Abstract
Studies in Drosophila have defined a new growth inhibitory pathway mediated by Fat (Ft), Merlin (Mer), Expanded (Ex), Hippo (Hpo), Salvador (Sav)/Shar-pei, Warts (Wts)/Large tumor suppressor (Lats), and Mob as tumor suppressor (Mats), which are all evolutionarily conserved in vertebrate animals. We previously found that the Mob family protein Mats functions as a coactivator of Wts kinase. Here we show that mats is essential for early development and is required for proper chromosomal segregation in developing embryos. Mats is expressed at low levels ubiquitously, which is consistent with the role of Mats as a general growth regulator. Like mammalian Mats, Drosophila Mats colocalizes with Wts/Lats kinase and cyclin E proteins at the centrosome. This raises the possibility that Mats may function together with Wts/Lats to regulate cyclin E activity in the centrosome for mitotic control. While Hpo/Wts signaling has been implicated in the control of cyclin E and diap1 expression, we found that it also modulates the expression of cyclin A and cyclin B. Although mats depletion leads to aberrant mitoses, this does not seem to be due to compromised mitotic spindle checkpoint function.
CANCER arises from defective regulation in diverse cellular activities such as cell cycle, apoptosis, signal transduction, maintenance of cell polarity, and cell adhesion. Recent research in Drosophila has contributed to characterizing the Hippo (Hpo) and Warts/Large tumor suppressor (Wts/Lats) signaling pathway that controls both cell proliferation and apoptosis (reviewed in Edgar 2006; Hariharan and Bilder 2006; Harvey and Tapon 2007; Pan 2007). Components in Hpo/Wts signaling are evolutionarily conserved as Drosophila mutants can be functionally rescued by their respective human homologs (Tao et al. 1999; Wu et al. 2003; Lai et al. 2005).
Two upstream components implicated in the Hpo/Wts signaling are the FERM-domain-containing membrane-associated factors, Merlin/Expanded, whose activity can increase the kinase function of Hpo (Hamaratoglu et al. 2006). The Fat (Ft) protein may function as a receptor farther upstream or in parallel with Hpo/Wts signaling (Harvey and Tapon 2007; Pan 2007). Hpo associates with an adaptor protein Salvador (Sav), and this interaction is shown to increase Hpo phosphorylation to another kinase Wts/Lats (Kango-Singh et al. 2002; Tapon et al. 2002; Wu et al. 2003; Colombani et al. 2006). We have previously identified Mob as tumor suppressor (Mats) as a coactivator for Wts/Lats kinase (Lai et al. 2005) and have shown that Hpo enhances Mats function via phosphorylation (Wei et al. 2007). Loss of function in any of these genes results in upregulation of cyclin E and Drosophila inhibitor of apoptosis (DIAP1), causing cell overproliferation and defective cell death in mosaic tissues (Justice et al. 1995; Xu et al. 1995; Kango-Singh et al. 2002; Tapon et al. 2002; Harvey et al. 2003; Jia et al. 2003; Pantalacci et al. 2003; Udan et al. 2003; Wu et al. 2003; Lai et al. 2005). As a major downstream target, the growth-promoting transcriptional cofactor Yorkie (Yki) is negatively regulated by the Hpo/Wts growth inhibitory pathway (Huang et al. 2005).
Studies of homologs in yeast and human cells have shown potential cellular activities of factors involved in the Hpo/Wts pathway. Budding yeast homologs of Wts and Mats (Dbf2 and Mob1, respectively) are components of the mitotic exit network (Komarnitsky et al. 1998; Luca and Winey 1998; Luca et al. 2001). Intracellular localization of these proteins are regulated during the cell cycle such that, until anaphase, they are localized at the spindle pole bodies but they move to the bud neck prior to actin ring assembly in a functionally interdependent manner (Frenz et al. 2000; Luca et al. 2001; Yoshida and Toh-e 2001). In mammalian cells, both Wts homologs, LATS1 and LATS2, were found to regulate G2/M-phase and G1/S-phase transitions, respectively (Tao et al. 1999; Yang et al. 2001; Xia et al. 2002; Li et al. 2003). Both LATS1 and LATS2 are found in the centrosome, and their loss of function results in multinucleation, centrosomal amplification, and genomic instability, suggesting that they are involved in some aspects of cell cycle progression (Nishiyama et al. 1999; Morisaki et al. 2002; McPherson et al. 2004; Toji et al. 2004).
Although mutations in wts or mats result in obvious overgrowth, mitotic defects associated with mutations in these factors have not been reported in Drosophila. Here we show that mats is an essential gene that is required for early embryonic development. The Mats protein is a centrosomal component that appears to be critical for maintaining genome stability and the disruption of mats function results in aberrant mitoses. However, this does not seem to be due to compromised mitotic spindle checkpoint function. Moreover, our data suggest that Mats regulates expression not only of cyclin E, but also of cyclin A and cyclin B, which are key regulators of cell cycle progression in both invertebrate and vertebrate animals.
MATERIALS AND METHODS
Analysis of homozygous mats mutant:
To assess the lethal stage of homozygous mats− mutants, w1118; FRT82B mats−/CyO·TM32xhs-GFP was crossed with w1118; Df(3R)17D1/CyO·TM32xhs-GFP to generate mats−/Df(3R)17D1 larvae, which are equivalent to the homozygous mats mutant. Expression of green fluorescent proteins (GFP) was induced by heat treatment of larvae at 37° for 15 min. Larvae of genotypes mats−/Df(3R)17D1 and mats−/CyO·TM32xhs-GFP [or Df(3R)17D1/CyO·TM32xhs-GFP] were placed on a microscope slide. Both matse235 and matsroo alleles were used. Images were taken with the Nikon Coolpix990 digital camera mounted on the Nikon Eclipse TS100 inverted scope.
