Abstract
MHC class I antigen expression is necessary for CD8+ T-cell-mediated recognition of tumors. Recently, several mechanisms leading to loss or decreased expression of MHC antigens on the tumor cell surface have been described that may account for tumor escape from immune recognition. It is yet unknown whether tumor recognition by CTL occurs at a threshold amount of MHC molecules or correlates with the level of HLA-allele expression. In this study, a model was developed in which clones derived from the 624-MEL melanoma cell line and expressing varying amounts of HLA-A2 molecules were lysed in a standard 51Cr release assay by an HLA-A2-restricted CTL clone (A42) or a bulk culture of tumor-infiltrating lymphocytes. The A42 clone and the tumor-infiltrating lymphocyte culture were characterized previously as specifically recognizing the melanoma antigen MART-127–35 peptide. A marked heterogeneity in the susceptibility to lysis by A42 was observed in tumor clones and was not due to heterogeneous expression of MART-1 by the clones or loss of accessory molecules involved in the lymphocyte-target interaction. Lysis by A42 and by the tumor-infiltrating lymphocyte culture significantly correlated with the level of HLA-A2 expression, evaluated as mean channel number of fluorescence by flow cytometry (P < 0.001). Transfection of an HLA-A2-negative clone (624.28) with the HLA-A2.1 gene produced a panel of clones expressing different levels of HLA-A2, the lysis of which was highly correlated with the expression of HLA-A2 (P < 0.001). The addition of exogenous MART-127–35 peptide enhanced lysis of clones expressing intermediate amounts of HLA-A2 but did not affect clones with high expression. These data suggest that the number of HLA molecules present on the surface of tumor cells can quantitatively affect their lysis by CTL in situations with borderline amounts of peptide and/or MHC.
INTRODUCTION
The expression of MHC antigens is necessary for tumor recognition by CTLs (1-3). Although the level of surface expression of MHC antigens on tumor cells has been studied extensively (4-16), most studies have limited the analysis to the monomorphic component of HLA (5), leading to an underestimation of the frequency of locus and allele-specific aberrations of expression. Only recently, thanks to the development of locus- and allele-specific mAbs, we and others could describe several mechanisms leading to loss of expression of HLA class I antigens or to their down-regulation (13-19). Indeed, in addition to the loss of expression of all MHC class I antigens due to faulty expression of β2-microglobulin (13-16), allele-specific loss has been detected in vivo (20). In vitro studies have shown that losses of variable portions of genomic DNA in the short arm of chromosome 6 frequently cause allele (17)- or haplotype (18)-specific loss of expression in tumor cell lines. Furthermore, expression of MHC antigens, when detectable, was noted to be variable due to locus- or allele-specific down-regulation (18, 19). Despite the extensive analysis of potential mechanisms for MHC loss in tumor cells, the majority of the studies have evaluated MHC expression on tumor cells as an all-or-none phenomenon, paying little attention to the amount of expression of specific HLA alleles. In this study, we questioned whether to understand the complex interactions between MHC class-I-restricted tumor specific CTL and their targets, researchers may have to pay attention to quantitative differences in HLA antigen expression.
It has been shown that a small fraction of the total number of MHC class I-peptide complexes expressed on the cell surface is necessary to sensitize a target cell for lysis by CTLs (21). In the antiviral immune response, this may be relatively easy to achieve, since viral proteins are overexpressed during viral replication. However, peptides derived from self proteins are likely to be less vigorously expressed while they have to compete with more than 1000 other peptides for a specific MHC allele (22). Therefore, not all possible endogenous epitopes can be presented with a density sufficient for recognition by CTL (23), and tolerance may be allowed in spite of the presence, in the same organism, of a CTL population specific for that epitope/MHC complex (24). Recently described epitopes recognized by autologous anti-melanoma CTL are products of self proteins expressed in melanoma cells and, frequently, in epithelial melanocytes (25-30). These peptides may be expressed in variable quantities and have a different affinity for different HLA alleles (28). Although approximately 90% of melanoma cell lines have detectable amounts of a specific HLA allele (18), the level of allele-specific expression, as detected by fluorescent cell sorter (FACS3) analysis, may vary greatly (18, 19). Because of the highly competitive characteristics of MHC-peptide binding (24, 31), variations in MHC molecule expression may significantly affect recognition of self antigenic peptides by MHC-restricted CTLs. This may be particularly important for poorly immunogenic human tumors in which a combination of a low level of peptide production, variable affinity of the peptide for MHC allele, and reduced expression of the allele may switch the capability of a specific CTL to recognize tumor cells on and off.
In this study, we present a model that allowed us to analyze the susceptibility of cells expressing various amounts of HLA-A2 molecules to lysis by CTL. A CD8+ T-cell clone and a CTL line, both recognizing the MART-1 melanoma antigen in the context of HLA-A2, were used as the effector cells (32). In this model, recognition of melanoma targets was not based on a threshold expression of HLA antigens but instead on variations in the level of expression of HLA on the cell surface, in a range commonly seen among different melanoma cell lines, and was highly correlated with lysis by CTL in a continuous fashion. This in vitro model suggests that future studies of MHC antigen expression on tumor cells in vitro or in vivo should take into account not simply whether a specific MHC allele is expressed but also the amount of its expression. Partial reduction of the level of allele expression may also represent an additional mechanism for immune escape of tumor cells.
