Abstract
NAC1 is a novel member of the POZ/BTB (Pox virus and Zinc finger/Bric-a-brac Tramtrack Broad complex) but varies from other proteins of this class in that it lacks the characteristic DNA-binding motif, suggesting a novel role. We have employed constitutive gene deletion to elucidate the role of NAC1 in vivo. Nac1 mutant mice are viable with no obvious developmental or physiological impairments. Previous studies suggest a role for NAC1 in cocaine-mediated behaviors. Therefore, we evaluated a variety of behaviors associated with psychomotor stimulant effects in Nac1 mutant mice. Acute locomotor activating effects of cocaine or amphetamine are absent in Nac1 mutant mice, however longer exposure to these psychomotor stimulants result in the development of behavioral sensitization. Acute rewarding properties of cocaine and amphetamine are also blunted in mutant mice, yet repeated exposure resulted in conditioned place preference similar to that observed in wild type mice. Lastly, increases in extracellular dopamine in the nucleus accumbens, which accompany acute cocaine administration, are blunted in mutant mice, but following chronic cocaine extracellular dopamine levels are increased to the same extent as in wild-type mice. Together these data indicate involvement of NAC1 in the acute behavioral and neurochemical responses to psychomotor stimulants.
Introduction
Addiction is associated with long-term behavioral changes produced by repeated exposure to drugs of abuse. In particular, chronic exposure to psychomotor stimulants like cocaine and amphetamine produce adaptations in brain circuitry similar to those associated with long-term plasticity. Molecules that contribute to these enduring structural and behavioral changes elicited by addictive drugs may provide novel pharmacotherapeutic targets for treating addiction. One such molecule is the protein NAC1, a member of the POZ/BTB family of transcription factors (Pox virus and Zinc finger/Bric-a-brac Tramtrack Broad complex). In other proteins this motif mediates interactions among several other transcriptional regulators [1] however the Nac1 gene does not encode the characteristic DNA-binding motif domain [4], suggesting a unique function for this protein.
NAC1 was first discovered as an mRNA up-regulated in the nucleus accumbens by self-administered cocaine [4], and later shown to exist in two isoforms, long (lNAC1) and short (sNAC1; [14]). Relative to other known cocaine-regulated proteins, the expression of NAC1 has a unique expression profile in response to cocaine with increased expression evident at both an early and late phase of drug exposure. For example, levels of NAC1 rise shortly after acute administration and return to baseline within 12 hours after injection [4], similar to expression patterns seen for a variety of immediate early genes (IEGs) [3,4,17]. However, unlike most IEGs that return to baseline levels within a few days or weeks after drug exposure, levels of NAC1 in the nucleus accumbens are elevated over control one week following withdrawal from cocaine administration, and remain elevated for at least 3 months. This unique profile poses the possibility that the cocaine-induced changes in NAC1 expression may influence the behavioral response to acute cocaine, as well as the expression of addicted behaviors produced by long-term cocaine administration, such as behavioral sensitization, craving and paranoia [7,8].
Two splice variants of NAC1 have been identified based on cDNA cloning: long NAC1 (lNAC1) and a short form that has 27 fewer amino acids (sNAC1) [14]. The sNAC1 expression increases rapidly and transiently in the nucleus accumbens 2 hours following acute cocaine administration [14]. In contrast lNAC1 expression is increased with repeated administration of cocaine. Moreover, when levels of lNAC1 and sNAC1 are correlated with the development of sensitization, animals that did not sensitize had significantly higher levels of lNAC1 in the nucleus accumbens compared to animals that did sensitize [22]. These data suggest that NAC1 may serve to prevent the development of sensitization to repeated cocaine. This inverse correlation appears to be causally relevant in light of antisense experiments, though these studies do not differentiate the contribution of specific NAC1 isoforms. Regardless, rats microinjected with two different antisense oligonucleotides into the nucleus accumbens demonstrate an increase in locomotor activity elicited by acute cocaine [11]. Increased expression of genes or proteins is usually associated with a role in enhancing behavioral effects, however, in the case of NAC1, the induction may be a compensatory response that will decrease consequences of cocaine administration. Indeed, adenoviral-mediated overexpression of NAC1 (Ad-NAC1) protein in the nucleus accumbens of rats blocked the development of behavioral sensitization to the locomotor activating effects of cocaine [16].
Gene targeting is a powerful approach to investigate gene function in vivo. By generating mice homozygous for a null allele for Nac1 we are able to study the role of NAC1 with respect to psychostimulant-induced behaviors. Given our previous findings that interrupted expression of NAC1 via antisense oligonucleotide injection augments the behavioral responses to cocaine and that adenoviral-mediated overexpression of NAC1 protein in the rat nucleus accumbens prevents the development but not the expression of cocaine behavioral sensitization, we hypothesized that NAC1-deficient mice would show deficits in the development of behavioral sensitization and reward learning. The present study examines the effects of two psychomotor stimulants cocaine and amphetamine. In both cases, mice deficient in NAC1 protein demonstrated significant reductions in the behavioral and neurochemical effects of acute cocaine and amphetamine administration, but no alterations in chronic responses to these drugs.
Materials and Methods
Generation of Nac1 mutant mice
The mouse Nac1 gene was cloned from a 129SvJ BAC library (Research Genetics, Inc., Huntsville, AL, USA) as described previously [15]. The targeting vector containing the Escherichia coli lacZ gene encoding β-galactosidase and the neomycin-resistance gene driven by the mouse phosphoglycerate kinase promoter [10] was inserted into exon 2 (figure 1A). Exon 2 was chosen as it encodes for the POZ/BTB functional domain of the NAC1 protein. Thus, deletion of exon 2 of the Nac1 gene produces a functional null allele. The construct was electroporated into mouse 129SvTac embryonic stem (ES) cells (a gift from Dr. P. Labosky, Vanderbilt University) and neomycin resistant colonies were selected for Southern blot analysis. 198 resistant clones were analyzed by PCR using a common 5' primer (5'-GGCCGCTAGTAGCTCTTACTT-3'), and specific 3' primers derived from exon 2 (5'-GTCGCAACTTGGAGAGCTAAC-3') for the wild type allele, resulting in an amplified fragment of 243 bp and the lacZ gene (5'-CAAAGCGCCATTCGCCATTCA-3') for the mutant allele resulting in an amplified fragment of 340 bp. Targeted ES cells were injected into C57BL/6N blastocycts to obtain 8 germ line chimeras. Chimeric mice were mated with C57BL/6N mice (Taconic, USA) to obtain heterozygote mice, which were mated to give rise to mice of all three genotypes (figure 1B).