Analysis of maternally mats null embryos:
Dominant female sterility (DFS) technique takes advantage of dominant ovoD1 mutation that renders sterility to oocytes (Chou and Perrimon 1996). Thus, for heterozygous females carrying ovoD1 to be able to lay eggs, somatic recombination needs to happen to generate clones of homozygous oocytes that have eliminated ovoD1. When used in combination with the third chromosome that harbors the mats mutation, this technique generates homozygous mats mutant oocytes in heterozygous females, and only these oocytes can generate eggs. Female flies of y, hs-FLP; FRT82B matse235/TM6B were crossed with males of w1118; FRT82B ovoD1/TM3, and embryos were collected for 24 hr. Hatched larvae were heat-shocked for 20–30 min at 37° in L2 stage, and resulting virgin females of hs-FLP; FRT82B matse235/FRT82B ovoD1 were collected. As ovoD1 gives dominant female sterility, for females of this genotype to be able to lay eggs, they must undergo somatic recombination in the ovary to generate FRT82B matse235/FRT82B matse235 cells, eliminating ovoD1 and functional the mats allele from their genome. These flies were crossed with males of w1118; FRT82B matse235/CyO·TM32xhs-GFP and resulting embryos were heat-shocked at 37° for 15 min. Embryos and larvae were handpicked and placed on the microscope slide, and GFP autofluorescence was observed under a Zeiss microscope. The animals were categorized by developmental stage (embryo or larva) and the presence or absence of GFP, and their numbers were scored.
For immunostaining, embryos were collected for 2–3 hr, and handpicked embryos were washed in PBS and dechorinated in mild bleach for 2 min. After washes, embryos were fixed in a 1:1 mix of paraformaldehyde-lysine-phosphate (PLP: 2% paraformaldehyde, 0.75 m poly-l-lysine, 0.25% sodium periodate) and heptane on the bench top for 20 min. Then PLP was replaced by methanol, and the samples were vigorously shaken to remove vitelline membrane. Embryos were then rehydrated with balanced salt solution (BSS) Triton 0.3% (Ashburner 1989) and stained with anti-Cnn antibodies (a gift of Thomas Kaufman; Megraw et al. 2002) at 4° overnight. The secondary anti-rabbit Alexa Fluor (AF) 488 antibodies (Molecular Probes, Eugene, OR) were used at 1:500 dilutions. Images were taken with an Olympus FluoView 300 confocal microscope.
Cell culture and immunostaining:
HEK293T cells were cultured in DMEM containing 10% fetal bovine serum (FBS). Transfection was mediated through Polyfect transfection reagent (QIAGEN, Valencia, CA). Transfected cells were seeded on coverslips coated with FBS and fixed with methanol (−20°). Cells were washed and stained with either anti-Myc antibodies or anti-CycE antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) in BSS with 0.3% Triton. Anti-rabbit AF594 (1:500) and anti-mouse AF680 (1:200) antibodies were used for secondary staining. Draq5 (Biostatus, Leiscestershire, UK) was used for DNA staining.
Genetic interactions of Wts/Lats signaling:
UAS-myc-wts6R on the second chromosome is a relatively strong allele and was recombined with ey-Gal4 to generate w1118; ey-Gal4, UAS-myc-wts6R/SM6·TM6B. Females of this genotype were crossed with w1118, UAS-cycE, UAS-diap1, UAS-cycA, UAS-cycB, cyclin EAR95/TM3, diap14/TM3, cyclin AC8LR1/TM3, cyclin A03946/TM3, cyclin B2/CyO, or cyclin BKG08886/CyO male flies. UAS transgenic flies were crossed with w1118; ey-Gal4/SM6·TM6B to generate flies for comparison. Flies carrying loss-of-function alleles of cyclin E, diap1, cyclin A, and cyclin B were also crossed with w1118 flies to determine their heterozygous phenotypes.
Somatic homozygous mats mutant cells were generated by crossing w1118; FRT82B matse235/TM6B males with y,w, ey-FLP; FRT82B arm-lacZ/TM6B females. For control, w1118 larvae of the same developmental stage were used. The eye discs of Tb+ larvae were dissected and fixed with PLP on ice for 45 min and washed with BSS with 0.3% Triton twice. Peripodial membranes were removed, and discs were stained with primary antibodies [mouse anticyclin A (1:5 dilution) and mouse anticyclin B (1:5 dilution) antibodies; Developmental Studies Hybridoma Bank at the University of Iowa] followed by AF 680 fluorescent secondary antibodies (1:200). Clonal expression of Yki in larval discs was achieved using hsFLP122 actin>CD2>Gal4 UAS-lacZ/UAS-yki-V5 flies, which were treated at the early second instar larval stage for 1 hr at 37° to allow FLP expression. Wing and eye discs were isolated from matured third instar larvae. Antibodies for cyclin A, cyclin B, and β-galactosidase were used for immunostaining of the larval tissues.
Mitotic checkpoint analysis:
Third instar larval eye discs containing control or mps1 or mats homozygous mutant clones were generated by crossing w1118; FRT82B 90E P[w+] or w1118; FRT82B mps11/TM6B (a gift of Christian Lehner; Fischer et al. 2004) or w1118; FRT82B matse235/TM6B males with UAS-GFP, ey-FLP; Tub-Gal4, FRT82B Tub-Gal80/TM6B females. Dissected eye discs were incubated for 3 hr in Schneider S2 medium (Invitrogen, Carlsbad, CA) either with or without 1 μm colcemid (Sigma, St. Louis) before staining with phosphohistone H3 antibodies (1:200; a gift of Esther Siegfried). Clones were identified by the presence of GFP signals.
