the p38 MAPK pathway (Troemel et al. 2006). In addition, there is almost no overlap between P. aeruginosa-elicited gene expression and genes that are activated by DAF-16, even though in both cases the activated genes appear to function as immune effectors (Shapira et al. 2006; Troemel et al. 2006). For example, lys-7 or dod-6 were not differentially regulated in worms feeding on P. aeruginosa compared to worms feeding on E. coli (Shapira et al. 2006; Troemel et al. 2006), even though both lys-7 and dod-6 have been implicated in a role in immunity (Mallo et al. 2002; O'Rourke et al. 2006; Shapira et al. 2006; Troemel et al. 2006).
DAF-16 could still play a very important role in immunity in the wild, even if it is not required for the immune response to infection under the particular conditions we use to carry out killing assays in the laboratory. Indeed, DAF-16-regulated genes likely do play a role in immunity, as illustrated by the fact that upregulation of DAF-16, either through transgenic overexpression (Singh and Aballay 2006) or through a daf-2 mutation (Garsin et al. 2003), causes marked enhanced pathogen resistance. However, as discussed above, we cannot rule out the possibility that a DAF-16-independent mechanism that targets the same effector genes as DAF-16 could be involved in mediating pathogen resistance in sterile mutants.
Immune effectors downstream of DAF-16:
Our analysis of several DAF-16-dependent genes revealed that lys-7 and dod-6, but not sod-3 or mtl-1, were highly expressed in 1-day-old adult sterile mutants compared to wild type (Figure 8A). Previously, it was shown that two other pathogens, M. nematophilum and S. marcescens, also induce lys-7 (Mallo et al. 2002; O'Rourke et al. 2006), suggesting that it plays an important role as an immune effector. However, inactivation of neither lys-7 nor dod-6 by RNAi had a significant effect on P. aeruginosa (data not shown). A likely explanation for these negative results is functional redundancy at the level of downstream effectors.
Our results also suggest that DAF-16 activity must be differentially modified to orchestrate expression of different subsets of DAF-16-activated genes in these sterile mutants and in a daf-2 mutant. Our analyses of DAF-16-dependent gene expression also highlighted that glp-1 exhibited higher levels of expression of the DAF-16-dependent genes tested in young adult animals unlike the other sterile mutants (Figure 8). This may be due to the fact that glp-1 mutants are anatomically different than wild-type animals virtually their entire lives, whereas mutants that are sterile because of a lack of oocytes or sperm are not different from wild-type animals until adulthood. Indeed, glp-1 mutants have already been shown to upregulate DAF-16 via the KRI-1 signaling pathway, which is thought to be specific to mutants like glp-1 that are deficient for germ-line stem cells from hatching onward (Hsin and Kenyon 1999; Berman and Kenyon 2006).
DAF-16-independent resistance of fem-3:
Unlike the other sterile mutants examined in this study, the pathogen-resistant phenotype of fem-3 was only partially suppressed by daf-16. Previously, it has been shown that male C. elegans exhibit enhanced resistance to the fungal pathogen C. neoformans and that this resistance is only partially dependent on DAF-16 (van den Berg 2006). The fem-3gf strain, which was used in this study, is a gain-of-function mutation that results in production of excess sperm but not oocytes (Barton et al. 1987). One interesting possibility is that the fem-3gf strain behaves similarly to true males and its pathogen resistance relies on both DAF-16-dependent and DAF-16-independent factors.