Figure 1.
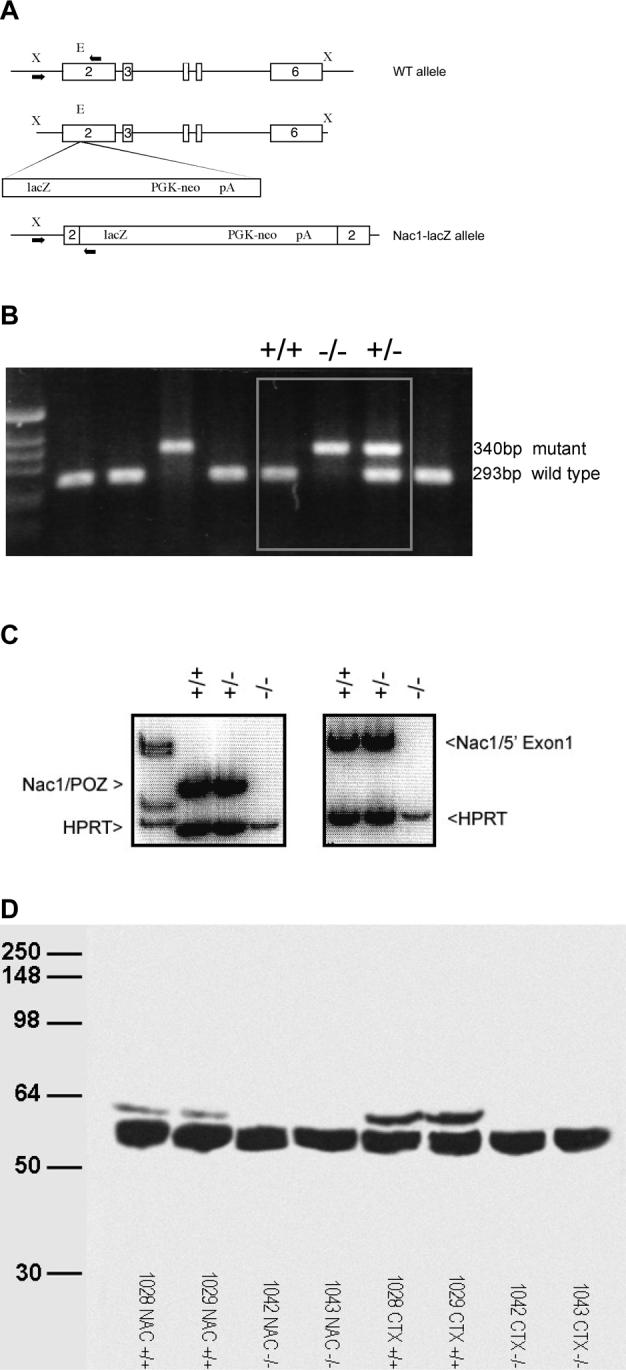
Generation of Nac1 mutant mice. A. The targeting vector of Nac1 a 5kb lacZ-PGK-neo cassette was inserted into exon 2; Enzyme restriction sites are designated as X;Xba and E; EagI, the positions of primersets used to detect wild-type and mutant alleles are indicated by black arrows, geneotypes of mice were identified using PCR primers corresponding to 5' and 3' in wild type allele, where the 3' primer is located just downstream of the EagI site where the targeting vector was inserted. B. DNA analysis in wild type and mutant mice. Wild-type band is 293bp, mutant band 340bp that is amplified using the same 5'primer and a primer located within the lacZ-PGK-neo cassette. C. The absence of Nac1 mRNA was confirmed by RT-PCR analysis using primer sets corresponding to either the POZ/BTB domain or a 5' region of Exon 1. HPRT is used as an internal control. D. The absence of Nac1 protein was confirmed in mutant mice by Western analysis in both nucleus accumbens (NAC-/-) and cortex (CTX-/-).
RNA Isolation and cDNA Synthesis
All RNA was isolated using TRIzol (GIBCO-BRL, Gaithersburg, MD) according to manufacturer's instructions. The quality of the RNA samples was determined by ethidium bromide staining of 18S and 28S rRNAs following fractionation on denaturing agarose gels. Contaminating genomic DNA was removed using 1 μL RNase-free DNase I (Boehringer)/10 μg of RNA at 37°C for 30 min. cDNA was synthesized using MMLV-RT (GIBCO-BRL) with deoxyribonucleotides (dNTPs) and random hexamer primers RT (GIBCO-BRL) at 42°C for 30 min.
Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Conditions used for RT-PCR followed the method of Wilson and Melton (1994) with minimal modifications. The mouse HPRT (hypoxanthine guanine phosphoribosyl transferase) gene was used as an internal control, as the mRNA of this gene is expressed in most tissues in roughly equal amounts. cDNA synthesized from total brain RNA provided templates for PCRs under the following conditions: one cycle of 95°C for 5 min, followed by 25 cycles of 95°C for 1 min, 60°C for 1 min, 72°C for 2 min, and one cycle of 72°C for 5 min in a buffer containing 1.5 mmol/L MgCl2, 10 μmol/L primers , 0.05 μCi 32P-dATP, and 200 μmol/L dNTPs. Primer sequence available upon request. PCR products were separated on 5% acrylamide gels that were dried and exposed to phosphorimager screens overnight.
Western blot analysis
Tissues from individual animals were homogenized in 200 μL of ice-cold extraction buffer containing phosphate buffered saline (PBS), 1 mM EGTA, 1 mM EDTA, 1% SDS, and 1 mM PMSF. Protein concentrations were determined using a Bradford assay, with bovine serum albumin as the standard. Equivalent amounts of protein (50 μg) for each sample were resolved in 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, proteins were transferred to Immobilon-P membranes (Millipore). Membranes were incubated in PBS with 0.5% Tween 20 (PBS-T) containing 5% non-fat milk for 1 hr at room temperature to block non-specific binding. The blots were reacted with primary antibodies) overnight at 4 °C. After washing in PBS-T, the blots were incubated in secondary antibody in PBS-T for 1 hr. Membranes were then washed three times with PBS-T. Immunolabeling was detected by enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ, USA). The blots were stripped using the standard protocol, washed and reprobed with the reference antibody (α-tubulin). The antibody for NAC1 was generated using a C-terminal peptide (TASHDGEAGPSAEVLQ) (Pro-Sci Inc., Poway, CA). Primary antibodies used were Nac-1 and α-tubulin (1:1000) (Sigma, St. Louis, MO). The secondary antibodies used were goat anti-rabbit IgG-HRP (1:500) and goat anti-mouse IgG-HRP (1:1000) (Santa Cruz Biotechnology, Santa Cruz, CA). Densitometric analysis was conducted to quantify the immunoreactivity in Western blotting with a scanner and ImageQuant software (Amersham Biosciences, Piscataway, NJ). The NAC1 bands were detected at ∼59 kDa, α-tubulin bands at 50 kDa.