RESULTS
Mats is essential for embryonic development:
mats has been previously shown to be a growth inhibitor and its clonal loss of function promotes tissue outgrowth (Lai et al. 2005). mats is an essential gene required for normal development, as development of zygotic homozygous mats mutant animals did not proceed beyond the second instar larval stage. These animals became sluggish and eventually died. Because mats mRNA exists in young embryos (data from Berkeley Drosophila Genome Project, http://www.fruitfly.org), it is possible that maternal contribution of mats mRNA and/or protein product can rescue the homozygous mutant animals to a certain extent, delaying the lethal stage. To test this possibility, germline clones of mats mutant cells were made to eliminate this maternal loading of mats to the embryos. Using a DFS technique (Chou and Perrimon 1996), maternally and zygotically mats null mutants were generated and identified by the absence of GFP expression upon heat shock. On the other hand, mats heterozygotes collected after midblastula transition would produce GFP upon heat treatment due to heat-shock-inducible GFP contained in the balancer chromosome. This analysis revealed that maternally and zygotically null embryos did not hatch. Thus, maternal mats is indeed critical for viability. Interestingly, maternally null and zygotically heterozygous animals were viable and survived to the adult stage. Of 116 larvae observed, 113 of them were alive and exhibited GFP reporter expression. They grew up to become fertile adults without any obvious morphological defects. The remaining three larvae were dead and had no GFP expression and were probably escapers of mats maternal and zygotic null mutants. Alternatively, these may be carcasses of mats heterozygotes that lost GFP signals after death. These results suggest that mats maternal function can be rescued by zygotic mats.
Mats is required for proper chromosomal segregation:
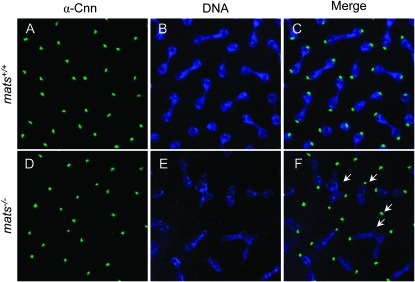
Up to the midblastula transition, the cellular function of Drosophila embryos relies on maternally loaded transcripts and protein products (Edgar et al. 1986). Therefore, in maternally mats null embryos, the cell divisions up to the 16th cycle occur in the total absence of Mats function. To observe cell division phenotype in the absence of Mats, embryos from the maternally null crosses were collected before midblastula transition (at this point embryos included both maternally and zygotically null and heterozygous zygotes) and the behavior of a centrosomal marker Centrosomin (Cnn) (Megraw et al. 2002) and DNA (Draq5) was examined. While in wild-type embryos, an equal amount of DNA was segregated to two centrosomal poles (Figure 1, A–C), all embryos generated from maternal null oocytes showed aberrant DNA segregation (Figure 1, D–F). Maternally mats null embryos also appeared to have DNA fragmentation in some divisions and occasionally three or more centrosomes were associated with one pool of segregating DNA (Figure 1F). Thus Mats appears to play a role in ensuring the proper chromosomal segregation during mitosis.
Figure 1.—
mats mutations cause DNA segregation defect. Control (w1118) (A–C) and maternally mats null mutant (D–F) embryos were stained with anti-Cnn antibodies and the DNA dye Draq5. Centrosomes were identified by Cnn (A and D) and DNA by Draq5 (B and E). Compared to control embryos (C), maternally mats-depleted embryos (F) exhibited DNA segregation defects. Arrows highlight aberrant DNA segregation observed in mats mutant embryos.
Mats is ubiquitously expressed at low levels in developing tissues:
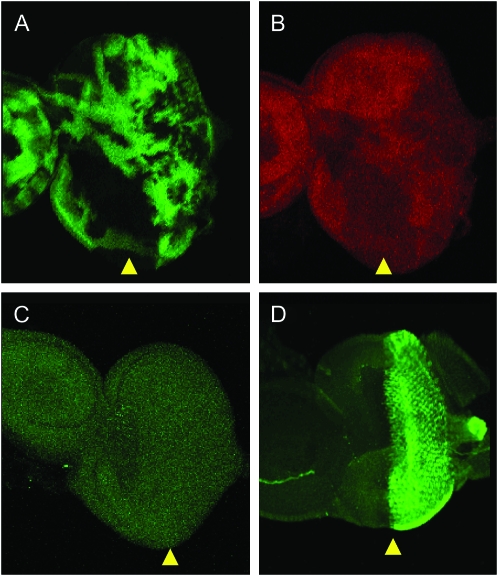
To better understand the developmental role of Mats, the expression pattern of the endogenous Mats protein has been analyzed. For this purpose, third instar larval imaginal discs were dissected and stained with anti-Mats antibodies (Lai et al. 2005). To ensure the specificity of the antibodies, mats homozygous null mutant clones were generated by FLP/FRT-mediated somatic recombination (Xu and Rubin 1993). As shown in Figure 2, A and B, the staining level was reduced in the mats null mutant clones. In addition, the endogenous staining was compared with a positive control, in which two copies of full-length mats transgenes were under the control of GMR-Gal4 for expression in cells posterior to the morphogenetic furrow. As a result, very strong staining signals were observed posterior to the morphogenetic furrow, while endogenous Mats staining in the anterior region of the eye disc and in other larval tissues such as leg and wing discs was low (Figure 2, C and D; data not shown). Staining level observed in Figure 2C is comparable to the anterior eye disc staining in Figure 2D. These results suggest that endogenous mats is ubiquitously expressed at a low level and that the Mats antibody can specifically recognize the Mats protein.
