Abstract
The recessive black plumage mutation in the Japanese quail (Coturnix japonica) is controlled by an autosomal recessive gene (rb) and displays a blackish-brown phenotype in the recessive homozygous state (rb/rb). A similar black coat color phenotype in nonagouti mice is caused by an autosomal recessive mutation at the agouti locus. An allelism test showed that wild type and mutations for yellow, fawn-2, and recessive black in Japanese quail were multiple alleles (*N, *Y, *F2, and *RB) at the same locus Y and that the dominance relationship was Y*F2 > Y*Y > Y*N > Y*RB. A deletion of 8 bases was found in the ASIP gene in the Y*RB allele, causing a frameshift that changed the last six amino acids, including a cysteine residue, and removed the normal stop codon. Since the cysteine residues at the C terminus are important for disulphide bond formation and tertiary structure of the agouti signaling protein, the deletion is expected to cause a dysfunction of ASIP as an antagonist of α-MSH in the Y*RB allele. This is the first evidence that the ASIP gene, known to be involved in coat color variation in mammals, is functional and has a similar effect on plumage color in birds.
THE yellow mutation in the Japanese quail (Coturnix japonica) is controlled by an autosomal incomplete dominant gene (Y). Adult heterozygotes (Y/+, yellow) display a straw plumage color but the dominant homozygotes (Y/Y) display an early embryonic lethality (Homma et al. 1967). The fawn-2 mutation at the Y locus is also controlled by an autosomal incomplete dominant gene (Yf2), which is allelic to and incompletely dominant over the Y gene. Adult homozygotes (Yf2/Yf2, fawn-2) show a whitish light-brown color in males and a creamier plumage color in females than in males. The heterozygotes (Yf2/+, dark fawn-2) show a deeper brown color than the homozygotes in each sex (Tsudzuki et al. 1996). The recessive black plumage color in the Japanese quail is controlled by an autosomal recessive gene (rb) and gives a blackish-brown plumage to homozygotes (rb/rb, recessive black) (Fujiwara et al. 2005), which is very similar to that of homozygotes for the extended brown plumage mutation controlled by an autosomal incomplete dominant gene (E) (Somes 1979) at the MC1R locus (Nadeau et al. 2006).
There are interesting similarities between the phenotypic effects and dominance relationships of mutations at the agouti locus in the house mouse (Mus musculus). In nonagouti mice, a black coat color phenotype (a/a) is caused by an autosomal recessive mutation at the agouti (ASIP) locus (Silvers 1979). Embryonic lethality of a dominant homozygous yellow mutation (Ay/Ay) is associated with a deletion upstream of agouti that removes the coding exons of the Raly (hnRNP protein that is associated with the lethal yellow) gene (Michaud et al. 1993). The plumage color of the yellow (Y/+) Japanese quail is similar to the coat color of the yellow (Ay/+) mouse. The Y locus in the Japanese quail was mapped on the QL10 linkage group homologous to GGA20 in chicken (Gallus gallus) (Miwa et al. 2005), where an ASIP-like sequence was found (Klovins and Schiöth 2005). Recently we studied the growth of yellow quails and found that they shared similar phenotypic characteristics (increased body fat, decreased body temperature) with lethal yellow mice (Minvielle et al. 2007). Because of these points, the ASIP gene was considered to be a candidate gene for the yellow mutation in the Japanese quail.
Mutations in the ASIP gene have been shown to cause a wide variety of coat colors in mammals. For instance, standard silver color in red foxes (Vulpes vulpes), nonagouti black in Norway rats (Rattus norvegicus), recessive black in horses (Equus caballus), and black coloration in cats (Felis catus) are all associated with deletions in exon 2 of ASIP that cause a loss of the agouti function (Våge et al. 1997; Kuramoto et al. 2001; Rieder et al. 2001; Eizirik et al. 2003). In dogs (Canis familiaris), separate substitutions in exon 3 are associated with nonagouti black (a) and fawn or sable (ay) colors (Kerns et al. 2004; Berryere et al. 2005). In the chicken, however, no plumage color variation was found to be associated with ASIP up to now, which has led to the hypothesis that birds had no functional agouti gene (Boswell and Takeuchi 2005).