Behavior Analysis
Animals
All mice (3−5 months old, in a mixed genetic background of 129SvEvTac;C57BL/6N F2 generation; 23−40g) were housed in groups of 4 per cage and maintained on a 12 hr light-dark cycle with food and water available ad libitum in accordance with the University of Pennsylvania Animal Care and Use Committee. Mice of both sexes were used in all studies. All experimental testing sessions were conducted between 12.00 and 18.00 hours, with animals randomly assigned to treatment conditions and tested in counterbalanced order.
Drugs
All drug doses were calculated as mg/kg base weight and were dissolved in 0.9% saline. Cocaine hydrochloride and amphetamine was obtained from NIDA Drug Supply (Research Triangle Park, NC). Saline (0.9%) and dopamine hydrochloride were obtained from Sigma Aldrich, St Louis, MO.
Locomotor Activity
Locomotor activity in response to intraperitoneal drug administration was analyzed in a “home cage” activity monitoring system (MedAssociates, St. Albans, VT). The home cage (28.9 × 17.8 × 12 cm) was placed in a photo-beam frame (30 × 24 × 8 cm) with sensors arranged in an 8-beam array strip. To avoid effects of novelty during testing, mice were injected i.p. with saline (0.9% sodium chloride) and individually placed in the cages for 3 days prior to drug administration. Beam break data were read into MedAssociates personal computer-designed software and monitored for 30 min. For acute studies, mice were injected i.p. with either saline, cocaine (5.0 mg/kg, 10.0 mg/kg or 20.0 mg/kg) or amphetamine (2.0 mg/kg) and individually placed in the cages. For behavioral sensitization studies, baseline activity was recorded for 3 days to reduce novelty induced hyperlocomotion similar to the procedure for acute administration. Drugs were administered on day 4 and repeated once a day for 7 days (day 4−10, cocaine) or 4 days (day 4−7, amphetamine) and locomoter activity was recorded. All mice remained in their home cages with no drug administration during the development of sensitization. To evaluate cocaine induced sensitization, mice were tested on day 29 with an i.p. injection of either saline (saline control groups) or a challenge dose of cocaine (5 mg/kg). A second cocaine challenge was administered on day 40 to evaluate long lasting effects of sensitization. During the course of the amphetamine study, compliance with animal facility regulation on moving mice prevented us from testing on day 29, therefore mice were tested on day 90 with saline only and then on day 91 with an i.p. injection of a challenge dose of amphetamine (2.0 mg/kg). Beam break data was monitored and recorded for 30min.
Conditioned Place Preference
10-day Conditioned Place Preference
To facilitate adaptation to novel surroundings, mice were transported to the testing room at least one hour prior to testing. Briefly, for preconditioning, day 1, mice were allowed to explore both sides of a 2-chambered CPP apparatus. The place conditioning boxes consisted of two distinct plastic sides (20×20×20 cm) differing in lighting intensity; one with stripes on the walls and a metal grid over cage bedding (referred to as striped side) and the other with gray walls and smooth flooring (referred to as solid side). A partition separated the two sides with an opening that allowed access to either side of the chamber during preconditioning and test days. This partition was closed off during pairing days. Preconditioning phase: On day 1 mice were placed randomly on the striped or solid side and allowed to explore both sides of the place conditioning boxes for 900 sec; time spent in each side was determined using the criteria of placement of head and front paws in the chamber. These data were used to separate mice into groups with approximately equal biases for each side. None of the mice exhibited a significant preference for one side over the other. Conditioning phase: Mice were paired for 8 days (days 2−9), with the saline group receiving injections (0.9% sodium chloride) on both sides of the boxes, while the drug paired group received cocaine (10mg//kg) or amphetamine (2mg/kg) on one side and saline on the other side. For cocaine experiments, drug was paired for 30min, while amphetamine was paired for 45 min. Drug-paired sides were randomized among all groups. Testing phase: On day 10, all animals were given a saline injection and placed on the same side they were originally introduced on pre-conditioning day (striped or solid). Because of the randomization, approximately half of the drug-paired mice were introduced into the side that was paired with drug, and the other half were introduced into the side that was paired with saline. Animals were allowed to roam freely between the two sides, and time spent on each side was recorded.
3-day Conditioned Place Preference
These studies were carried out essentially as described above with respect to preconditioning and testing days. However, conditioning took place on one day, in two sessions. Animals were exposed to one side of the box in the morning session and the opposite side in the afternoon session. Animals were paired with either a saline injection on each side (saline paired group), cocaine (20 mg/kg), or amphetamine (2.0mg/kg) on one of the sides (drug paired group) and saline on the opposite side. Drug-paired mice were randomized with respect to session time and box side among all groups.
Surgery
Naïve mice were anesthetized with a combination of ketamine (100 mg/kg, i.p.) and xylazine (5 mg/kg, i.p.). Using coordinates derived from Franklin and Paxinos (1997) atlas (in millimeters: AP, +1.42; ML, ± 0.8; DV, −2.8 relative to bregma) microdialysis guide cannulae (20 gauge, 10mm; Small Parts, Miami Lakes, FL) were implanted bilaterally above the nucleus accumbens. The animal was placed in a stereotaxic alignment system (David Kopf Instruments, Tujunga, CA)with a Cunnigham mouse adapter. The skull was exposed and two holes were drilled. Cannulae were positioned and the skull was prepared for resin application and a light-cured dental resin was used to secure guide cannulae in position (Kerr Corporation, Orange, CA). The incision was closed with a tissue adhesive. After surgery, mice were allowed to recover at least 7 days prior to microdialysis.
Microdialysis
A microdialysis probe (24 gauge; including 1.5−2 mm of active membrane) was lowered into the guide cannula and perfused with a microdialysis buffer (5 mM glucose, 2.7 mM KCl, 140 mM NaCl, 1.4 mM CaCl2, 1.2 mM MgCl2, 0.15% phosphate-buffered saline [pH=7.4]) at a rate of 2 μl/min via a syringe pump (BAS Bioanalytical System, West Lafayette, IN). After four hours, dialysis samples were collected every 20 min, beginning 2 hours prior to an i.p. injection of 20 mg/kg cocaine (0.01 ml/g) and for 120 min thereafter. Samples were collected into vials containing 10 ml of 0.05 M HCl and were frozen at −80°C until analysis.