MATERIALS AND METHODS
HLA Typing of Patients' Lymphocytes and Tumor Lines
The original HLA phenotypes of patients were established on peripheral blood lymphocytes using the Amos modified microcytotoxicity test (33). Expression of HLA molecules on the surface of relevant clones of 624 MEL was tested as described previously (34) by using monoclonal trays for the microcytotoxicity test (One Lambda, Canoga Park, CA) after culturing cell lines in the presence or absence of rIFN-γ (specific activity, 1 × 107 viral inhibition units/mg; Sigma Chemical Co., St. Louis, MO) at the dose of 500 units/ml for 48 h. This dosage schedule allowed maximal up-regulation of HLA in all cell lines tested in previous studies (18) and is routinely recommended for typing of cell lines at our institution.
TIL Culture and TIL Clone
The bulk culture 1088 TIL recognizes MART-1-expressing targets in a HLA-A2 restricted fashion (27). The CD8+ T-cell clone A42 was established from a 31-day bulk TIL culture from patient 501 by limiting dilution in the presence of 120 Cetus units/ml interleukin 2 (Cetus-Oncology Division, Chiron Corp., Emeryville, CA), irradiated (10,000 rads) autologous EBV-B cells (104 cells/well), irradiated (30,000 rads) autologous tumor cells (103 cells/well) and pooled allogeneic PBL from three donors (total 3 × 104 cells/well; Ref. 26). This clone recognizes the immunodominant nonamer peptide MART-127–35 (AAGIGILTV)of the MART-1 human melanoma antigen as well as two decamer peptides (MART-126–35 and MART-127–36), which contain the MART-127–35 sequence plus an additional amino acid, either at the NH2 (glutamic acid) or at the COOH terminus (isoleucine), as described previously (27). The A42 clone was maintained in culture by weekly restimulation with autologous tumor cells and interleukin 2.
624-MEL and Clones
The 624-MEL melanoma cell line was derived from a metastatic lesion. The tumor lines were maintained in monolayer culture in CM consisting of RPMI 1640 (Biofluids, Rockville, MD) supplemented with 0.03% glutamine, 100 units/ml penicillin (both from NIH media unit), 0.5 μg/ml amphotericin B (Flow Laboratories, McLean, VA), and 10% heat-inactivated FCS (Biofluids) as described previously (18). When relevant, to induce expression of HLA class I antigens, cells were also incubated for 48 h in CM containing recombinant rIFN-γ at the dose of 500 units/ml. Clones from 624-MEL were obtained at passage 35 by limiting dilution in microtiter plates. Clones used for bioassay were grown from 0.3 cells/well dilutions.
Flow Cytometric Analysis
Surface Expression of HLA or Other Markers
To examine MHC antigen expression, all cultured cells were harvested as described previously using a technique that has no effect on the detection of HLA class I molecules by FACS analysis (18, 19). When cultures were approximately 70% confluent, cells were incubated for 5 min with 0.05% trypsin and 0.02% EDTA (Biofluids, Rockville, MD) at 37°C; washed twice in ice-cold HBSS free of Ca2+, Mg+ +, and phenol red, with 5% heat inactivated FCS (Biofluids), and with 0.2% sodium azide (FACS buffer); and incubated for 45 min at 4°C with 20 μl of the appropriate mAb. Staining was performed with 20 μl (50 μg/ml) of the appropriate FITC-conjugated secondary antibody for 45 min at 4°C. Fluorescence was analyzed by a FACScan (Becton Dickinson, San Jose, CA) by the same operator (A. M.) throughout the duration of the study to allow maximal standardization of the results. Nonviable cells were gated out with propidium iodide. FACS data are presented as MCN of fluorescence or percent positivity, as appropriate. FACS positivity was defined as the percentage of cells with a MCN above the 97th percentile of the staining of the negative control sample (FITC-secondary antibody alone). Ten thousand events were acquired for each individual analysis. FACS analyses were performed, when relevant, within 0–5 days from the evaluation of lysability by the A42 clone. Comparisons of MCN among different clones discussed in this study refer to data obtained within the same experiment. Comparisons were never made among different experiments.
Intracellular Expression of the MART-1 Gene Product
Expression was analyzed with a routine protocol for staining of intracellular epitopes. Cells were harvested and counted; washed twice in ice-cold HBSS free of Ca2+, Mg+ +, and phenol red; and fixed with 1% paraformaldehyde (Sigma) in HBSS for 20 min at room temperature. Then cells were washed three times at room temperature in HBSS containing 0.1% saponin (Sigma). The first antibody was then added in 0.1% saponin/FACS buffer, and cells were incubated at room temperature for 1 h. After washing twice with FACS buffer, the relevant second antibody was added, and a second incubation was performed at room temperature for at least 1 h. Cells were then washed twice and run through the FACS scanner.