In this study, we investigated whether rb was a fourth allele at the Y locus by segregation analysis and whether variation in the coding sequence of ASIP was associated with the Y, Yf2, and rb mutations. The Glu92Lys substitution (c.272G > A SNP) in MC1R, the specific missense mutation for the extended brown plumage, was also genotyped to confirm that this mutation was not involved in the recessive black phenotype.
MATERIALS AND METHODS
Allelism test:
Under the hypothesis that the yellow, fawn-2, and recessive black mutations were caused by different alleles at the same locus, single-pair mating experiments were performed to test the allelism between yellow and recessive black mutations and between fawn-2 and recessive black mutations, in two successive generations (Table 1). The observed segregation data on down colors of newly hatched chicks were analyzed by a chi-square test.
TABLE 1.
Segregation data on mating experiments between yellow or dark fawn-2 and recessive black plumage color
| Mating typea
|
No. of matings | No. of newly hatched chicks
|
Expected ratiob
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hen | × | Cock | Y (Y/rb) | DFA2 (Yf2/rb) | WT (+/rb) | RB (rb/rb) | Y | DFA2 | WT | RB | χ2 | d.f. = 1 | ||
| Y (Y/+) | × | RB (rb/rb) | 2 | 9 | — | 17 | — | 1 | : | 1 | 2.46 | 0.20 > P > 0.10 | ||
| DFA2 (Yf2/+) | × | RB (rb/rb) | 1 | — | 10 | 12 | — | 1 | : | 1 | 0.18 | 0.70 > P > 0.60 | ||
| Y (F1) (Y/rb) | × | RB (rb/rb) | 4 | 31 | — | — | 46 | 1 | : | 1 | 2.92 | 0.10 > P > 0.05 | ||
| RB (rb/rb) | × | Y (F1) (Y/rb) | 2 | 28 | — | — | 24 | 1 | : | 1 | 0.31 | 0.60 > P > 0.50 | ||
| Total | 6 | 59 | — | — | 70 | 1 | : | 1 | 0.94 | 0.40 > P > 0.30 | ||||
| DFA2 (F1)(Yf2/rb) | × | RB (rb/rb) | 3 | — | 43 | — | 33 | 1 | : | 1 | 1.32 | 0.30 > P > 0.20 | ||
| RB (rb/rb) | × | DFA2 (F1) (Yf2/rb) | 2 | — | 11 | — | 11 | 1 | : | 1 | 0 | |||
| Total | 5 | — | 54 | — | 44 | 1 | : | 1 | 1.02 | 0.40 > P > 0.30 | ||||
Y, yellow; DFA2, dark fawn-2 (see text); WT, wild type; RB, recessive black.
Under the hypothesis that yellow or dark fawn-2 and recessive black are controlled by alleles Y, Yf2, and rb, with Y and Yf2 being dominant over rb.
Cloning and sequencing of ASIP cDNA:
Complementary DNA (cDNA) sequences of ASIP from wild-type (+/+), yellow (Y/+), fawn-2 (Yf2/Yf2), and recessive black (rb/rb) neonatal chicks of the Japanese quail maintained at Gifu University were compared. Total RNA was purified from homogenized dorsal skin samples by using the PureLink Micro-to-Midi total RNA purification system (Invitrogen, Carlsbad, CA).
A reverse-transcription (RT) reaction was performed, using the first-standard cDNA synthesis kit (Amersham Biosciences, Piscataway, NJ) and a poly(T) primer with an anchor region (5′-AACTGGAAGAATTCGCGGCCGCAGGAAT18-3′). The chicken expressed sequence tags (ESTs) homologous to the ASIP and Raly genes have already been sequenced (BBSRC ChickEST database: http://www.chick.manchester.ac.uk/) and were mapped on GGA20 by BLAST search (http://www.ncbi.nlm.nih.gov/projects/genome/guide/chicken/). To sequence the whole coding region, PCR primers were designed against the 5′- and 3′-untranslated regions (UTR) of the ASIP-like gene in chicken (Table 2). Sequence reactions for RT–PCR products were performed with ASIP_F and ASIP_R, using an ABI Prism 3100 DNA sequencer (PE Applied Biosystems, Foster City, CA).
TABLE 2.