Figure 2.—
mats is expressed at low levels ubiquitously. A mosaic third instar larval eye disc containing mats null clones is shown (A and B). Mutant clones of mats were identified by the absence of β-gal staining in A. Anti-Mats antibody staining in B showed lower levels of Mats protein in mats mutant cells. (C) Endogenous mats staining in the w1118 eye disc showed very low signals. (D) When mats transgenes were expressed in GMR-Gal4; UAS-matsB121B142, high levels of Mats staining were observed posterior to the morphogenetic furrow. The anterior half of the eye disc showed very low levels of staining similar to that of endogenous staining observed in C. Arrowheads identify the morphogenetic furrow. Anterior is to the left in A–D.
It is known that cells in an eye disc are synchronized with respect to the progression of the morphogenetic furrow, such that cells in the furrow are in G1 phase, and as cells emerge from the furrow, they undergo S phase synchronously and complete one cell cycle prior to the terminal differentiation (Baker 2001). Since there is no particular endogenous expression level change adjacent to the morphogenetic furrow, mats expression does not appear to fluctuate throughout the cell cycle. Similarly, microarray analysis indicates that mRNA levels of human MATS genes do not dramatically alter during the cell cycle (Whitfield et al. 2002). These observations suggest that regulation of mats may occur mainly through protein modifications.
Mats and Wts are colocalized at the centrosome:
To determine subcellular localization of Mats and its binding partner Wts kinase, Mats and Wts proteins were tagged and expressed in cultured cells. Specifically, human embryonic kidney (HEK) 293T cells were used to transfect mats-GFP and myc-wts fusion genes under the control of the cytomegalovirus promoter. We found that Mats-GFP was localized in the cytosol as well as in the nucleus, whereas Wts was exclusively cytosolic during interphase (Figure 3). It was also clear that both Mats and Wts accumulated at the perinuclear region in a dot-like pattern. This dot-like pattern was duplicated once the mitotic DNA segregation commenced. These accumulations appeared to be centrosomes, as they colocalized with γ-tubulin signals (Figure 3, A–H). This observation is consistent with the centrosomal localization of human LATS1 and MOB1A (MATS2) reported elsewhere (Nishiyama et al. 1999; Bothos et al. 2005). Mats and Wts are also localized at the midbody area during cytokinesis (Figure 3, B, D, F, and H). Moreover, Mats and Wts colocalize with endogenous human cyclin E at the centrosome (Figure 3, I–P). Thus, Mats and Wts proteins appear to function together in subcellular organelles such as centrosomes.
Figure 3.—
Mats and Wts accumulate at the centrosome throughout the cell cycle. (A–H) In transfected HEK293T cells, Mats and Wts colocalize with γ-tubulin at the centrosome during mitosis (A, C, E, and G) and cytokinesis (B, D, F, and H). After formation of the nuclear membrane, Mats was found in both the cytosol and the nucleus, while Wts was excluded from the nucleus. Both during mitotic phase and after nuclear membrane formation, Mats (A and B) and Wts (C and D) were accumulated at the centrosome together with γ-tubulin (E–H). (I–P) Mats and Wts also colocalized with cyclin E at the centrosome. During interphase, Mats and Wts accumulated at the centrosome in the perinuclear region and this pattern was also seen in the endogenous cyclin E staining (I, K, M, and O). This pattern was maintained throughout mitosis (J, L, N, and P). The centrosome is indicated by arrows.
Genetic interactions among wts, diap1, and cyclin E genes:
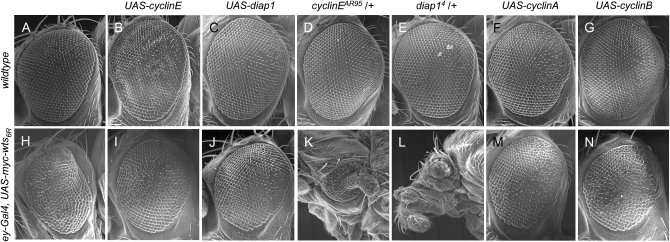
cyclin E and diap1 are considered to be two downstream targets of the Hpo/Wts pathway (reviewed in Harvey and Tapon 2007; Pan 2007). To further observe functional interactions between Hpo/Wts signaling and its output, ey-Gal4 was used to express UAS-myc-wts in the developing eye. Compared to the previously reported line 16B (Lai et al. 2005), the 6R line exhibited stronger phenotypes. Thirty percent of the ey-Gal4 UAS-myc-wts6R/+ flies died at the late pupal stage and the surviving flies typically had their eyes severely reduced in size and shaped like a cone (Figure 4H). Overexpression of cyclin E showed a slightly rough eye (Figure 4B), whereas ey-Gal4-driven diap1 expression showed relatively normal eye morphology (Figure 4C). When combined with ey-Gal4 UAS-myc-wts6R, expression of cyclin E and diap1 effectively suppressed the cone-shape and small-eye phenotypes caused by wts overexpression (compare Figure 4I and 4J with 4H). All of the ey-Gal4 UAS-myc-wts6R/UAS-cycE and ey-Gal4 UAS-myc-wts6R/UAS-diap1 flies survived to the adult stage (Table 1). Moreover, the effects of loss-of-function alleles of cyclin E and diap1 on Wts-induced mutant phenotypes were examined. While cycEAR95 and diap14 heterozygotes were phenotypically normal (Figure 4, D and E), Wts-induced mutant phenotypes were strongly enhanced by the reduction of endogenous cyclin E or diap1 function such that animals had underdeveloped head tissue and their eyes were extremely reduced in size with some of them exhibiting a rod-like structure extending from the center of the eye (compare Figure 4K and 4L with 4H). The lethal phenotype was also enhanced as 60% of the ey-Gal4 UAS-myc-wts6R/cycEAR95 flies and 85% of the ey-Gal4 UAS-myc-wts6R/diap14 flies died at the pupal stage (Table 1). These results further support cyclin E and diap1 as critical targets of Hpo/Wts signaling.