Antibodies
The following primary mAbs were used: W6/32 (Sera Labs, Westbury, NY) (mouse IgG2a) reacting with a monomorphic determinant on the HLA class I molecules (Ref. 35; 20 μg/ml); IVA12 (100 μg/ml; IgG1; ATCC HB 145) reacting against HLA class II antigens (36); MA2.1 (IgG1) recognizing HLA-A2; and B17 was used at a concentration of 20 μg/ml (37). MB40.2 (anti HLA-B7; B40; 20 μg/ml) mouse IgG mAb was purchased from Incstar Corp. (Stillwater, MN). 277-HA1 (anti-HLA-B14) mouse IgM mAb was purchased from One Lambda and used undiluted. The following mAbs were purified from hybridoma supernatants (Lofstrand Co., Gaithersburg, MD): GAP A3 mAb (mouse IgG2ak) specific for HLA-A3 (38); and mAb SFR8-B6 (rat IgG2b) directed against the HLA-Bw6 super-specificity (39). These mAbs were used at a concentration of 10 μg/ml. Anti-human β2-microglobulin mouse IgG1 mAb was purchased from Boehringer Mannheim Co. (Indianapolis, IN) and used at a concentration of 10 μg/ml. Anti-human ICAM-1 mouse IgG2a mAb was obtained from Endogen, Inc. (Boston, MA) and used at a concentration of 20 μg/ml. Anti-human costimulatory molecule B7/BB-1(CD28) mouse IgG1 mAb was purchased from Becton Dickinson and used undiluted. Secondary antibodies for FACS included: FITC-conjugated goat anti-mouse IgG1, IgG2a, IgG2b, and IgG3 (Becton Dickinson, San Jose, CA) at a concentration of 50 μg/ml; FITC-conjugated goat anti-mouse IgM (μ chain specific; Jackson Immune Research, Inc. West Grove, PA) at a dilution of 1:20; and FITC-conjugated mouse anti-rat IgG2b,3b, F(ab′)2 fragment-specific (MARF; Jackson Immune Research) at a dilution of 1:30. For analysis of expression of MART-1 antigen, a polyvalent serum obtained from a rabbit immunized with MART-1 protein was used. The anti-MART-1 and the preimmunization serum from the same rabbit were Na2SO4 fractionated and used at a final dilution of 1:160. The specificity of this serum was tested by extensive analysis of cell lines or strains of nonmelanocytic origin including breast, colon, prostate, and cervical carcinoma cell line and a melanoma cell line (A 375) not expressing MART-1 by Northern blot and reverse transcription-PCR (29). None of these lines stained with this serum in the experimental conditions described. As a secondary antibody, FITC-conjugated goat anti-rabbit IgG (Jackson Immune Research) was used at a dilution of 1:10. As a further positive control for the intracellular staining technique HMB45 (anti-GP-100), mouse mAb was used (Enzo Diagnostics, New York, NY) at a dilution of 1:10 in otherwise identical conditions. This antibody is specific for cells of melanocytic lineage, and most melanoma cell lines express detectable amounts of the correspondent antigenic determinant. As a negative control for HMB45, an isotype-matched murine IgG was used, and FITC-conjugated goat anti-mouse IgG (50 μg/ml) was used as a secondary antibody. Both anti-MART-1 serum and HMB45 mAb did not stain viable cells, suggesting that they do not recognize epitopes included among the relevant peptides present on the cell surface.
5lCr Release Assay
Cytotoxicity was measured by 51Cr release assay as described previously (40). Effector cells were prepared by harvesting CTL cultures and lymphokine-activated killer cells, resuspending them in CM, and plating at different E:T ratios in 96-well, U-bottomed plates (Costar). One hundred mCi of 51Cr were added per ml of target cell suspension; the mixture was incubated for 2 h at 37°C and then washed three times in HBSS, suspended in CM, and 5 × 103 cells were added to each well. Targets and effectors were centrifuged at 500 rpm for 5 min, then incubated for 4 h at 37°C. The supernatant samples were harvested by using the Skatron apparatus (Skatron, Sterling, VA) and were counted in a gamma counter. The percentage of lysis was calculated as follows:
In some experiments, an attempt was made to enhance the lysis of tumor targets by pulsing the cell lines with 1 μg/ml of MART-127–35 peptide for 2 h at 37°C.
Stable Transfection of the HLA-A2.1 Gene into the 624.28 MEL Clone
To test whether restoration of surface expression of HLA-A2 was sufficient to restore recognition of the 624.28 HLA-A2-negative clone by A42, the pcDNA3-HLA-A2.1 plasmid (Invitrogen, San Diego, CA) was transfected by the modified calcium phosphate method (Stratagene, La Jolla, CA) as described previously (41). Briefly, 2 × 105 exponentially growing melanoma cells were cultured in a 60-mm dish for 16 h in DMEM + 10% FCS. Twenty μg of pcDNA-HLA-A2.1 were added and incubated for 20 min at room temperature in CaCl2, N,N-bis-(2-hydroxyethyl)-2-aminoethane-sulfonic acid, and buffered saline solution. This DNA precipitate mixture was added to melanoma cell cultures and incubated for 6 to 16 h at 37°C in a 3 to 5% CO2, incubator. After incubation, cells were washed, medium was replaced, and G418 (Geneticin; GIBCO-BRL, Grand Island, NY) at a final concentration of 500 μg/ml was added for selection of transfectants. Transfection efficiency was 10−5 to 2 × 10−4. Individual tumor cells were isolated and grown as clones using the limiting dilution technique. Individual clones were then screened for HLA-A2 allele expression by FACS analysis using MA2.1 mAb. Each transduced line had very homogeneous expression of HLA-A2 antigens, suggesting that clonal populations were indeed obtained.
Northern Blot Analysis of Expression of the MART-1 Melanoma Antigen
Different clones of 624-MEL were tested by Northern blot analysis for expression of the MART-1 antigen. Total RNA was isolated with the guanidine isothiocyanate-cesium chloride centrifugation method. Ten to 20 μg of RNA per lane were subjected to electrophoresis through a 1% agarose-formaldehyde gel and transferred into a nylon membrane (Duralon-UV membranes; Stratagene). The SalI-digested fragment containing the full-length cDNA from the MART-1 and the β-actin cDNA (Clontech) were labeled by random priming and used as a probe. Hybridizations were performed at 68°C in Quikhyb rapid hybridization solution (Stratagene), and filters were then washed two times for 30 min with 2X SSC-0.1% SDS at 60°C for 15 min and once with 0.1X SSC at 60°C for 30 min (1× SSC = 150 μm NaCl + 15 μm Na citrate, pH 7.0). The filters were then autoradiographed in a standard fashion.