Pathogen resistance and longevity:
Determining whether there are mechanistic differences underlying increased longevity and enhanced resistance in C. elegans is complicated because the readout for both pathogen resistance and longevity is survival. Thus, if a particular mutant has both a long-lived and a pathogen-resistant phenotype, is pathogen resistance simply a reflection of increased longevity, or vice versa? One argument that longevity and pathogen resistance are distinct phenomena is the poor correlation between the degree of pathogen resistance and the degree of increased longevity. For example, inx-14(ag17) is one of the most pathogen-resistant mutants that we examined, but it only exhibits a modest, although statistically significant, increase in longevity (Figure 1). [Previously, a genomewide RNAi screen in C. elegans revealed that RNAi inactivation of inx-14 extends longevity in a DAF-16-dependent manner (Hamilton et al. 2005)]. Likewise, as previously reported (Garsin et al. 2003), although daf-2 mutants exhibit both increased longevity and pathogen resistance, daf-2 mutations have different quantitative effects on longevity and resistance. The daf-2(e1370) allele extends life span when feeding on E. coli about twofold but confers a fivefold increase in life span when feeding on S. aureus (Garsin et al. 2003). Similarly, even though all of the previously characterized sterile mutants analyzed in this study exhibited pathogen resistance, some of them are reported to have essentially wild-type longevity (fog-2 and fer-15;fem-1) (Arantes-Oliveira et al. 2002; McCarroll et al. 2004). There are also examples of long-lived mitochondrial mutants that do not exhibit pathogen resistance (D. Kim, personal communication). Finally, the gene expression data shown in Figure 8 suggest that particular DAF-16-regulated genes can be differentially activated in long-lived daf-2 mutants and pathogen-resistant sterile mutants. Taken together, these results suggest distinct molecular mechanisms underlying life-span extension and pathogen resistance. On the other hand, it is certainly possible that increased longevity per se can contribute to pathogen resistance as measured by worm survival in the presence of a pathogen. Because of this, additional mechanistic understanding, defining independent measures of innate immunity, will be key to distinguishing longevity and immune-related phenomena.
Conclusion:
We found that certain sterile mutants have enhanced pathogen resistance due to upregulation of the transcription factor DAF-16. Unlike the signaling pathway between the germ line and DAF-16, which involves germ cells that suppress life span, this proposed pathway seems to involve a signal generated during embryonic development that suppresses immunity in adult animals. This proposed new signaling pathway functions in adults with developing embryos, but not in younger or older adults that lack embryos. Identifying the pathway by which embryonic development suppresses the pathogen resistance of C. elegans may shed light on the interconnections between reproduction and immunity and the general mechanism by which animals maintain a systemwide energy balance.
Acknowledgments
We gratefully acknowledge Rhonda Feinbaum and Jennifer Powell for helpful discussions and critically reading the manuscript. We thank Eyleen O'Rourke and Sean Curran for critically reading the manuscript and Alison Frand for helpful suggestions. We also thank Renaud Rinaldi for technical help in reformatting the data for statistical analysis. We thank the Caenorhabiditis Genetics Center, funded by National Institutes of Health, for providing a variety of mutants used in this study, and the Wellcome Trust Sanger Institute for providing the cosmid F07A5. Two inx-14 alleles, inx-14(tm2864/+) and inx-14(tm2593/+), were generated by the Japanese National Bioresource Project, which is supported by the Ministry of Education, Culture, Science, Sports, and Technology. This work was supported by a Leukemia/Lymphoma Society fellowship awarded to E.R.T. and National Institutes of Health grant R01-AI064332 awarded to F.M.A.
References
- Aballay, A., P. Yorgey and F. M. Ausubel, 2000. Salmonella typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr. Biol. 10 1539–1542. [DOI] [PubMed] [Google Scholar]
- Akira, S., S. Uematsu and O. Takeuchi, 2006. Pathogen recognition and innate immunity. Cell 124 783–801. [DOI] [PubMed] [Google Scholar]
- Arantes-Oliveira, N., J. Apfeld, A. Dillin and C. Kenyon, 2002. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science 295 502–505. [DOI] [PubMed] [Google Scholar]
- Arantes-Oliveira, N., J. R. Berman and C. Kenyon, 2003. Healthy animals with extreme longevity. Science 302 611. [DOI] [PubMed] [Google Scholar]
- Ausubel, F. M., 2005. Are innate immune signaling pathways in plants and animals conserved? Nat. Immunol. 6 973–979. [DOI] [PubMed] [Google Scholar]
- Bairoch, A., and R. Apweiler, 2000. The SWISS-PROT protein sequence database and its supplement TrEMBL in 2000. Nucleic Acids Res. 28 45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bany, I. A., M. Q. Dong and M. R. Koelle, 2003. Genetic and cellular basis for acetylcholine inhibition of Caenorhabditis elegans egg-laying behavior. J. Neurosci. 23 8060–8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton, M. K., T. B. Schedl and J. Kimble, 1987. Gain-of-function mutations of fem-3, a sex-determination gene in Caenorhabditis elegans. Genetics 115 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman, J. R., and C. Kenyon, 2006. Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell 124 1055–1068. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez, V., A. Mohri-Shiomi, A. Maadani, L. A. Vega and D. A. Garsin, 2007. Oxidative stress enzymes are required for DAF-16 mediated immunity due to generation of reactive oxygen species by Caenorhabditis elegans. Genetics 176 1567–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, M. W., M. Hammarlund, T. Harrach, P. Hullett, S. Olsen et al., 2005. Rapid single nucleotide polymorphism mapping in C. elegans. BMC Genomics 6 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernburg, A. F., J. Zalevsky, M. P. Colaiacovo and A. M. Villeneuve, 2000. Transgene-mediated cosuppression in the C. elegans germ line. Genes Dev. 14 1578–1583. [PMC free article] [PubMed] [Google Scholar]
- Dillin, A., D. K. Crawford and C. Kenyon, 2002. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science 298 830–834. [DOI] [PubMed] [Google Scholar]
- Epstein, H. F. S., and D. C. Shakes (Editors), 1995. Caenorhabditis elegans: Modern Biological Analysis of an Organism. Academic Press, New York.