Primer sequence and position
| Nucleotide no.
|
||||
|---|---|---|---|---|
| Primer name | Sequence (5′–3′) | 5′-terminal | 3′-terminal | Position in the chicken sequence |
| ASIP_F | attttcatgacagtgggatt | −33 | −14 | 5′-UTR (exon 3) |
| ASIP_R | acacttgagaagctactga | +445 | +427 | 3′-UTR (exon 5) |
| ASIP_F2 | aaatgctgaactgaagacac | +219 | +238 | Exon 5 |
| MC1R_F3 | ttgggcgcacgggggcttt | +368 | +386 | Exon 1 |
| MC1R_92Glu | gcagcatgaagagcgtctc | +750 | +732 | Exon 1 |
| MC1R_92Lys | gcagcatgaagagcgtctt | +750 | +732 | Exon 1 |
+1 corresponds to the A at the start codon of each mRNA.
Genotyping:
The specific mutation for rb was genotyped in 34 recessive black mutants (rb/rb; e+/e+), seven wild-type quail (Rb+/Rb+; e+/e+), and 7 extended brown mutants (Rb+/Rb+; E/E). DNA was extracted from the peripheral blood, using an AquaPure genomic DNA blood kit (Bio-Rad, Hercules, CA). DNA samples were amplified by ASIP_F2 and ASIP_R primers, using AmpliTaq Gold. PCR products were electrophoresed on an ABI Prism 3100 DNA sequencer and were analyzed using GeneScan 3.7 and Genotyper 3.7 (PE Applied Biosystems).
The Glu92Lys substitution (c.272G > A SNP) in MC1R, a specific missense mutation for E, was also genotyped in those samples, using allele-specific primers (Table 2). DNA samples were amplified by allele-specific primers named MC1R_F3-MC1R_92Glu and MC1R_F3-MC1R_92Lys. PCR products were analyzed by 1.5% agarose gel electrophoresis.
Quantitative RT–PCR:
Gene expression levels in the skin were compared among four phenotypes (wild type, yellow, fawn-2, and recessive black). cDNA of three chicks from each of the four phenotypes was amplified using the SYBR ExScript RT–PCR kit, including SYBR Premix Ex Taq with 0.2 μm each of ASIP_F2 and ASIP_R primers. The GAPDH gene was used as a control with GAPDH_F (5′-GGAGAAACCAGCCAAGTATGATG-3′) and GAPDH_R (5′-AAAGGTGGAGGAATGGCTGTCA-3′) primers. Three replications were carried out.
RESULTS
Allelism test:
Table 1 shows the segregation data obtained in the F1 and backcross generations, respectively. In Table 1, from matings between yellow (Y/+) or dark fawn-2 (Yf2/+) hens and recessive black cocks, both yellow or dark fawn-2 and wild-type F1 chicks were obtained, and the observed segregation did not differ from that of the null hypothesis (with similar expectations, however, under one- and two-locus modes of inheritance of the Y and Yf2 alleles and the rb gene). Only yellow or dark fawn-2 and recessive black backcross chicks, in equal proportions, were obtained from reciprocal matings of yellow or dark fawn-2 F1 with recessive black quails. This segregation and the fact that no quail with wild-type plumage were obtained among a total of 227 backcross chicks indicate that the pattern of segregation is satisfactorily explained by a one-locus mode of inheritance and that Y, Yf2, and rb are alleles.
Sequencing of ASIP cDNA:
cDNA of ASIP was successfully amplified from mRNA derived from skin samples of the Japanese quail using primers designed from the chicken ASIP ortholog. The cDNA was sequenced starting 13 bp upstream of the start codon (−13) and finishing 33 bp downstream of the stop codon (+426) for a total of 439 bp. Sequence identity between the Japanese quail and chicken was 95% for nucleotides of the coding region and 96% for the deduced 130 amino acids. From the comparison of sequences from one bird each of wild-type, yellow, fawn-2, and recessive black phenotype, no SNP was detected in yellow quail, two synonymous SNPs (c.31T > C and c.100C > T) were detected in fawn-2 quail, and an 8-bp deletion was detected in recessive black quail in the coding region (Figure 1). No amino acid substitutions corresponding to the yellow and fawn-2 phenotypes were found. The frameshift mutation (c.373_380del) specific to the recessive black mutation resulted in changes in amino acids from 125 to the end, located at the C-terminal cysteine residues domain, which is conserved in mammalian ASIP.