PCR
Extraction of genomic DNA was performed by the salting out method, and after ethanol precipitation, the DNA pellet was reconstituted in distilled H2O at a final concentration of 40 μg/ml. PCR amplification of HLA-A alleles was done using a modification of the technique reported by Browning et al. (17). PCR primers were selected according to heterologous sequences within the second exon of the class I gene or within the following intron. Specifically for the PCR conditions used in this study, the selected HLA-A2 upstream coding primer was 5′-GTG GAT AGA GCA GGA GGG T-3′, and the downstream noncoding primer was 5′-CCA AGA GCG CAG GTC CTC T-3′, yielding an expected fragment of 487 bp. Other primers for typing of HLA-A were kindly provided by Dr. Michael Browning (University of Oxford, Oxford, United Kingdom). Internal control primers specific for the adenomatous polyposis gene were upstream for the coding region 5′-ATG ATG TTG ACC TTT CCA GGG-3′ and downstream for the noncoding region 5′-ATT GTG TAA CTT TTC ATC AGT TGC-3′ (256-bp fragment; Ref. 42). These primers were synthesized by Lofstrand. PCR reactions contained 1X PCR II buffer (Perkin-Elmer), 0.15 mm magnesium chloride, 200 μm of each deoxynucleotide triphopshate, 0.2 μm of each primer, 200 ng of genomic DNA, and 1.0 unit of Taq DNA polymerase (Amplitaq; Perkin-Elmer). The amplifications were done in a Perkin-Elmer model 9600 thermal cycler. DNA denaturation was performed by heating the solution at 95°C for 3 min (1 cycle); then DNA amplification was performed in 30 cycles using constant denaturation conditions (95°C for 30 s), varying annealing temperatures (cycles 1–10, 65°C for 50 s; cycles 11–20, 63°C for 50 s; and cycles 21–30, 60°C for 50 s) and constant extension conditions (72°C for 50 s). The amplified DNA was loaded on a 2% agarose gel and then run for 90 min at 150 V. Gels were visualized under UV illumination and documented by photography. PCR for HLA class II was performed using a routine procedure as described previously (18).
Statistical Analysis
Correlation analysis comparing different variables against lysis of target cells in a 51Cr release assay was performed using a simple regression model. MCN data were always compared within the same experiment. Lysis of targets by 5lCr release assay in different conditions was compared by two-tailed Student's t test.
RESULTS
HLA Phenotype of Clone A42 and 624-MEL
The HLA phenotype was tested on patient peripheral blood lymphocytes. Clone A42 was generated from patient 501, whose HLA phenotype corresponded to HLA-A2, 24, -B18, 35, -Cw4, 7, -DR5, -DQ3, -DRw52. TIL 1088 were generated from a patient whose HLA phenotype corresponded to HLA-A1, 2, -B8, 44, -Cw5. Patient 624 phenotype corresponded to HLA-A2, 3, -B7, 14, -Cw7, -DR7, -DQ2, -DRw53. The patients were, therefore, totally mismatched except for HLA-A2 and HLA-Cw7. However, the tumor line 624-MEL does not constitutionally express any of the HLA-B, HLA-C, or class II alleles as determined by FACS or complement mediated cytotoxicity as described previously (18, 19). In the absence of stimulation with rIFN-γ, the only restriction elements in common between the effector cells (A42 and 1088 TIL) and the 624-MEL target cell was HLA-A2.
Heterogeneous HLA-A2 Expression in 624-MEL Clones
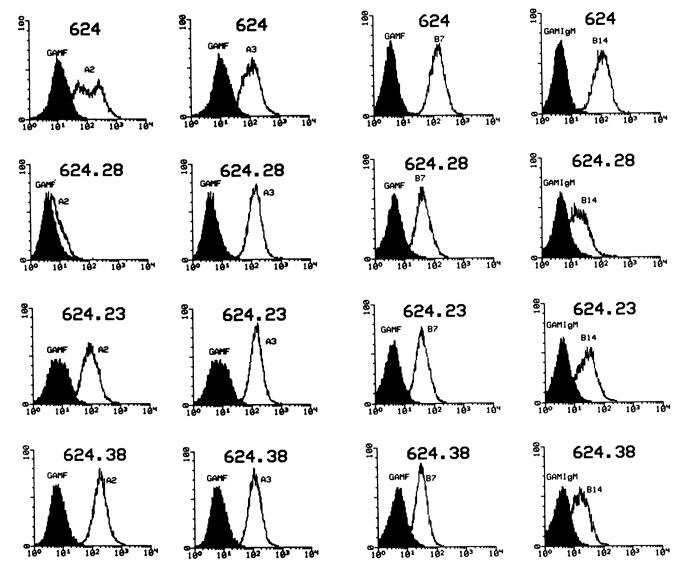
The original bulk melanoma line 624-MEL was noted by FACS analysis with MA2.1 mAb to contain a mixed population of tumor cell clones with a broad range of expression of HLA-A2 molecules (Fig. 1, first row). Consequently, to obtain tumor cell clones with a different expression of HLA-A2, 624-MEL was cloned in limiting dilution conditions. The clones obtained in this fashion were then screened for HLA-A expression by FACS analysis using MA2.1 mAb. Expression of HLA-A2 and HLA-A3 molecules on the cell surface of these clones is presented in Table 1. Although the expression of HLA-A3 was comparable (MCN range, 65 to 130), a wide distribution in the expression of HLA-A2 was noted among the different clones (MCN range, 10–247). In spite of this wide range of expression of HLA-A2 antigens, all clones, with the exception of 624.28, were more than 90% positive for HLA-A2.