- Fedorka, K. M., M. Zuk and T. A. Mousseau, 2004. Immune suppression and the cost of reproduction in the ground cricket, Allonemobius socius. Evolution 58 2478–2485. [DOI] [PubMed] [Google Scholar]
- Fire, A., S. Xu, M. K. Montgomery, S. A. Kostas, S. E. Driver et al., 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391 806–811. [DOI] [PubMed] [Google Scholar]
- Fowler, K., and L. Partridge, 1989. A cost of mating in female fruitflies. Nature 338 760–761. [Google Scholar]
- Garsin, D. A., J. M. Villanueva, J. Begun, D. H. Kim, C. D. Sifri et al., 2003. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science 300 1921. [DOI] [PubMed] [Google Scholar]
- Gottlieb, S., and G. Ruvkun, 1994. daf-2, daf-16, and daf-23: genetically interacting genes controlling Dauer formation in Caenorhabditis elegans. Genetics 137 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan, J. A., H. Cheng, J. E. Harris and D. Greenstein, 2006. Galphao/i and Galphas signaling function in parallel with the MSP/Eph receptor to control meiotic diapause in C. elegans. Curr. Biol. 16 1257–1268. [DOI] [PubMed] [Google Scholar]
- Gravato-Nobre, M. J., and J. Hodgkin, 2005. Caenorhabditis elegans as a model for innate immunity to pathogens. Cell Microbiol. 7 741–751. [DOI] [PubMed] [Google Scholar]
- Hamilton, B., Y. Dong, M. Shindo, W. Liu, I. Odell et al., 2005. A systematic RNAi screen for longevity genes in C. elegans. Genes Dev. 19 1544–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, M., A. L. Hsu, A. Dillin and C. Kenyon, 2005. New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet. 1 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, T. W., R. Lee, E. Schwarz, K. Bradnam, D. Lawson et al., 2003. WormBase: a cross-species database for comparative genomics. Nucleic Acids Res. 31 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautekeete, N. C., Y. Piquot and H. Van Dijk, 2001. Investment in survival and reproduction along a semelparity-iteroparity gradient in the Beta species complex. J. Evol. Biol. 14 795–804. [Google Scholar]
- Henderson, S. T., and T. E. Johnson, 2001. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr. Biol. 11 1975–1980. [DOI] [PubMed] [Google Scholar]
- Hsin, H., and C. Kenyon, 1999. Signals from the reproductive system regulate the lifespan of C. elegans. Nature 399 362–366. [DOI] [PubMed] [Google Scholar]
- Hsu, A. L., C. T. Murphy and C. Kenyon, 2003. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300 1142–1145. [DOI] [PubMed] [Google Scholar]
- Kamath, R. S., A. G. Fraser, Y. Dong, G. Poulin, R. Durbin et al., 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421 231–237. [DOI] [PubMed] [Google Scholar]
- Kenyon, C., J. Chang, E. Gensch, A. Rudner and R. Tabtiang, 1993. A C. elegans mutant that lives twice as long as wild type. Nature 366 461–464. [DOI] [PubMed] [Google Scholar]
- Kim, D. H., and F. M. Ausubel, 2005. Evolutionary perspectives on innate immunity from the study of Caenorhabditis elegans. Curr. Opin. Immunol. 17 4–10. [DOI] [PubMed] [Google Scholar]
- Kim, D. H., R. Feinbaum, G. Alloing, F. E. Emerson, D. A. Garsin et al., 2002. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297 623–626. [DOI] [PubMed] [Google Scholar]
- Klein, S. L., and R. J. Nelson, 1999. Influence of social factors on immune function and reproduction. Rev. Reprod. 4 168–178. [DOI] [PubMed] [Google Scholar]
- Kurz, C. L., and J. J. Ewbank, 2003. Caenorhabditis elegans: an emerging genetic model for the study of innate immunity. Nat. Rev. Genet. 4 380–390. [DOI] [PubMed] [Google Scholar]