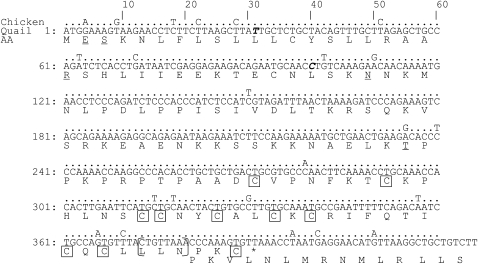
Figure 1.—
Nucleotide and deduced amino acid sequence of Japanese quail ASIP. Nucleotide and deduced amino acid sequences in the wild-type allele (Y*N) are shown. Nucleotide substitutions in chicken are shown at the tops of the sequences. Two synonymous nucleotide substitutions (c.31T > C SNP and c.100C > T SNP) observed in the fawn-2 allele (Y*F2) are italicized. Amino acid substitutions compared with chicken sequence are underlined. They are KRSSA in the chicken sequence. C-terminal cysteine residues conserved in a wide range of species are boxed. Nucleotides deleted in the recessive black allele (Y*RB) resulting in a frameshift mutation are indicated in brackets and changed amino acids in recessive black are shown at the bottoms of the sequences.
Quantitative RT–PCR:
The average level of relative expression of ASIP in the recessive black, yellow, and fawn-2 quail compared to wild-type quail is shown in Table 3. The relative expression level in the recessive black quail was significantly lower than that of the wild-type quail (P = 0.004). Fawn-2 quail showed ∼13 times higher relative expression, whereas yellow and wild-type quail had similar relative expression.
TABLE 3.
Relative expression of ASIP
| Wild type (mean ± SD) | Mutants (mean ± SD) | F | P |
|---|---|---|---|
| recessive black | |||
| 1.000 ± 0.348 | 0.075 ± 0.056 | 20.7 | 0.004 |
| yellow | |||
| 1.159 ± 0.219 | 0.4 | 0.528 | |
| fawn-2 | |||
| 12.987 ± 9.488 | 4.8 | 0.071 |
Total RNA was purified from homogenized dorsal skin of three chicks from each of four phenotypes (wild type, yellow, fawn-2, and recessive black). Complementary DNA (cDNA) was amplified using ASIP_F2 and ASIP_R primers. The GAPDH gene was used as a control. Three replications were carried out.
Genotyping:
For the c.373_380del in ASIP, we confirmed that all 7 wild-type and 7 extended brown quail showed no deletion, whereas all 34 recessive black quail had the 8-bp deletion genotype.
For the c.272G > A SNP in MC1R, all 7 extended brown quail showed the A/A SNP genotype, whereas the 7 wild-type and 34 recessive black quail had the G/G SNP genotype. Thus the recessive black mutation was associated with the deletion in ASIP, and it was not affected by MC1R.
DISCUSSION
The allelism test showed that recessive black (rb) is a fourth allele at the Y locus. Therefore, alleles for wild type, yellow, fawn-2, and recessive black were named Y*N, Y*Y, Y*F2, and Y*RB, respectively, according to the gene nomenclature of Crittenden et al. (1996), and the dominance relationship was Y*F2 > Y*Y > Y*N > Y*RB.
A deletion of 8 bases was found in ASIP in the recessive black allele (Y*RB). Genotyping from a large population confirmed that the deletion is associated with the recessive black allele. The frameshift caused by this mutation changed the last six amino acids and the stop codon. Among the 10 cysteine residues conserved in a wide range of animals, the last one was missing in the recessive black allele because of this mutation. Since the cysteine residues are important to build the disulphide bond, the deletion might cause dysfunction of ASIP as an antagonist of α-MSH in the Y*RB allele. Several studies of black coat color mutations in mammals have found an association of the coat color with mutations in the coding regions of ASIP. Among them, the black coat color of German shepherd dogs was associated with a SNP to cause an arginine to cysteine substitution at the C-terminal cysteine-rich residues (Kerns et al. 2004). In mice, several amino acid substitutions at cysteine-rich residues are known to be related to the black phenotype (Miltenberger et al. 2002), and an insertion in the first intron was reported in nonagouti mice (Bultman et al. 1994), but no deletion in the coding sequence has been reported yet.