Fig. 1.
Flow cytometric analysis of allele-specific surface expression of HLA antigens in representative clones of 624-MEL. Data are presented as MCN of fluorescence. The following mAbs were used: MA2.1, anti-HLA-A2 and B17 (37); MB40.2, anti-HLA-B7, B40; 277-HA1, anti-HLA-B14; GAP A-3. anti-HLA-A3 (38). The original bulk culture (624-MEL) was HLA-A2, A3, -B7, B14 positive (first row) expressing the A loci in standard culture conditions (first two columns) and the B alleles only under stimulation with 500 units/ml of rIFN-γ for 48 h (last two columns). Clone 626.28 totally lost expression of HLA-A2 (second row), clone 624.23 has intermediate expression of HLA-A2 (third row), and clone 624.38 expresses the highest amount of HLA-A2 molecules on its cell surface (fourth row).
Table 1. Expression of surface molecules on 624-MEL clones and lysis by A42 CTL clone.
Expression of surface markers is given as intra-experimental MCN of fluorescence by FACS analysis.
| Clone no. | HLA-A2/-A3 | Class I | β2-ma | ICAM-1 | % lysisb | |
|---|---|---|---|---|---|---|
| 1 | 53 | 86 | 141 | 146 | 105 | 31 (58) |
| 3 | 149 | 80 | 244 | 274 | 81 | 65 (42) |
| 9 | 186 | 120 | 173 | 207 | 62 | 79 (42) |
| 10 | 109 | 71 | 259 | 238 | 92 | 52 (31) |
| 13 | 119 | 103 | 141 | 137 | 63 | 55 (34) |
| 16 | 241 | n.d. | 136 | 126 | 100 | 89 (65) |
| 18 | 96 | 67 | 166 | 90 | 72 | 41 (26) |
| 23 | 42 | 99 | 198 | 156 | 159 | 24 (66) |
| 26 | 36 | 123 | 242 | 134 | 31 | 30 (57) |
| 28 | 10 | 104 | 137 | 78 | 193 | 0 (67) |
| 30 | 75 | 130 | 166 | 148 | 83 | 35 (48) |
| 38 | 247 | 65 | 214 | 151 | 123 | 88 (60) |
| 45 | 41 | 106 | 237 | 283 | 97 | 31 (44) |
| 48 | 36 | 93 | 129 | 161 | 94 | 25 (65) |
| 49 | 35 | 102 | 126 | 161 | 94 | 19 (59) |
| Bulk | 31 | n.d. | 141 | 146 | 57 | 32 (48) |
β2-m, β2-microglobulin.
Lysis evaluated in a 51Cr release assay refers to the E:T ratio of 20:1. In parentheses, the values of lysis of the same target by lymphokine-activated killer cells at 40:1 E:T ratio. Correlation coefficient for the linear regression analysis between lysis of target cells and each other parameter are r = 0.91, P < 0.001 for HLA-A2; r = 0.1, P > 0.1 for HLA-A3, r = 0.2, P > 0.1 for HLA class I monomorphic component (detected by mAb W6/32), r = 0.2, P > 0.1 for β2…microglobulin, and r = 0.03, P > 0.1 for ICAM-1.
To further analyze the pattern of reduction or loss of allele expression of the clones, more detailed phenotypic analysis of HLA-A alleles under standard culture conditions, and HLA-B alleles after stimulation with rIFN-γ, was performed on selected clones. Examples of the phenotypic analyses of three representative 624-MEL clones are shown in Fig. 1. Expression of HLA-A3 was not significantly different in the melanoma clones tested; clone 624.28 totally lost expression of HLA-A2 (Fig. 1, second row), clone 624.23 showed intermediate expression (Fig. 1, third row), and clone 624.38 had the highest amount of surface expression (Fig. 1, fourth row). Expression of HLA-A2 was not enhanced by stimulation with rIFN-γ in any of the clones tested (data not shown). HLA-B7 and HLA-B14 were not detectable under standard culture conditions, as often seen in melanoma cell lines (18), but their expression was elicited with rIFN-γ that generated a similar pattern in all 624-MEL clones. HLA class II molecules were not detectable by FACS under routine culture conditions in any of the clones tested but were similarly elicited in all clones with rIFN-γ. Loss of HLA-A2 expression in clone 624–28 by FACS was consistent with complement-mediated cytotoxicity using both the Amos modified method (three multiparous sera per allele) and monoclonal trays (two mAbs for each allele). Therefore, loss of HLA-A2 surface expression was confirmed using a total of six independent reagents against HLA-A2 (data not shown). No difference in expression of HLA-A3 or other HLA class I or II was noted between 624.28 and the original 624 MEL by FACS (Fig. 1) and complement-mediated cytotoxicity (data not shown).