HPLC analysis of dopamine
Dopamine in the dialysate sample was measured using a HPLC system with electrochemical detection. The mobile phase consisted of 4.76 mM citric acid, 150 mM NaH2PO4, 50 μM EDTA, 2.5 mM sodium dodecyl sulfate, 10% methanol (v/v), 17% acetylnitrile (v/v) at a pH = 5.6. Dopamine was separated using a reversed-phase column (HR-80, ESA) and oxidized/reduced using coulometric detection (Coulochem II; ESA Inc.). Three electrodes were used: a guard cell (+0.40 V), a reduction analytical electrode (E1, −0.10 V), and an oxidation analytical electrode (E2, +0.22 V). The peak area was measured with ESA 501 Chromatography Data System and the dopamine values were compared with an external standard curve for quantification.
Histology and Statistical Analysis
After completion of microdialysis experiments, mice were deeply anesthetized using pentobarbital (50mg/kg, i.p.) and perfused transcardially with saline and 4% formaldehyde solution. The brains were stored in the latter solution until sectioning. Placement of the microdialysis probes within the nucleus accumbens were verified in cresyl violet-stained tissue sections (50 μm). Only those mice with probes located in the nucleus accumbens were included in the data analysis. For all data, statistical analyses were performed using StatView (SAS). Behavioral sensitization locomotor data and conditioned place preference data were analyzed for significance with ANOVAs using a Bonferroni–Dunn post hoc test. The microdialysis data were analyzed using a two-way ANOVA with repeated measures over time followed by an LSD multiple comparisons post hoc test.
Results
Nac1 mutant mice are viable
A null allele of the Nac1 gene was generated by gene targeting and homologous recombination (figure 1A). Mice carrying the Nac1 mutant allele were derived by blastocyst injection and mated to homozygosity to produce adult mice of all three genotypes in normal Mendelian ratios of +/+ 25%; +/−50%; −/−25% (figure 1B). Successful gene ablation was confirmed by RT-PCR analysis that demonstrated the absence of NAC1 mRNA using primers directed against the POZ domain as well as a 5' region of exon 2 (figure 1C). In addition, Western Blot analysis revealed no protein of the appropriate size is produced in mutant mice (figure 1D). Nac1 mutant mice exhibit normal weight gain, fertility and home cage exploratory activity compared to wildtype controls (data not shown).
Acute locomotor activating effects of psychomotor stimulants are absent in Nac1 mutant mice
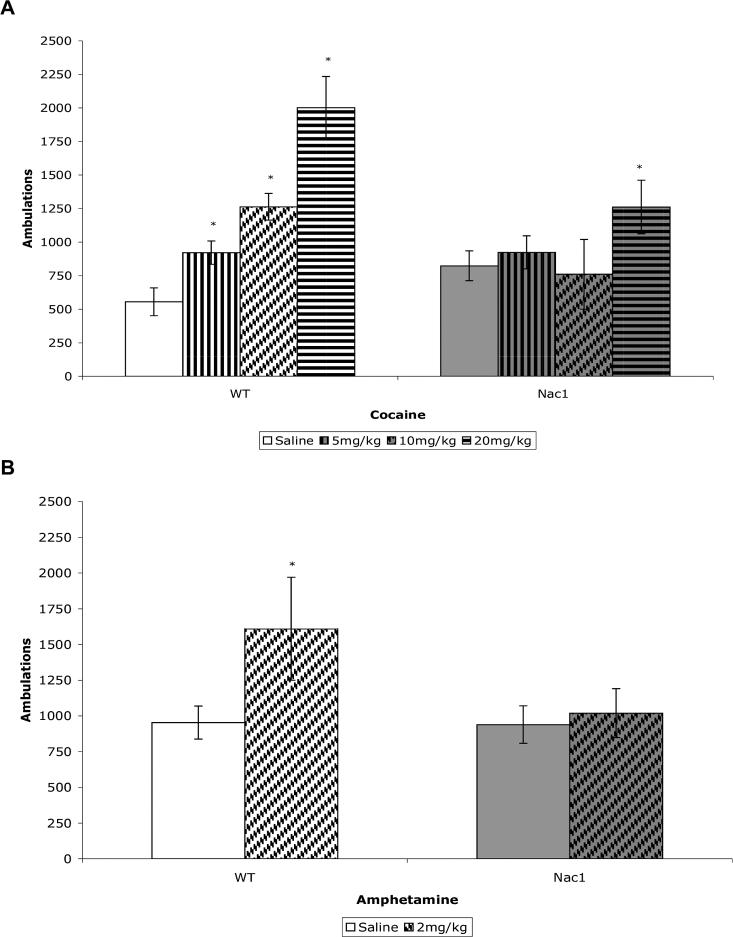
Naïve wild type and mutant mice were injected with saline, cocaine (5.0, 10.0, or 20.0 mg/kg, ip) or amphetamine (2.0 mg/kg, ip) and locomotor activity monitored. Wild type mice showed a dose-dependent increase in locomtor activity to cocaine, while the response was blunted in Nac1 mutant mice (figure 2A). Similarly, the acute motor stimulant effect of amphetamine was absent in the mutant mice (figure 2B).
Figure 2.
A. Nac1 mutant mice do not show a significant increase in ambulatory behavior at 5mg/kg or 10mg/kg acute cocaine administration (i.p. injection), but do show a significant increase at 20mg/kg. Wild-type mice show a significantly increased behavioral response in response to all doses of acute cocaine administration. *p < 0.05 from corresponding saline group (n = 6 per group). (ANOVA; Fisher's PLSD post hoc test). B. Nac1 mutant mice do not show a significant increase in ambulatory behavior while wild-type mice do show a significantly increased behavioral response in response to acute amphetamine administration (2mg/kg i.p. injection). *p < 0.05 from corresponding saline group (n = 6 per group). (ANOVA; Fisher's PLSD post hoc test).