- Lee, R. Y., J. Hench and G. Ruvkun, 2001. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr. Biol. 11 1950–1957. [DOI] [PubMed] [Google Scholar]
- Libina, N., J. R. Berman and C. Kenyon, 2003. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell 115 489–502. [DOI] [PubMed] [Google Scholar]
- Lin, K., J. B. Dorman, A. Rodan and C. Kenyon, 1997. daf-16: an HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 278 1319–1322. [DOI] [PubMed] [Google Scholar]
- Lin, K., H. Hsin, N. Libina and C. Kenyon, 2001. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat. Genet. 28 139–145. [DOI] [PubMed] [Google Scholar]
- Mahajan-Miklos, S., M. W. Tan, L. G. Rahme and F. M. Ausubel, 1999. Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell 96 47–56. [DOI] [PubMed] [Google Scholar]
- Mallo, G. V., C. L. Kurz, C. Couillault, N. Pujol, S. Granjeaud et al., 2002. Inducible antibacterial defense system in C. elegans. Curr. Biol. 12 1209–1214. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer, A., J. B. Anderson, P. F. Cherukuri, C. DeWeese-Scott, L. Y. Geer et al., 2005. CDD: a conserved domain database for protein classification. Nucleic Acids Res. 33 D192–D196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll, S. A., C. T. Murphy, S. Zou, S. D. Pletcher, C. S. Chin et al., 2004. Comparing genomic expression patterns across species identifies shared transcriptional profile in aging. Nat. Genet. 36 197–204. [DOI] [PubMed] [Google Scholar]
- McElwee, J., K. Bubb and J. H. Thomas, 2003. Transcriptional outputs of the Caenorhabditis elegans forkhead protein DAF-16. Aging Cell 2 111–121. [DOI] [PubMed] [Google Scholar]
- McKean, K. A., and L. Nunney, 2001. Increased sexual activity reduces male immune function in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 98 7904–7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello, C., and A. Fire, 1995. DNA transformation. Methods Cell Biol. 48 451–482. [PubMed] [Google Scholar]
- Millet, A. C., and J. J. Ewbank, 2004. Immunity in Caenorhabditis elegans. Curr. Opin. Immunol. 16 4–9. [DOI] [PubMed] [Google Scholar]
- Morris, J. Z., H. A. Tissenbaum and G. Ruvkun, 1996. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature 382 536–539. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay, A., S. W. Oh and H. A. Tissenbaum, 2006. Worming pathways to and from DAF-16/FOXO. Exp. Gerontol. 41 928–934. [DOI] [PubMed] [Google Scholar]
- Murakami, S., and T. E. Johnson, 1996. A genetic pathway conferring life extension and resistance to UV stress in Caenorhabditis elegans. Genetics 143 1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, C. T., S. A. McCarroll, C. I. Bargmann, A. Fraser, R. S. Kamath et al., 2003. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424 277–283. [DOI] [PubMed] [Google Scholar]
- Nelson, D. W., and R. W. Padgett, 2003. Insulin worms its way into the spotlight. Genes Dev. 17 813–818. [DOI] [PubMed] [Google Scholar]
- O'Quinn, A. L., E. M. Wiegand and J. A. Jeddeloh, 2001. Burkholderia pseudomallei kills the nematode Caenorhabditis elegans using an endotoxin-mediated paralysis. Cell. Microbiol. 3 381–393. [DOI] [PubMed] [Google Scholar]
- O'Rourke, D., D. Baban, M. Demidova, R. Mott and J. Hodgkin, 2006. Genomic clusters, putative pathogen recognition molecules, and antimicrobial genes are induced by infection of C. elegans with M. nematophilum. Genome Res. 16 1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl, M. W., 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29 e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan, P., 2005. Innexins: members of an evolutionarily conserved family of gap-junction proteins. Biochim. Biophys. Acta 1711 225–245. [DOI] [PubMed] [Google Scholar]