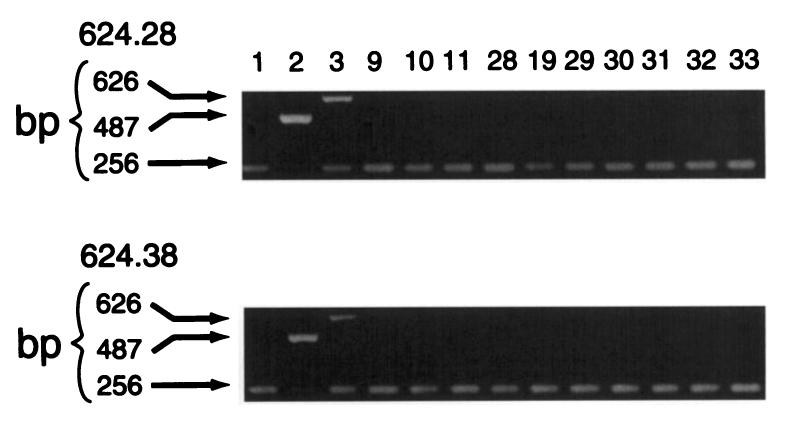
PCR reaction amplified the HLA-A2-specific, 487-bp fragment in both 624.28 and 624.38 melanoma clones, while no amplification was noted of HLA-A2 and HLA-A3, respectively, from DU-145 (HLA-A3, 33) and LN-CAP (HLA-A1, 2) prostatic cancer lines (Fig. 2). Similarly, analysis of the HLA class II genomic DNA for the two clones showed an identical pattern with both lines being DRβ1 0401, 0701, DQβ1 0201, 0301, and DRβ4 0101. Thus, the lack of surface expression of HLA-A2 observed in 624.28 was not due to loss of a large genomic unit of DNA encompassing a full haplotype as described previously for other lines (18). These data, however, do not exclude deletion of other exons and/or introns within the HLA-A2 gene that might be responsible for loss of expression of the HLA-A2 allele on the surface of 624.28.
Fig. 2.
Analysis of HLA-A genomic DNA in 624.28 and 624.38 clones of 624-MEL (HLA-A2, 3). Allele-specific amplification was done as described in the text (17, 18), using genomic DNA from the two clones and two relevant controls: DU-145 (HLA-A3, 33) and LN-CAP (HLA-A1, 2) prostatic cancer lines (data not shown). Included in each PCR reaction was an internal control primer that gave rise to a 256-bp fragment (42).
Role of the Level of HLA-A2 Expression in Determining the Susceptibility of 624-MEL Clones to A42 CTL
624-MEL clones were then tested for lysis by A42 CTL. This is an HLA-A2-restricted, melanoma-specific CTL clone shown previously to recognize the melanoma/melanocyte-specific antigen MART-1 (26) and more precisely the HLA-A0201 binding peptide MART-127–35 (27). Lysis of different representative clones of 624-MEL by A42 CTL at 20:1 E:T ratio is presented in Table 1. Similar results were obtained with E:T ratios of 5:1 (Fig. 3). A marked heterogeneity in the susceptibility to lysis by A42 was observed. Lysis ranged from 0 to 89% lysis, in a 4-h 51Cr release assay, at an E:T ratio of 20:1. Clone 624.28 (HLA-A2 negative) was not recognized by A42 CTL but still was lysed by MHC-unrestricted LAK cells (Table 1). A total of 60 clones of 624-MEL were tested; lysis of these clones was quite heterogeneous, but no other CTL-resistant (0% lysis) clone could be identified (data not shown).
Fig. 3.
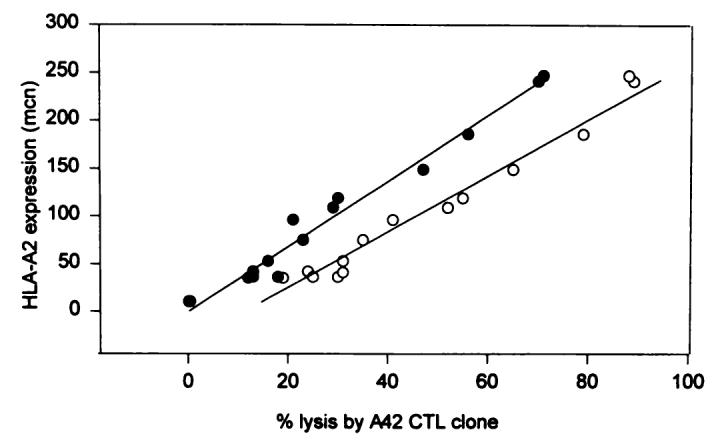
Correlation between the level of expression of HLA-A2 surface antigens in clones of 624-MEL and lysis by an HLA-A2-restricted CTL clone A42 recognizing the melanoma MART-127–35 peptide. Cytotoxicity was measured in a standard 4-h 51Cr release assay as described previously (40). The percent lysis was calculated (see “Materials and Methods”). Data are presented at 20:1 (○) and 5:1 (●) E:T ratio. To simplify the analysis, a simple linear correlation was assumed for this range of lytic activity and HLA-A2 allele expression. The level of expression is described in a semiquantitative fashion by analyzing the MCN of fluorescence within the same experiment.