Behavioral sensitization is normal in Nac1 mutant mice
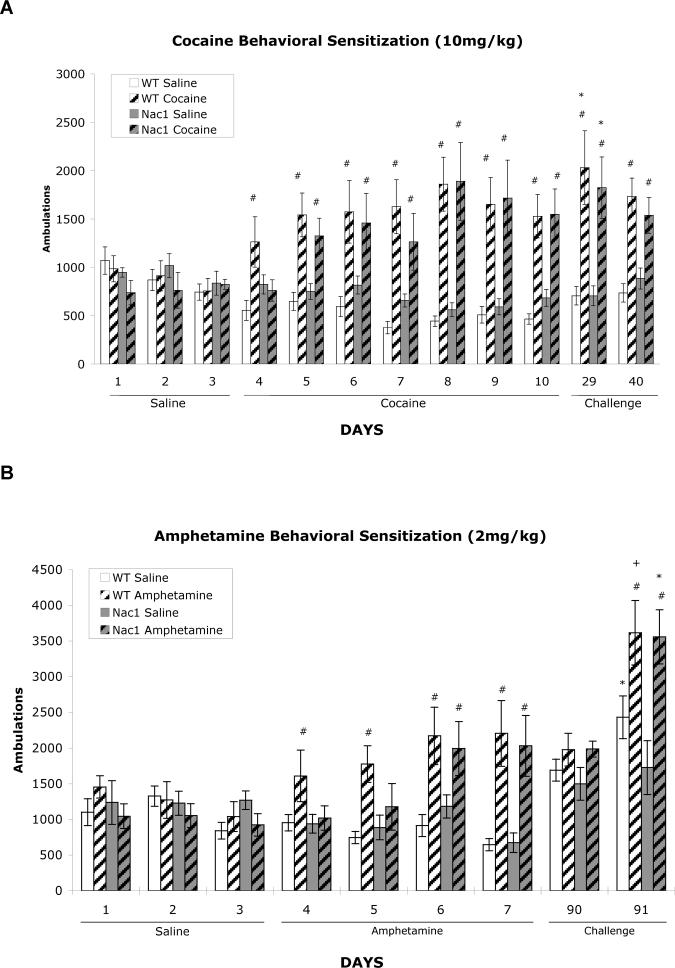
Although Nac1 mutant mice show a blunted acute behavioral response to cocaine or amphetamine when either drug was administered acutely, behavioral sensitization to the activating effects developed in both wild type and mutant mice (figure 3). By the last day of daily drug administration there was no genotypic difference in locomotor activity to cocaine (see day 10 in figure 3A) or amphetamine (day 7 in figure 3B). Cocaine (10mg/kg i.p) was administered daily from day 4−7. Importantly, animals were challenged with a lower dose of cocaine (5mg/kg,i.p) on day 29 (19 days drug free) or day 40 (30 days drug free). The sensitized locomotor response remained intact in both genotypes.
Figure 3.
A. Behavioral sensitization in Nac1 mutant and wild-type mice. No significant differences between the wild type and mutant mice during the habituation period (days 1−3; saline injections only). A geneotype effect is present on the first day of cocaine administration (day 4). During the development of sensitization (days 5−10) cocaine-treated (10mg/kg i.p. injection) wild-type mice and Nac1 mutant mice exhibit significantly more ambulatory activity than saline-treated mice Both cocaine-treated groups exhibit sensitization on the challenge days (days 29 and 40; 5mg/kg cocaine). # p < 0.05 from corresponding saline group on same day, and * p < 0.05 from corresponding group on day 4 (n = 6 per group). (ANOVA; Fisher's PLSD post hoc test). B. Behavioral sensitization to amphetamine (2 mg/kg) in Nac1 mutant mice (NAC1−/−) and wild-type mice. No significant differences appear between geneotypes during the habituation period (days 1−3; saline injections only). During the development of sensitization (days 4−7) amphetamine-treated mice exhibit significantly more ambulatory activity than saline-treated mice in all cases except for Nac1 mutant mice on the first and second days of development (days 4 and 5). Both groups show no difference in ambulation in response to saline after development of sensitization (day 90) and both amphetamine-treated groups exhibit sensitization on the challenge days (day 91; 2mg/kg amphetamine). # p < 0.05 from corresponding saline group on same day, * p < 0.05 and + p < 0.01 from corresponding group on day 90 (n = 6 per group). (ANOVA; Fisher's PLSD post hoc test).
Similarly, behavioral sensitization developed to the repeated injections of amphetamine (2.0 mg/kg, i.p.). Acute locomotor effects of amphetamine are evident in wild type mice starting on day 4 (first day of drug administration). Of interest, Nac1 mutant mice do not show a behavioral response for the first two days of drug administration but only demonstrate increased locomotor activation on day 6. The sensitization paradigm was altered from that used for cocaine due to an animal quarantine in the vivarium that prevented animals from being removed from their housing room on the appropriate testing days. To continue the study once quarantine was lifted, we exposed all mice to the locomotor testing chambers on day 90 (84 days after the last daily amphetamine injection) and administered saline to all groups. Both wild type and mutant mice previously exposed to saline demonstrate slightly higher baseline locomotor activity that is not significantly different from mice that had pre-exposure to amphetamine (figure 3B). To evaluate the state of sensitization in the drug treated mice all mice were administered amphetamine (2.0mg/kg) the following day (day 91). Wild type mice previously administered saline show an increased response to amphetamine, as expected, however Nac1 mutant mice previously administered saline throughout the study do not show this augmentation, consistent with previous results. All mice that had pre-exposure to amphetamine show significantly greater activity compared to day 4 (wild type) or day 6 (mutant) demonstrating expression of behavioral sensitization is intact.