The result of RT–PCR in this study showed that gene expression of recessive black quail was remarkably lower than that of wild-type quail, which was 13.3 times higher. Although this result might be an indication that there is another mutation at the promoter region of the Y*RB allele, it is rather unlikely that a second loss-of-function mutation would have accumulated in the same gene. It is much more likely that the suppression of the termination codon in the 3′-UTR of rb-ASIP due to the frameshift mutation was associated with mRNA decay and, consequently, low expression of rb-ASIP. Indeed, the existence of a mechanism to degrade mRNA lacking a termination codon was described by Van Hoof et al. (2002), and nonstop mRNA decay was further confirmed as one way to safeguard the cell from abnormal mRNA function (Isken and Maquat 2007; Ito-Harashima et al. 2007). Moreover, the absence of mutation in the ASIP sequence of yellow quail is consistent with the recent finding in an accompanying article by Nadeau et al. (2008, this issue) that the yellow mutation in quail was associated with a large deletion upstream of ASIP, placing its expression under the control of the Raly promoter, as is the case for the yellow coat color in Ay/+ mice (Michaud et al. 1993). Finally, the synonymous SNPs identified in all fawn-2 quail might indicate that they are in linkage disequilibrium with a still unknown regulatory mutation causing higher ASIP mRNA expression and therefore are dominant over the yellow mutation. In any case, this study has shown that the recessive black plumage color in quail was closely associated with the ASIP gene, and the results on the three alleles yellow, fawn-2, and recessive black are consistent with the existence of regulatory or structural mutations for this gene. Together with the study by Nadeau et al. (2008), we have given here the first evidence that ASIP is functional in birds and that its effects on plumage color parallel that of ASIP on mouse coat color.
Acknowledgments
This study was partly funded by 2004 and 2005 grants from Gifu University and by a 2006–2007 grant from the Japan Livestock Technology Association.
References
- Berryere, T. G., J. A. Kerns, G. S. Barsh and S. M. Schmutz, 2005. Association of an Agouti allele with fawn or sable coat color in domestic dogs. Mamm. Genome 16 262–272. [DOI] [PubMed] [Google Scholar]
- Boswell, T., and S. Takeuchi, 2005. Recent developments in our understanding of the avian melanocortin system: its involvement in the regulation of pigmentation and energy homeostasis. Peptides 26 1733–1743. [DOI] [PubMed] [Google Scholar]
- Bultman, S. J., M. L. Klebig, E. J. Michaud, H. O. Sweet, M. T. Davisson et al., 1994. Molecular analysis of reverse mutations from nonagouti (a) to black-and-tan (at) and white-bellied agouti (Aw) reveals alternative forms of agouti transcripts. Genes Dev. 8 481–490. [DOI] [PubMed] [Google Scholar]
- Crittenden, L. B., J. J. Bitgood, D. W. Burt, F. A. Ponce De Leon and M. Tixier-Boichard, 1996. Nomenclature for naming loci, alleles, linkage groups and chromosomes to be used in poultry genome publications and databases. Genet. Sel. Evol. 28 289–297. [Google Scholar]
- Eizirik, E., N. Yuhki, W. E. Johnson, M. Menotti-Raymond, S. S. Hannah et al., 2003. Molecular genetics and evolution of melanism in the cat family. Curr. Biol. 13 448–453. [DOI] [PubMed] [Google Scholar]
- Fujiwara, A., M. Mizutani, T. Ono and H. Kagami, 2005. “Recessive black”: a plumage color mutant in Japanese quail. J. Poult. Sci. 42 64–69. [Google Scholar]
- Homma, K., S. Shumiya and M. Jinno, 1967. Yellow-feathered Japanese quail (Coturnix coturnix japonica). Jpn. J. Zootech. Sci. 38 163–166. [Google Scholar]