Figure 4.—
wts overexpression phenotype is effectively modified by dosage change of diap1 and cyclin genes. SEM micrograph of (A) w1118 adult eye is used as a positive control. Overexpression of cyclin E caused a slightly rough-eye phenotype (B, ey-Gal4/UAS-cycE), while expression of diap1 was normal (C, ey-Gal4/+; +/UAS-diap1). Heterozygosity of cyclin E (D, cycEAR95/+) or diap1 (E, diap14/+) did not show eye phenotypic change. (F) ey-Gal4/UAS-cyclin A. (G) ey-Gal4/UAS-cyclin B. (H) Overexpression of wts in ey-Gal4 UAS-myc-wts6R/+ reduced viability and eyes became smaller and cone shaped. Coexpression of either cyclin E (I, ey-Gal4 UAS-myc-wts6R/UAS-cycE) or diap1 (J, ey-Gal4 UAS-myc-wts6R/+; +/UAS-diap1) effectively suppressed wts-induced small and cone-shaped eye phenotypes. On the other hand, reduction of either cyclin E (K, ey-Gal4 UAS-myc-wts6R/cycEAR95) or diap1 (L, ey-Gal4 UAS-myc-wts6R/+; +/diap14) function resulted in strong enhancement of growth inhibition caused by Wts. These flies were lethal at the late pupa stage; thus SEM pictures were taken from flies dissected from the pupa case. (M) ey-Gal4 UAS-myc-wts6R/UAS-cyclin A. (N) ey-Gal4 UAS-myc-wts6R/UAS-cyclin B. Anterior is to the left in A–N.
TABLE 1.
wts genetically interact with diap1and cyclin genes
| Genotype | % of flies that died at the pupal stage |
|---|---|
| ey-Gal4, UAS-myc-wts6R/+ | 30 (n = 198) |
| ey-Gal4, UAS-myc-wts6R/UAS-cyclin E | 0 (n = 65) |
| ey-Gal4, UAS-myc-wts6R/UAS-diap1 | 0 (n = 101) |
| ey-Gal4, UAS-myc-wts6R/cyclin EAR95 | 60 (n = 70) |
| ey-Gal4, UAS-myc-wts6R/diap14 | 85 (n = 20) |
| ey-Gal4/UAS-cyclin A | 53 (n = 66) |
| ey-Gal4, UAS-myc-wts6R/UAS-cyclin A | 63 (n = 46) |
| ey-Gal4, UAS-myc-wts6R/UAS-cyclin B | 0 (ND) |
Viability of cyclin EAR95/+ and diap14/+ heterozygotes is normal. Similarly, overexpression of cyclin E, diap1, and cyclin B driven by ey-Gal4 did not affect viability. ND, not determined.
Expression of cyclin A and cyclin B is negatively regulated by Hpo/Wts signaling:
To examine whether mats also regulates expression of other cyclin genes such as cyclin A and cyclin B, immunostaining experiments using cyclin A and cyclin B antibodies were conducted. We found that loss of mats function in larval eye discs resulted in elevated levels of both cyclin A (Figure 5, B–B″) and cyclin B proteins (Figure 5, D–D″). Moreover, clonal expression of Yki in developing tissues such as wing discs was able to increase the levels of cyclin A and cyclin B proteins (Figure 5, F–F″ and H–H″). These results support the idea that cyclin A and cyclin B genes are targets of Hpo/Wts signaling.
Figure 5.—
Regulation of cyclin A and cyclin B expression by mats and yki. In normal larval eye discs, both cyclin A and cyclin B are upregulated in the second mitotic wave area just posterior to the morphogenetic furrow (A and C, respectively). Probed with cyclin A and cyclin B antibodies, higher levels of cyclin A (B–B″) and cyclin B (D–D″) proteins were found in mats mutant clones, which were identified in the absence of β-galactosidase expression. The morphogenetic furrow is indicated by yellow arrowheads (A–D″). Anterior is to the left in A–D″. Expression of endogenous cyclin A (E) and cyclin B (G) in wild-type third instar larval wing discs. (F–F″) Expression of the yki-V5 transgene was clonally induced, which caused elevated levels of cyclin A (F–F″) and cyclin B (H–H″). Yki-V5 expression clones were identified by the presence of β-galactosidase expression. Examples of mats mutant clones and Yki overexpression clones are indicated by white arrows.
Using the ey-Gal4 UAS-myc-wts6R assay, we have examined how cyclin A and cyclin B genes might genetically interact with wts. To do this, UAS-myc-wts6R was ectopically expressed in combination with cyclin A or cyclin B. Fifty-three percent of the ey-Gal4/UAS-cycA flies died at the pupal stage (Table 1) and those that survived to the adult stage (18%) showed slightly reduced eyes (Figure 4F). On the contrary, expression of cyclin B driven by ey-Gal4 in the wild-type background had no effect on viability and showed normal-eye phenotypes (Figure 4G). Coexpression of cyclin B with wts6R effectively suppressed Wts-induced pupal lethality as all flies survived to the adult stage (Table 1), and it suppressed cone-shape and small-eye phenotypes as well (Figure 4N). For cyclin A, it was also able to suppress Wts-induced eye phenotypes (Figure 4M), while there were still 63% of the ey-Gal4 UAS-myc-wts6R/UAS-cycA flies that died at the pupal stage (Table 1). Thus, results from these gain-of-function alleles of cyclin A and cyclin B are consistent with the model that cyclin A and cyclin B are targets of the Hpo/Wts signaling pathway. When loss-of-function alleles of cyclin A and cyclin B genes were tested in this assay, however, no significant modification of the eye phenotype of ey-Gal4 UAS-myc-wts6R flies was observed (data not shown). It appears to be that the eye phenotype of ey-Gal4 UAS-myc-wts6R flies is not sensitive to the reduction of cyclin A and cyclin B function.