- Rual, J. F., J. Ceron, J. Koreth, T. Hao, A. S. Nicot et al., 2004. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 14 2162–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, C. A., A. Huffaker and Y. Yamaguchi, 2007. New insights into innate immunity in Arabidopsis. Cell Microbiol. 9 1902–1908. [DOI] [PubMed] [Google Scholar]
- Schedl, T., and J. Kimble, 1988. fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics 119 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenburg, H., C. L. Kurz and J. J. Ewbank, 2004. Evolution of the innate immune system: the worm perspective. Immunol. Rev. 198 36–58. [DOI] [PubMed] [Google Scholar]
- Shapira, M., B. J. Hamlin, J. Rong, K. Chen, M. Ronen et al., 2006. A conserved role for a GATA transcription factor in regulating epithelial innate immune responses. Proc. Natl. Acad. Sci. USA 103 14086–14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifri, C. D., J. Begun, F. M. Ausubel and S. B. Calderwood, 2003. Caenorhabditis elegans as a model host for Staphylococcus aureus pathogenesis. Infect. Immun. 71 2208–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, V., and A. Aballay, 2006. Heat-shock transcription factor (HSF)-1 pathway required for Caenorhabditis elegans immunity. Proc. Natl. Acad. Sci. USA 103 13092–13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starich, T., M. Sheehan, J. Jadrich and J. Shaw, 2001. Innexins in C. elegans. Cell Commun. Adhes. 8 311–314. [DOI] [PubMed] [Google Scholar]
- Stroeher, V. L., B. P. Kennedy, K. J. Millen, D. F. Schroeder, M. G. Hawkins et al., 1994. DNA-protein interactions in the Caenorhabditis elegans embryo: oocyte and embryonic factors that bind to the promoter of the gut-specific ges-1 gene. Dev. Biol. 163 367–380. [DOI] [PubMed] [Google Scholar]
- Tan, M. W., S. Mahajan-Miklos and F. M. Ausubel, 1999. a Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. USA 96 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, M. W., L. G. Rahme, J. A. Sternberg, R. G. Tompkins and F. M. Ausubel, 1999. b Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc. Natl. Acad. Sci. USA 96 2408–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi, M. F., 2005. Innate immune responses to infection. J. Allergy Clin. Immunol. 116 241–249 quiz 250. [DOI] [PubMed] [Google Scholar]
- Troemel, E. R., S. W. Chu, V. Reinke, S. S. Lee, F. M. Ausubel et al., 2006. p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. 2 e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg, M. C., J. Z. Woerlee, H. Ma and R. C. May, 2006. Sex-dependent resistance to the pathogenic fungus Cryptococcus neoformans. Genetics 173 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorp, R. G., and T. B. Kirkwood, 1998. Human longevity at the cost of reproductive success. Nature 396 743–746. [DOI] [PubMed] [Google Scholar]
- Whitten, S. J., and M. A. Miller, 2007. The role of gap junctions in Caenorhabditis elegans oocyte maturation and fertilization. Dev. Biol. 301 432–446. [DOI] [PubMed] [Google Scholar]
- Wolff, S., H. Ma, D. Burch, G. A. Maciel, T. Hunter et al., 2006. SMK-1, an essential regulator of DAF-16-mediated longevity. Cell 124 1039–1053. [DOI] [PubMed] [Google Scholar]
- Wolkow, C. A., K. D. Kimura, M. S. Lee and G. Ruvkun, 2000. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science 290 147–150. [DOI] [PubMed] [Google Scholar]
- Yochem, J., T. Gu and M. Han, 1998. A new marker for mosaic analysis in Caenorhabditis elegans indicates a fusion between hyp6 and hyp7, two major components of the hypodermis. Genetics 149 1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]