Single exposure to psychomotor stimulants induced conditioned place preference in wild type but not Nac1 mutant mice
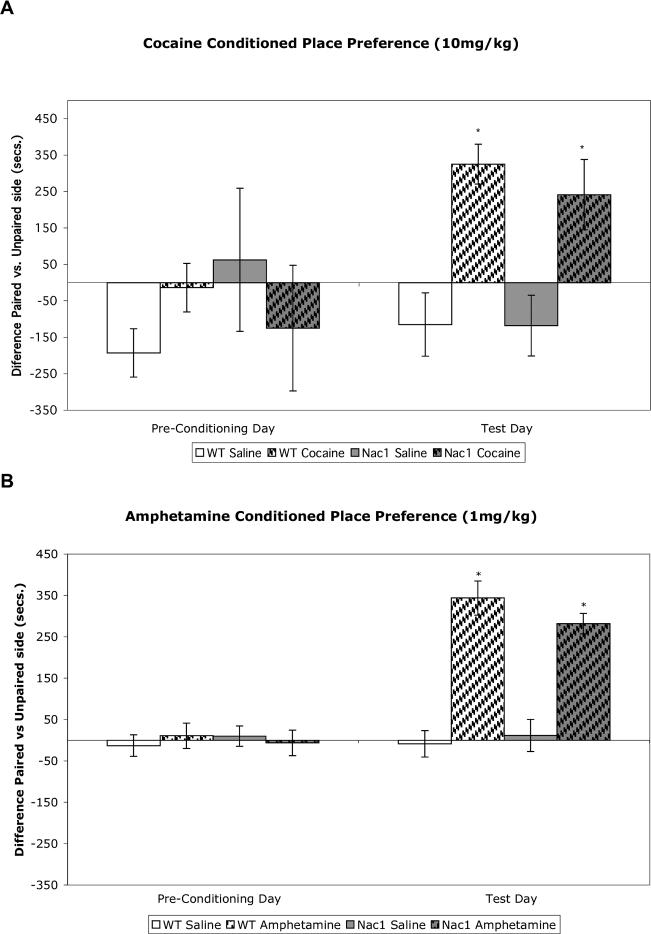
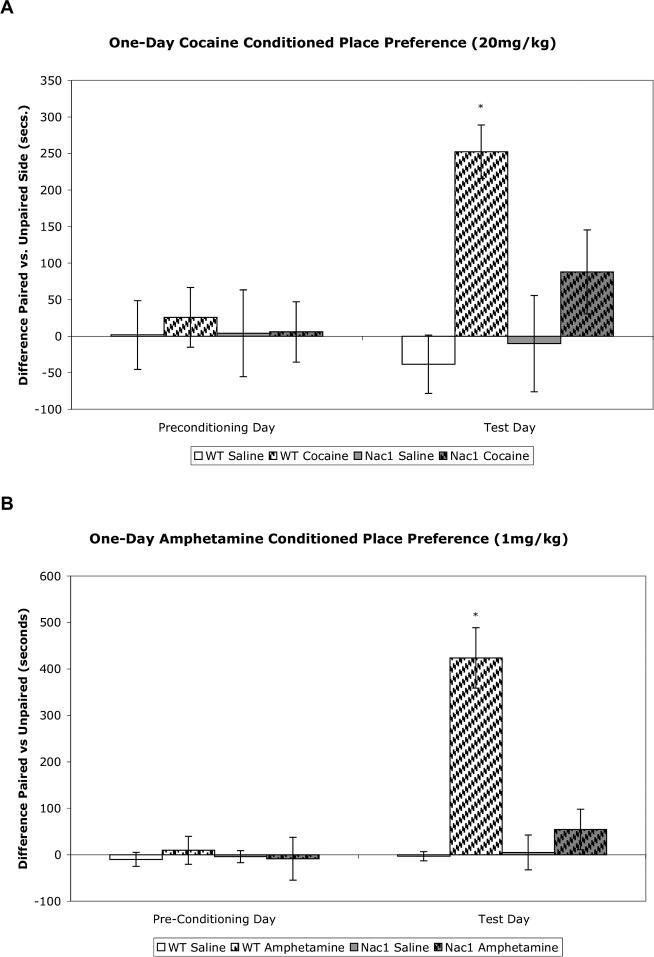
In a conditioned place preference paradigm in which animals were paired over 8 consecutive days with either drug or saline, both Nac1 mutant and wild type mice showed significant preference for cocaine at 10.0 mg (figure 4A) as well as amphetamine at 2.0mg/kg (figure 4B). As this paradigm uses multiple exposures to the apparatus and repeated drug administration, it is not possible to evaluate a deficit of an acute response to drug. Therefore, we modified this paradigm to evaluate rewarding properties of a single drug exposure in the conditioning apparatus. Twenty-four hours following cocaine or amphetamine administration we tested mice for place preference. When trained under the one-day conditioned place preference conditions (1 day; 1 morning/1 afternoon session) wild type mice show significant place preference for the side of the box paired with either cocaine or amphetamine. In contrast, Nac1 mutant mice did not show significant preference for one side of the box or the other (figure 5A and B).
Figure 4.
A. Nac1 mutant mice and wild-type mice exhibit significant cocaine-conditioned place preference. The left side of the graph indicates that no initial preference for either side of the testing box exists for any of the groups. The right side of the graph shows that both mutant and wild-type mice show a preference for the side of the testing box that was paired with 10 mg/kg cocaine. There is no significant genotype effect * p < 0.05 from corresponding saline group (n = 6 per group). (ANOVA; Fisher's PLSD post hoc test). B. Nac1 mutant mice and wild-type mice exhibit significant amphetamine-conditioned place preference. The left side of the graph indicates that no initial preference for either side of the testing box exists for any of the groups. The right side of the graph shows that both mutant mice and wild-type mice show a preference for the side of the testing box that was paired with 2 mg/kg amphetamine. There is no significant difference between the amphetamine-treated Nac1 mutant mice and the wild-type mice. * p < 0.05 from corresponding saline group (n = 6 per group). (ANOVA; Fisher's PLSD post hoc test).
Figure 5.
A. Nac1 mutant mice do not exhibit significant one-day cocaine-conditioned place preference. The left side of the graph indicates that no initial preference for either side of the testing box exists for any of the groups. The right side of the graph shows that wild-type mice show a preference for the side of the testing box that was paired with a single injection of 20 mg/kg cocaine whereas the mutant mice do not show a significant preference. There is a significant difference between the cocaine-paired wild-type mice and the cocaine-paired Nac1 mutant mice. * p < 0.05 from all groups (n = 6 per group). (ANOVA; Fisher's PLSD post hoc test). B. Nac1 mutant mice do not exhibit significant one-day amphetamine-conditioned place preference. The left side of the graph indicates that no initial preference for either side of the testing box exists for any of the groups. The right side of the graph shows that wild-type mice show a preference for the side of the testing box that was paired with a single injection of 1 mg/kg amphetamine whereas the mutant mice do not show a significant preference. There is a significant difference between the amphetamine-paired wild-type mice and the amphetamine-paired Nac1 mutant mice. * p < 0.05 from all groups (n = 6 per group) (ANOVA; Fisher's PLSD post hoc test).
Elevations in extracellular dopamine are reduced in Nac1 mutant mice following acute, but not repeated exposure to cocaine
Wild type and Nac1 mutant mice received three saline injections (1 per day), followed by 5 days of cocaine injections (20mg/kg, i.p./day). The first injection of cocaine was administered during a microdialysis session (Day 1). A second microdialysis session was conducted during the last cocaine injection (Day 5). Akin to the behavioral deficit in acute cocaine-induced locomotor activity, the elevation in accumbens extracellular dopamine normally produced by an acute injection of cocaine was significantly blunted during the first 60 min after injection (figure 6A). Also akin to the lack of genotypic behavioral difference in sensitized locomotor activity, the sensitized increase in extracellular dopamine in response to cocaine on day 5 was equivalent between genotypes (figure 6B).
Figure 6.