Mats is not involved in mitotic checkpoint:
The possibility that mats mutation would cause genomic instability suggests its involvement in cell cycle checkpoint function. To assess whether Mats plays a role in this cellular response, the MARCM system (Lee and Luo 1999) was used to generate eye discs containing control, mps1 mutant, or mats mutant clones, and the tissue was dissected and incubated in either the presence or absence of colcemid for 2 hr and the accumulation of M-phase cells was analyzed. Discs containing control clones accumulated M-phase cells in response to colcemid treatment especially at the posterior disc area adjacent to the morphogenetic furrow [Figure S1, A–F, at http://www.genetics.org/supplemental/; − colcemid (n = 7 eye discs), + colcemid (n = 10 eye discs)]. Discs containing mps1 mutant clones, however, showed accumulation of M-phase cells only in wild-type tissue (GFP negative) but accumulation was not seen at the mps1 mutant clones (GFP positive) [Figure S1, G–L; − colcemid (n = 8 eye discs), + colcemid (n = 12 eye discs)] as previously reported (Fischer et al. 2004). Thus, mutations in mps1 resulted in failure to initiate mitotic checkpoint response, and consequently mps1 mutant cells did not arrest at the M phase. Discs containing mats mutant clones, on the other hand, accumulated M-phase cells exactly as seen in the control group, especially posterior to the morphogenetic furrow [Figure S1, M–R; − colcemid (n = 6 eye discs), + colcemid (n = 8 eye discs)]. These results indicate that mat is not involved in the spindle checkpoint response.
DISCUSSION
Here we report that mats is an essential gene that regulates proper mitotic division. Mats is expressed at low levels ubiquitously, which is consistent with its role as a general regulator of tissue growth. Cellular localization analysis indicated that Mats is present in both the cytosol and the nucleus. Interestingly, Mats and Wts colocalize at the centrosome, suggesting that the centrosome is likely a functional site of the Mats/Wts kinase complex. In addition to cyclin E and diap1, cyclin A and cyclin B may also be targets of the Hpo/Wts signaling pathway.
Mats is essential for normal development as mats mutants stop their growth at the second instar larval stage and eventually die. In fact, this growth retardation phenotype facilitated identification of matsroo and matse235 mutant larvae for DNA sequence analysis (Lai et al. 2005). Using our matse235 allele and the P-element-induced allele matsPB, He et al. (2005) showed that mats homozygotes and hemizygotes grew slowly and their imaginal discs were much smaller than that of wild-type larvae at the same age. mats mutant cells in mosaic tissues acquire growth advantage likely through comparison and competition with neighboring wild-type cells. In contrast, the absence of wild-type cells in homozygous mats mutant animals renders no competitive growth advantage to mutant cells. The mechanism by which mats mutants acquire growth advantage in the context of mosaic tissue still needs to be investigated. mats mutant embryos missing both maternal and zygotic mats functions failed to hatch, indicating that mats is essential for embryonic development. By analyzing mitotic cells, we found that maternally mats-depleted embryos showed aberrant DNA segregation such that uneven amounts of DNA were segregated toward opposing centrosomes. However, this did not appear to be due to the compromised function of mitotic spindle checkpoint, as mats mutant tissue still accumulated M-phase cells in response to inhibition of mitotic spindle formation by colcemid treatment. Thus, mats is not required for mitotic spindle checkpoint, unlike mps1.
Cyclin E is a critical cell cycle regulator (Sherr and Roberts 2004). Through a Cdk2-dependent mechanism, cyclin E-Cdk2 plays a critical role in accelerating G1–S transition in the cell cycle. As a general rule, cyclin E is tightly regulated during the cell cycle by Cdk2 and GSK-mediated phosphorylation and subsequent degradation. A nondegradable cyclin E mutant can cause extra rounds of DNA synthesis and polyploidy, and overexpression of cyclin E is frequently detected in tumor cells exhibiting polyploidy. Intriguingly, cyclin E is a centrosomal protein that functions to promote S-phase entry and DNA synthesis in a Cdk2-independent manner (Matsumoto and Maller 2004). Loss of cyclin E expression in the centrosome inhibits DNA synthesis, whereas ectopic expression of cyclin E in the centrosome accelerates S-phase entry. Thus, the centrosome is an important subcellular organelle for cyclin E to regulate cell proliferation, and the level and activity of cyclin E in centrosomes must be tightly controlled. The fact that Mats and Wts colocalize with cyclin E at the centrosome raises the possibility that Mats may function together with Wts kinase to regulate cyclin E function in the centrosome for mitotic control. In support of this hypothesis, loss-of-function mutations in mats increase the levels of cyclin E protein and both gain- and loss-of-function mutant alleles of cyclin E modulate the eye phenotypes caused by Wts overexpression (Lai et al. 2005; this study). Although Mats/Wts-mediated inhibition of cyclin E could occur through Yki to regulate cyclin E transcription, a direct control of cyclin E at the protein level would allow a rapid response to an upstream signal.
The fact that both Mats and Wts show a intracellular localization pattern very similar to that of their respective yeast relatives Mob1 and Dbf2 suggests that their function is conserved. This conservation may extend to mammals, as it has been shown that human LATS1, LATS2, and MOB1A (MATS2) also localize at the centrosome (Nishiyama et al. 1999; Morisaki et al. 2002; McPherson et al. 2004; Toji et al. 2004; Bothos et al. 2005; Abe et al. 2006). In addition, localization at the bud neck/midbody appears to be conserved in humans (Nishiyama et al. 1999; Bothos et al. 2005). Interestingly, such centrosomal localization of Mats and Wts does not seem to rely on Wts kinase activity as kinase-inactive Wts and Mats can be still localized at the centrosome (our unpublished observation). To examine whether endogenous Mats protein localizes at the centrosome, embryo immunostaining was done with Mats antibodies. As in larval tissues, expression of Mats protein in developing embryos does not exhibit any obvious pattern and Mats expression level is low and ubiquitous. Although we have not been able to show centrosomal localization of endogenous Mats protein, likely due to some technical problems, Mats (CG13852/Mob4) has been recently reported to be a centrosomal protein (Domingues et al. 2005).