- Isken, O., and L. E. Maquat, 2007. Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function. Genes Dev. 21 1833–1856. [DOI] [PubMed] [Google Scholar]
- Ito-Harashima, S., K. Kuroha, T. Tatematsu and T. Inada, 2007. Translation of the poly(A) tail plays crucial roles in nonstop mRNA surveillance via translation repression and protein destabilization by proteasome in yeast. Genes Dev. 21 519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns, J. A., J. Newton, T. G. Berryere, E. M. Rubin, J. F. Cheng et al., 2004. Characterization of the dog Agouti gene and a nonagouti mutation in German shepherd dogs. Mamm. Genome 15 798–808. [DOI] [PubMed] [Google Scholar]
- Klovins, J., and H. B. Schiöth, 2005. Agouti-related proteins (AGRPs) and agouti-signaling peptide (ASIP) in fish and chicken. Ann. NY Acad. Sci. 92 363–367. [DOI] [PubMed] [Google Scholar]
- Kuramoto, T., T. Nomoto, T. Sugimura and T. Ushijima, 2001. Cloning of the rat agouti gene and identification of the rat nonagouti mutation. Mamm. Genome 12 469–471. [DOI] [PubMed] [Google Scholar]
- Michaud, E. J., S. J. Bultman, L. J. Stubbs and R. P. Woychik, 1993. The embryonic lethality of homozygous lethal yellow mice (Ay/Ay) is associated with the disruption of a novel RNA-binding protein. Genes Dev. 7 1203–1213. [DOI] [PubMed] [Google Scholar]
- Miltenberger, R. J., K. Wakamatsu, S. Ito, R. P. Woychik, L. B. Russell et al., 2002. Molecular and phenotypic analysis of 25 recessive, homozygous-viable alleles at the mouse agouti locus. Genetics 160 659–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minvielle, F., D. Gourichon, S. Ito, M. Inoue-Murayama and S. Rivière, 2007. Effects of the dominant lethal yellow mutation on reproduction, growth, feed consumption, body temperature, and body composition of the Japanese quail. Poult. Sci. 86 1646–1650. [DOI] [PubMed] [Google Scholar]
- Miwa, M., M. Inoue-Murayama, B. B. Kayang, A. Vignal, F. Minvielle et al., 2005. Mapping of plumage color and blood protein loci on the microsatellite linkage map of the Japanese quail. Anim. Genet. 36 396–400. [DOI] [PubMed] [Google Scholar]
- Nadeau, N. J., F. Minvielle and N. I. Mundy, 2006. Association of a Glu92Lys substitution inMC1R with extended brown in Japanese quail (Coturnix japonica). Anim. Genet. 37 287–289. [DOI] [PubMed] [Google Scholar]
- Nadeau, N. J., F. Minvielle, S. Ito, M. Inoue-Murayama, D. Gourichon et al., 2008. Characterization of Japanese quail yellow as a genomic deletion upstream of the avian homolog of the mammalian ASIP (agouti) gene. Genetics 178 777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder, S., S. Taourit, D. Mariat, B. Langlois and G. Guérin, 2001. Mutations in the agouti (ASIP), the extension (MC1R), and the brown (TYRP1) loci and their association to coat color phenotypes in horses (Equus caballus). Mamm. Genome 12 450–455. [DOI] [PubMed] [Google Scholar]
- Silvers, W. K., 1979. The Coat Colors of Mice: A Model for Mammalian Gene Action and Interaction. Springer-Verlag, New York.
- Somes, Jr., R. G., 1979. Genetic bases for plumage color patterns in four varieties of Japanese quail. J. Hered. 70 205–210. [Google Scholar]
- Tsudzuki, M., S. Ito, K. Sato, S. Takahashi and H. Uchida, 1996. Fawn-2: a dominant plumage color mutation in Japanese quail. J. Hered. 87 248–252. [Google Scholar]
- Våge, D. I., D. Lu, H. Klungland, S. Lien, S. Adalsteinsson et al., 1997. A non-epistatic interaction of agouti and extension in the fox, Vulpes vulpes. Nat. Genet. 15 311–315. [DOI] [PubMed] [Google Scholar]
- Van Hoof, A., P. A. Frischmeyer, H. C. Dietz and R. Parker, 2002. Exosome-mediated recognition and degradation of mRNAs lacking a termination codon. Science 295 2262–2264. [DOI] [PubMed] [Google Scholar]