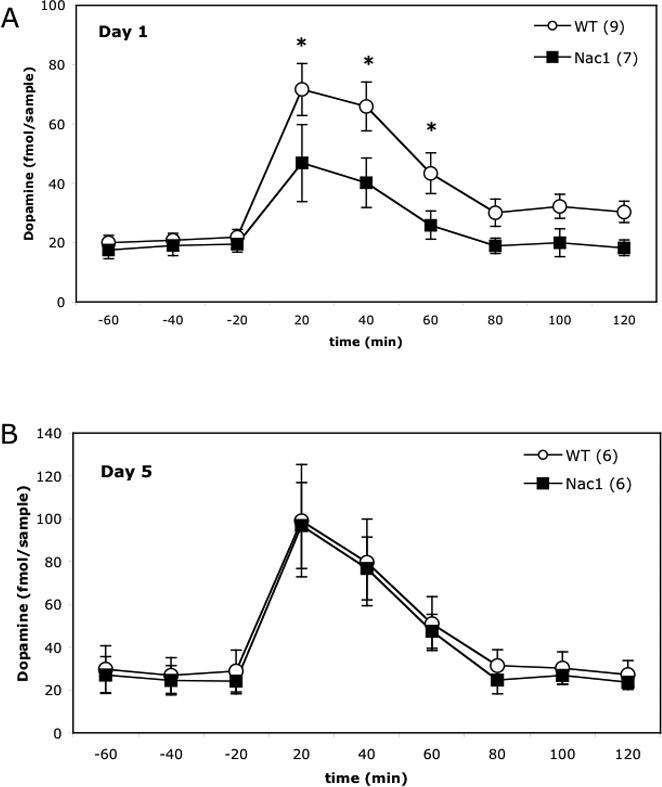
A. Nac1 mutant mice exhibit significantly decreased levels of dopamine in the nucleus accumbens following an acute injection of cocaine (20 mg/kg). Following the first injection, mutant mice show significantly reduced levels of dopamine in the nucleus accumbens as compared to their wild-type counterparts. There are no differences in the levels of dopamine in the nucleus accumbens of Nac1 mutant and wild-type mice following the last injection of cocaine. *p < 0.05 from mutant group (n = 6−9). B. Nac1 mutant mice and wild-type mice were exposed to 5 injections of cocaine over the course of 5 days. Both groups show equivalent increases in dopamine over the time-course examined.
Discussion
Current molecular and cellular theories surrounding addiction indicate that molecular changes in specific brain circuits are associated with the induction and expression of behavioral responses associated with chronic exposure to drugs of abuse [12]. While most of these changes have been examined as the effects of acute drug administration, some appear to be long-term adaptations [13,18]. Relative to other known cocaine-regulated genes, the expression of NAC1 is unique in response to cocaine. Like, c-fos, Homer1a and zif268, NAC1 is elevated shortly after acute administration and returns to baseline within 12 hours after drug injections [3,4,17]. However, levels of NAC1 are also significantly elevated one week following withdrawal from cocaine. While other genes demonstrate this longer withdrawal induced pattern of gene expression, such as the A1 adenosine receptor, homer1bc, activator of G-protein signaling 3 [2,20,21], these genes are not increased acutely like NAC1. Thus, this unique biphasic pattern of Nac1 gene expression suggests different roles for this protein throughout the development of addiction.
Previously we have shown that interrupted expression of NAC1 via antisense oligonucleotide injection augments the acute motor stimulant responses to cocaine [11], suggesting that the acute increase in NAC1 expression may be required for this behavioral response. Adenoviral-mediated overexpression of NAC1 protein in the nucleus accumbens in rat did not alter acute motor responses to cocaine, but did prevent the development of cocaine behavioral sensitization [16]. However, if virus is injected following the development of behavioral sensitization rats exhibit augmented behavioral response in locomotor activity similar to controls [16]. One caveat of these studies is the manipulation of NAC1 expression either in a gain of function or loss of function study was restricted to a single brain region, the nucleus accumbens.
Deletion of NAC1 by gene targeting blocked the acute locomotor activating response to cocaine as well as to amphetamine, but did not affect the development of sensitization. In parallel with the acute versus chronic effects of cocaine and amphetamine on locomotion, the conditioned rewarding properties of both drugs were absent in Nac1 mutant mice using a place preference paradigm involving a single drug pairing. In contrast, using a conditioning paradigm that involved multiple drug-context exposures no genotypic difference in preference was observed. When comparing the data between the present study using constitutive gene deletion with the results of previous studies using antisense oligonucleotide strategies, it is clear that nearly opposite effects on behavior and neurochemistry are produced. Indeed, there is precedent in other systems for discrepancies between antisense and gene targeting approaches where different outcomes are achieved in behavioral tasks depending on the method used to alter gene function [5, 6, 9, 19].
While many reasons may exist for these and other discrepancies in the literature between knock-out and antisense techniques, two primary differences stand out between strategies for changing gene expression. First, gene deletion is constitutive throughout development, while the changes in expression produced by antisense or over-expression are produced in adult animals. In addition, gene deletion experiments identify genes that are necessary for a given phenotype, while over-expression analysis identifies genes that are sufficient to confer a specific phenotype. Second, gene deletion experiments involve complete loss of NAC1 expression versus incomplete loss produced by antisense strategies. Both of these distinctions between gene deletion and more acute up or down-regulation of NAC1 expression are considered in detail below.
Constitutive gene deletion and developmental effects
The constitutive nature of the gene deletion experiment may result in functional redundancy in a studied system. The effects of deleting NAC1 early in development may be compensated for in the adult such that repeated exposure to cocaine or amphetamine is not affected by the loss of this protein. Of interest, acute responses to these drugs are altered in mice lacking the NAC1 protein. Thus, identification of changes that may have occurred in mice lacking NAC1 during development could lead to novel targets associated with acute psychomotor stimulant response. Overexpression of NAC1 in the nucleus accumbens was shown to prevent the development of cocaine induced behavioral sensitization [16]. This type of gain-of function experiment identifies NAC1 as a gene that could alter this drug response, but cannot determine if NAC1 is essential in mediating this response. The latter conclusion can only be made using loss-of-function experiments, such as gene deletion.