The different sensitivity to lysis by A42 could have been due to several mechanisms including: (a) differential expression of the MART-1 antigen; (b) heterogeneous expression of HLA molecules; (c) loss of other accessory molecules involved in lymphocyte-target interaction (43, 44); or (d) a combination of the above. Thus, the expression of adhesion molecules, and the expression of different components of the HLA class I molecule, including the monomorphic region recognized by the mAb W6/32 and β2-microglobulin, were analyzed by flow cytometry in 624-MEL clones. Expression was evaluated in a semiquantitative fashion by analyzing the MCN of fluorescence at the time when the lysis assay was performed. As shown in Table 1, no correlation between lysis by A42 CTL and the level of the expression on 624-MEL clones of HLA class I, β2-microglobulin, or ICAM-1 was identified (Ps shown in Table 1) The expression of B7 costimulatory molecule was not analyzed at the clonal level since the bulk 624-MEL line was totally negative for this antigen by FACS analysis (data not shown). However, a correlation was noted between the level of surface expression of HLA-A2 antigens as detected by MA2.1 mAb (37) and lysis by A42. In addition to clone 624.28, whose total loss of HLA-A2 expression could account for its resistance to lysis, clones with intermediate susceptibility to lysis (19–41% at 20:1 E:T ratio) expressed an intermediate amount of HLA-A2 (MCN range, between 35 and 96), and clones highly susceptible (52–89%) expressed a high level of HLA-A2 (119–247 MCN). The correlation between HLA-A2 expression and lysis of the clones by A42, analyzed with a simple linear regression model, was highly significant, as shown in Fig. 3 (r = 0.97, P < 0.001 for 20:1 E:T; r = 0.95, P < 0.001 for 5:1 E:T). These data suggested that the level of surface expression of an HLA class I allele may play a critical role in influencing the susceptibility of target cells to the lysis by HLA class I-restricted CTL.
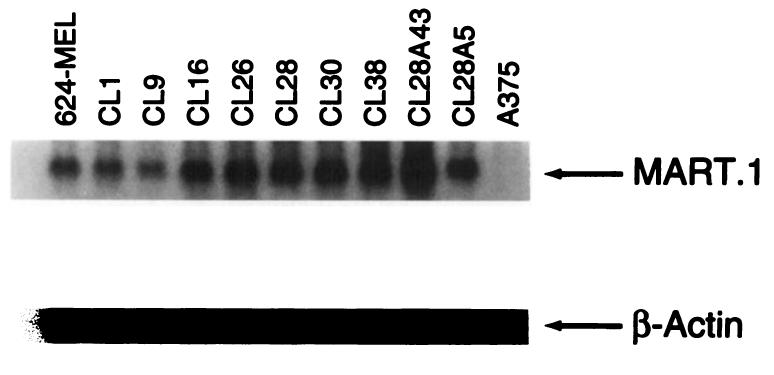
To rule out the possibility that heterogeneous amounts of expression of the antigen recognized by the effectors used in this study (MART-1) could be coincidentally associated with expression of HLA-A2 and possibly be responsible for the heterogeneous sensitivity to lysis of the different 624-MEL clones, the expression of MART-1 mRNA was evaluated by Northern blot in some representative clones (Fig. 4). Neither obvious semiquantitative difference in the level of MART-1 expression among clones nor correlation with lysability by A42 was observed. Ratios of Beta-plate counts (MART-1 versus β-actin) were in a close range between 4.5 and 5.9. In an effort to analyze the intracellular expression of the MART-1 protein, a polyvalent rabbit serum immunized against MART-1 was used for testing by FACS analysis after permeabilization of paraformaldehyde-fixed melanoma cells with saponin (Table 2). Expression of MART-1 as detected with this polyvalent serum was somewhat heterogeneous among the representative 624-MEL clones tested, but the amount of expression did not correlate with lysis (P > 0.1).
Fig. 4.
Expression of MART-1 mRNA in representative clones of 624-MEL as analyzed by Northern blot. The level of expression of the melanoma antigen MART-1 is semiquantitatively compared (10 to 20 μg of total RNA per lane) with a Sal/I-digested fragment containing the full-length cDNA from the MART-1 and the β-actin cDNA probe (Clontech). Although variable among the different clones, the level of expression does not correlate with their sensitivity to lysis by A42 CTL. Melanoma line A 375 represents a MART-1-negative control as already described (29).
Table 2.
Intracellular expression of MART-1 and GP100 melanoma antigens in representative clones of 624-MEL
| Clone no. | MART-1 (MCN)a |
GP100 |
|---|---|---|
| 1 | 254 | 297 |
| 9 | 109 | 175 |
| 16 | 87 | 139 |
| 23 | 85 | 198 |
| 26 | 119 | 137 |
| 28 | 62 | 189 |
| 30 | 193 | 319 |
| 38 | 231 | 274 |
| Sub-clones of 624.28 transduced with HLA-A2.1 gene | ||
| 28.A5 | 108 | 143 |
| 28.A43 | 91 | 142 |
| Control cell linesb | ||
| Wa Fib | 28 | 25 |
| MCF-7 | 18 | 25 |
| A-375 | 18 | 11 |
MCN of unstained controls were <10. Intracellular expression of MART-1 and GP100 proteins as detected by FACS analysis in 1% paraformaldehyde-fixed 624-MEL clones (see “Materials and Methods”). Linear regression between lysis by A42 clone (see Table 1) and expression of MART-1 was not significant.
Control cell lines included a fibroblast cell line (WaFib), a breast cancer cell line (MCF-7), and a melanoma cell line (A375) not expressing the MART-1 and GP100 antigens, as assessed by reverse transcription-PCR (29).