Both loss- and gain-of-function analysis supports a model in which cyclin E and diap1 are critical downstream targets of Hpo/Wts signaling. Evidence in this report suggests that Hpo/Wts signaling may also target cyclin A and cyclin B. Consistent with this notion, elevated levels of cyclin B were found in ex mutant cells (Pellock et al. 2007). In another study, wts has been shown to be required for a negative control of cyclin A but not cyclin B expression (Tao et al. 1999). In humans, LATS1 was shown to be a negative regulator of Cdc2/cyclin A (Tao et al. 1999) and to function at the G2/M-phase transition (Yang et al. 2001; Xia et al. 2002), while LATS2 affects cyclin E/Cdk2 activity and regulates G1/S phase passage (Li et al. 2003). Thus, the ability of Hpo/Wts signaling to target cyclin genes important for cell cycle progression appears to be evolutionarily conserved.
Acknowledgments
We thank W. Du, T. Kaufman, C. Lehner, E. Siegfried, J. Treisman, the Bloomington Drosophila Stock Center, and the Developmental Studies Hybridoma Bank at the University of Iowa for reagents and fly strains. This work was supported by a grant to Z.-C.L. from the National Science Foundation (IBN-0348262).
References
- Abe, Y., M. Ohsugi, K. Haraguchi, J. Fujimoto and T. Yamamoto, 2006. LATS2-Ajuba complex regulates gamma-tubulin recruitment to centrosomes and spindle organization during mitosis. FEBS Lett. 580 782–788. [DOI] [PubMed] [Google Scholar]
- Ashburner, M., 1989. Drosophila: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Baker, N. E., 2001. Cell proliferation, survival, and death in the Drosophila eye. Semin. Cell Dev. Biol. 12 499–507. [DOI] [PubMed] [Google Scholar]
- Bothos, J., R. L. Tuttle, M. Ottey, F. C. Luca and T. D. Halazonetis, 2005. Human LATS1 is a mitotic exit network kinase. Cancer Res. 65 6568–6575. [DOI] [PubMed] [Google Scholar]
- Chou, T. B., and N. Perrimon, 1996. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics 144 1673–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani, J., C. Polesello, F. Josue and N. Tapon, 2006. Dmp53 activates the hippo pathway to promote cell death in response to DNA damage. Curr. Biol. 16 1453–1458. [DOI] [PubMed] [Google Scholar]
- Domingues, C., A. Wainman, C. Florindo, D. Glover and A. Tavares, 2005. Centrosomal protein Mob4 is a new tumor suppressor. Eur. Dros. Res. Conf. 19 DR6.
- Edgar, B. A., 2006. From cell structure to transcription: Hippo forges a new path. Cell 124 267–273. [DOI] [PubMed] [Google Scholar]
- Edgar, B. A., C. P. Kiehle and G. Schubiger, 1986. Cell cycle control by the nucleo-cytoplasmic ratio in early Drosophila development. Cell 44 365–372. [DOI] [PubMed] [Google Scholar]
- Fischer, M. G., S. Heeger, U. Hacker and C. F. Lehner, 2004. The mitotic arrest in response to hypoxia and of polar bodies during early embryogenesis requires Drosophila Mps1. Curr. Biol. 14 2019–2024. [DOI] [PubMed] [Google Scholar]
- Frenz, L. M., S. E. Lee, D. Fesquet and L. H. Johnston, 2000. The budding yeast Dbf2 protein kinase localises to the centrosome and moves to the bud neck in late mitosis. J. Cell Sci. 113(Pt. 19): 3399–3408. [DOI] [PubMed] [Google Scholar]
- Hamaratoglu, F., M. Willecke, M. Kango-Singh, R. Nolo, E. Hyun et al., 2006. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat. Cell Biol. 8 27–36. [DOI] [PubMed] [Google Scholar]
- Hariharan, I. K., and D. Bilder, 2006. Regulation of imaginal disc growth by tumor-suppressor genes in Drosophila. Annu. Rev. Genet. 40 335–361. [DOI] [PubMed] [Google Scholar]
- Harvey, K., and N. Tapon, 2007. The Salvador-Warts-Hippo pathway: an emerging tumour-suppressor network. Nat. Rev. Cancer. 7 182–191. [DOI] [PubMed] [Google Scholar]
- Harvey, K. F., C. M. Pfleger and I. K. Hariharan, 2003. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114 457–467. [DOI] [PubMed] [Google Scholar]
- He, Y., K. Emoto, X. Fang, N. Ren, X. Tian et al., 2005. Drosophila Mob family proteins interact with the related tricornered (Trc) and warts (Wts) kinases. Mol. Biol. Cell 16 4139–4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J., S. Wu, J. Barrera, K. Matthews and D. Pan, 2005. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 122 421–434. [DOI] [PubMed] [Google Scholar]
- Jia, J., W. Zhang, B. Wang, R. Trinko and J. Jiang, 2003. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 17 2514–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice, R. W., O. Zilian, D. F. Woods, M. Noll and P. J. Bryant, 1995. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 9 534–546. [DOI] [PubMed] [Google Scholar]
- Kango-Singh, M., R. Nolo, C. Tao, P. Verstreken, P. R. Hiesinger et al., 2002. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development 129 5719–5730. [DOI] [PubMed] [Google Scholar]