Partial versus complete loss of NAC1 expression
The manipulations of NAC1 expression in adult animals result in more graded changes in expression versus complete absence in knock-out mice. The unique bi-modal pattern of NAC1 expression following cocaine administration indicates this protein can influence both the acute and chronic effects of psychostimulant administration. While precise roles remain to be elucidated, previous studies using up or down regulation of NAC1 in adult rats indicate that it is perhaps the acute regulation of NAC1 that is most critical. For example, the acute motor activating effect of cocaine was enhanced by NAC1 down-regulation [11], however, if this up-regulation is manifested by viral overexpression of NAC1, the development of sensitization is blocked [16]. These data suggest that NAC1 may signal a sequence of events that initiates locomotor activating effects, but this signal must be repressed to enable the further development of sensitization. In contrast, acute motor activating effects of cocaine and amphetamine were blocked by gene deletion, while the expression of sensitization was not affected. Thus the complete absence of a NAC1 signal produces a very different phenotype than partial regulation where transient changes in NAC1 expression are still possible. In addition, both gene deletion and antisense studies suggest very different molecular roles for NAC1 during the initiation of psychomotor stimulant effects compared to those underlying the development of behavioral sensitization.
Summary
Deletion of the NAC1 gene resulted in a blunted locomotor stimulation and development of place conditioning in response to acute administration of cocaine and amphetamine. The attenuated behavioral efficacy of acute psychostimulants was associated with a reduction of cocaine-induced increases in extracellular dopamine in the nucleus accumbens. These results offer a likely neurochemical basis for the decreased behavioral response in NAC1 mutant mice. Interestingly, the sensitized locomotor response and place preference elicited by repeated drug administration showed no genotypic difference. The mechanism by which repeated psychostimulant treatment overcomes the initial blunted responding is unclear, but may rely on the recruitment of corticolimbic circuitry by repeated psychostimulants that restores the activity of mesoaccumbens dopamine cells.
Acknowledgements
This work was supported by the Veterans Administration (DVA-MR-393), the Joseph Alexander Foundation, NIDA DA 11809 (S.A.M.) and NIDA DA11649 (J.A.B). The authors wish to thank Misty Godfrey for technical expertise and Dana R. Crumpler for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albagli O, Dhordain P, Deweindt C, Lecocq G, Leprince D. The BTB/POZ domain: a new protein-protein interaction motif common to DNA- and actin-binding proteins. Cell Growth Differ. 1995;6:1193–8. [PubMed] [Google Scholar]
- 2.Bowers MS, McFarland K, Lake RW, Peterson YK, Lapish CC, Gregory ML, Lanier SM, Kalivas PW. Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron. 2004;42:269–81. doi: 10.1016/s0896-6273(04)00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–8. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- 4.Cha XY, Pierce RC, Kalivas PW, Mackler SA. NAC-1, a rat brain mRNA, is increased in the nucleus accumbens three weeks after chronic cocaine self-administration. J Neurosci. 1997;17:6864–71. doi: 10.1523/JNEUROSCI.17-18-06864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Contarino A, Picetti R, Matthes HW, Koob GF, Kieffer BL, Gold LH. Lack of reward and locomotor stimulation induced by heroin in mu-opioid receptor-deficient mice. Eur J Pharmacol. 2002;446:103–9. doi: 10.1016/s0014-2999(02)01812-5. [DOI] [PubMed] [Google Scholar]
- 6.Curzon P, Anderson DJ, Nikkel AL, Fox GB, Gopalakrishnan M, Decker MW, Bitner RS. Antisense knockdown of the rat alpha7 nicotinic acetylcholine receptor produces spatial memory impairment. Neurosci Lett. 2006;410:15–9. doi: 10.1016/j.neulet.2006.09.061. [DOI] [PubMed] [Google Scholar]
- 7.Dackis CA, O'Brien CP. Cocaine dependence: a disease of the brain's reward centers. J Subst Abuse Treat. 2001;21:111–7. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 8.Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–20. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hummel M, Schroeder J, Liu-Chen LY, Cowan A, Unterwald EM. An antisense oligodeoxynucleotide to the mu opioid receptor attenuates cocaine-induced behavioral sensitization and reward in mice. Neuroscience. 2006;142:481–91. doi: 10.1016/j.neuroscience.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Kaestner KH, Montoliu L, Kern H, Thulke M, Schutz G. Universal β-galactosidase vectors for promotor analysis and gene targeting. Gene. 1994;148:67–70. doi: 10.1016/0378-1119(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 11.Kalivas PW, Duffy P, Mackler SA. Interrupted expression of NAC-1 augments the behavioral responses to cocaine. Synapse. 1999;33:153–9. doi: 10.1002/(SICI)1098-2396(199908)33:2<153::AID-SYN5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 12.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 13.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 14.Korutla L, Wang PJ, Lewis DM, Neustadter JH, Stromberg MF, Mackler SA. Differences in expression, actions and cocaine regulation of two isoforms for the brain transcriptional regulator NAC1. Neuroscience. 2002;110:421–9. doi: 10.1016/s0306-4522(01)00518-8. [DOI] [PubMed] [Google Scholar]
- 15.Mackler SA, Homan YX, Korutla L, Conti AC, Blendy JA. The mouse nac1 gene, encoding a cocaine-regulated Bric-a-brac Tramtrac Broad complex/Pox virus and Zinc finger protein, is regulated by AP1. Neuroscience. 2003;121:355–61. doi: 10.1016/s0306-4522(03)00376-2. [DOI] [PubMed] [Google Scholar]
- 16.Mackler SA, Korutla L, Cha XY, Koebbe MJ, Fournier KM, Bowers MS, Kalivas PW. NAC-1 is a brain POZ/BTB protein that can prevent cocaine-induced sensitization in the rat. J Neurosci. 2000;20:6210–7. doi: 10.1523/JNEUROSCI.20-16-06210.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moratalla R, Elibol B, Vallejo M, Graybiel AM. Network-level changes in expression of inducible Fos-Jun proteins in the striatum during chronic cocaine treatment and withdrawal. Neuron. 1996;17:147–56. doi: 10.1016/s0896-6273(00)80288-3. [DOI] [PubMed] [Google Scholar]
- 18.Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10:201–17. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- 19.Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A. Alpha7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of Acra7-deficient mice. Learn Mem. 1998;5:302–16. [PMC free article] [PubMed] [Google Scholar]
- 20.Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci. 2001;21:9043–52. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toda S, Alguacil LF, Kalivas PW. Repeated cocaine administration changes the function and subcellular distribution of adenosine A1 receptor in the rat nucleus accumbens. J Neurochem. 2003;87:1478–84. doi: 10.1046/j.1471-4159.2003.02121.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang PJ, Stromberg M, Replenski S, Snyder-Mackler A, Mackler SA. The relationship between cocaine-induced increases in NAC1 and behavioral sensitization. Pharmacol Biochem Behav. 2003;75:49–54. doi: 10.1016/s0091-3057(03)00040-6. [DOI] [PubMed] [Google Scholar]