- Komarnitsky, S. I., Y. C. Chiang, F. C. Luca, J. Chen, J. H. Toyn et al., 1998. DBF2 protein kinase binds to and acts through the cell cycle-regulated MOB1 protein. Mol. Cell. Biol. 18 2100–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, Z.-C., X. Wei, T. Shimizu, E. Ramos, M. Rohrbaugh et al., 2005. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell 120 675–685. [DOI] [PubMed] [Google Scholar]
- Lee, T., and L. Luo, 1999. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22 451–461. [DOI] [PubMed] [Google Scholar]
- Li, Y., J. Pei, H. Xia, H. Ke, H. Wang et al., 2003. Lats2, a putative tumor suppressor, inhibits G1/S transition. Oncogene 22 4398–4405. [DOI] [PubMed] [Google Scholar]
- Luca, F. C., and M. Winey, 1998. MOB1, an essential yeast gene required for completion of mitosis and maintenance of ploidy. Mol. Biol. Cell 9 29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca, F. C., M. Mody, C. Kurischko, D. M. Roof, T. H. Giddings et al., 2001. Saccharomyces cerevisiae Mob1p is required for cytokinesis and mitotic exit. Mol. Cell. Biol. 21 6972–6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto, Y., and J. L. Maller, 2004. A centrosomal localization signal in cyclin E required for Cdk2-independent S phase entry. Science 306 885–888. [DOI] [PubMed] [Google Scholar]
- McPherson, J. P., L. Tamblyn, A. Elia, E. Migon, A. Shehabeldin et al., 2004. Lats2/Kpm is required for embryonic development, proliferation control and genomic integrity. EMBO J. 23 3677–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megraw, T. L., S. Kilaru, F. R. Turner and T. C. Kaufman, 2002. The centrosome is a dynamic structure that ejects PCM flares. J. Cell Sci. 115 4707–4718. [DOI] [PubMed] [Google Scholar]
- Morisaki, T., T. Hirota, S. Iida, T. Marumoto, T. Hara et al., 2002. WARTS tumor suppressor is phosphorylated by Cdc2/cyclin B at spindle poles during mitosis. FEBS Lett. 529 319–324. [DOI] [PubMed] [Google Scholar]
- Nishiyama, Y., T. Hirota, T. Morisaki, T. Hara, T. Marumoto et al., 1999. A human homolog of Drosophila warts tumor suppressor, h-warts, localized to mitotic apparatus and specifically phosphorylated during mitosis. FEBS Lett. 459 159–165. [DOI] [PubMed] [Google Scholar]
- Pan, D., 2007. Hippo signaling in organ size control. Genes Dev. 21 886–897. [DOI] [PubMed] [Google Scholar]
- Pantalacci, S., N. Tapon and P. Leopold, 2003. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat. Cell Biol. 5 921–927. [DOI] [PubMed] [Google Scholar]
- Pellock, B. J., E. Buff, K. White and I. K. Hariharan, 2007. The Drosophila tumor suppressors Expanded and Merlin differentially regulate cell cycle exit, apoptosis, and Wingless signaling. Dev. Biol. 304 102–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr, C. J., and J. M. Roberts, 2004. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 18 2699–2711. [DOI] [PubMed] [Google Scholar]
- Tao, W., S. Zhang, G. S. Turenchalk, R. A. Stewart, M. A. St. John et al., 1999. Human homologue of the Drosophila melanogaster lats tumour suppressor modulates CDC2 activity. Nat. Genet. 21 177–181. [DOI] [PubMed] [Google Scholar]
- Tapon, N., K. F. Harvey, D. W. Bell, D. C. Wahrer, T. A. Schiripo et al., 2002. salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 110 467–478. [DOI] [PubMed] [Google Scholar]
- Toji, S., N. Yabuta, T. Hosomi, S. Nishihara, T. Kobayashi et al., 2004. The centrosomal protein Lats2 is a phosphorylation target of Aurora-A kinase. Genes Cells 9 383–397. [DOI] [PubMed] [Google Scholar]
- Udan, R. S., M. Kango-Singh, R. Nolo, C. Tao and G. Halder, 2003. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat. Cell Biol. 5 914–920. [DOI] [PubMed] [Google Scholar]
- Wei, X., T. Shimizu and Z.-C. Lai, 2007. Mob as tumor suppressor is activated by Hippo kinase in growth inhibition in Drosophila. EMBO J. 26 1772–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield, M. L., G. Sherlock, A. J. Saldanha, J. I. Murray, C. A. Ball et al., 2002. Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol. Biol. Cell 13 1977–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S., J. Huang, J. Dong and D. Pan, 2003. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114 445–456. [DOI] [PubMed] [Google Scholar]
- Xia, H., H. Qi, Y. Li, J. Pei, J. Barton et al., 2002. LATS1 tumor suppressor regulates G2/M transition and apoptosis. Oncogene 21 1233–1241. [DOI] [PubMed] [Google Scholar]
- Xu, T., and G. M. Rubin, 1993. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117 1223–1237. [DOI] [PubMed] [Google Scholar]
- Xu, T., W. Wang, S. Zhang, R. A. Stewart and W. Yu, 1995. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 121 1053–1063. [DOI] [PubMed] [Google Scholar]
- Yang, X., D. M. Li, W. Chen and T. Xu, 2001. Human homologue of Drosophila lats, LATS1, negatively regulates growth by inducing G(2)/M arrest or apoptosis. Oncogene 20 6516–6523. [DOI] [PubMed] [Google Scholar]
- Yoshida, S., and A. Toh-e, 2001. Regulation of the localization of Dbf2 and Mob1 during cell division of Saccharomyces cerevisiae. Genes Genet. Syst. 76 141–147. [DOI] [PubMed] [Google Scholar]