Restoration of Surface Expression of HLA-A2 Molecules in 624.28 Quantitatively Correlates with Recognition of MART-1 Antigen by A42 CTL
Although the 624-MEL clones described above were derived from the same bulk culture, the correlation between the level of expression of HLA-A2 and lysis could have been due to known or unknown mechanism(s) other than the level of expression of the HLA allele or the MART-1 antigen. In order to create a model in which the only potential variable was the level of expression of HLA-A2, clone 624.28 (HLA-A2 negative) was stably transfected with the HLA-A2.1 gene to promote expression of HLA-A2. The transfected line was selected in G418 and subcloned. Clones were then screened for different expression of HLA-A2 by FACS analysis. A panel of twelve 624.28 subclones expressing different levels of HLA-A2.1 could be obtained and tested for lysability by A42 CTL (Table 3). HLA-A2.1 transfection did not significantly modify MART-1 mRNA expression (two representative subclones are shown in Table 2) or the expression of ICAM-1, HLA class I, and β2-microglobulin (data not shown). As for the parental line, the 624.28 subclones showing an intermediate expression of HLA-A2.1 (MCN, 43–102) were characterized by intermediate sensitivity to lysis by A42 CTL (28 and 41% at E:T of 20:1), while the subclones expressing higher levels of HLA-A2.1 (MCN< 283–478) showed a significantly higher susceptibility to lysis (62–83%). As expected, subclones lacking surface expression of HLA-A2.1 maintained their CTL resistance. A significant direct correlation was noted between the expression of HLA-A2 (expressed as MCN) and lysis in a 51Cr release assay by A42 CTL (r = 0.93, P < 0.001 at 20:1 E:T; r = 0.92, P < 0.001 for 5:1 E:T). In order to evaluate whether this phenomenon could be limited to a clonal population, we analyzed the same parameters in a lytic assay using as effectors a bulk TIL line from a different patient (Table 3). Lysis of the different melanoma clones by 1088 TIL also highly correlated with expression of HLA-A2 (P < 0.001 for both E:T ratios tested). Thus, heterogeneous expression of an HLA class I allele on target cells could influence their lysis by HLA class I-restricted CTL.
Table 3.
Expression of HLA-A2 by subclones of 624.28-MEL after stable transfection of the HLA-A2.1 gene: correlation with lysis by CTL
| % of lysisa | |||
|---|---|---|---|
| Clone no. | HLA-A2 (MCN)b |
1088 TIL E:T = 40 (10):1 |
A42 E:T = 20 (5):1 |
| Original clone | |||
| 624-28 | 5 | 0 (1) | −1 (−1) |
| 624.28 HLA-A2.1-transfected clones | |||
| 28.48 | 3 | 0 (−2) | −2 (−1) |
| 28.22 | 6 | 0 (0) | 1 (0) |
| 28.45 | 43 | 19 (11) | 28 (26) |
| 28.26 | 65 | 25 (6) | 34 (20) |
| 28.17 | 101 | 37 (9) | 34 (16) |
| 28.46 | 102 | 29 (10) | 41 (30) |
| 28.43 | 283 | 50 (22) | 72 (61) |
| 28.24 | 307 | 54 (24) | 69 (58) |
| 28.11 | 336 | 51 (28) | 62 (62) |
| 28.5 | 355 | 52 (22) | 82 (66) |
| 28.15 | 365 | 53 (16) | 69 (61) |
| 28.28 | 478 | 54 (21) | 79 (62) |
Results of a 51Cr release assay. Linear regression between lysis and expression of HLA-A2 was P < 0.001 for either E:T ratio for both CTLs (1088 TIL and A42 clone).
MCNs of unstained controls were <10.
Saturation of HLA-A2.1 Binding Sites with Exogenous MART-127–35 Peptide and Modification of CTL Recognition
The absolute number of MHC-peptide complexes required for triggering CTL activity has been estimated at around 200 (21), but the amount of MHC molecules needed to achieve that threshold is much higher (21, 22) and depends, in part, on the level of peptide available for binding (22, 23). By increasing the amount of MART-127–35 peptide available for binding, it may thus be possible to enhance the susceptibility of clones with intermediate expression of HLA-A2 to CTL lysis by increasing the total number of HLA-peptide complexes.
To test this hypothesis, the number of MHC-peptide complexes was artificially increased by the addition of exogenous MART-127–35 peptide. Six representative 624.28, HLA-A2.1-transduced subclones with heterogeneous expression of HLA-A2.1 were tested for lysability by A42 CTL under standard conditions and after a 2-h pulse with 1 μg/ml (approximately 1 μm) MART-127–35 peptide (Fig. 5). In the presence of exogenous peptide, lysis was significantly enhanced in subclones expressing intermediate amounts of HLA-A2.1 molecules, whereas a plateau of maximal lysis was observed for the subclones with high HLA-A2.1 expression, either in the absence or in the presence of MART-127–35 peptide. These data showed that the concentration of endogenous peptide may become a limiting factor when the expression of the restricting HLA class I antigen is low or intermediate. Clone 28.22, which was HLA-A2.1 negative, was not susceptible to CTL lysis, even after peptide pulsing. Since it has been reported that the addition of exogenous peptide may artificially enhance the expression of HLA-A2 molecules in cell lines, such as T2 cells, with defects of the antigen presentation machinery (45), expression of HLA-A2 was compared by FACS in the absence and presence of pulsing with MART-127–35 peptide. No up-regulation of HLA-A2 surface expression was caused by peptide pulsing (data not shown), showing that the enhancement of lysis observed in this experiment was not simply due to increased expression of HLA-A2 molecules on the cell surface. Furthermore, the enhancement of sensitivity to CTL lysis observed in 624-MEL clones with intermediate expression of HLA-A2 after pulse with exogenous peptide suggested that, at least in these experimental conditions, in the presence of saturating concentrations of antigenic peptide, the amount of allele-specific expression of HLA-A molecules is no longer a limiting factor for CTL recognition.
Fig. 5.
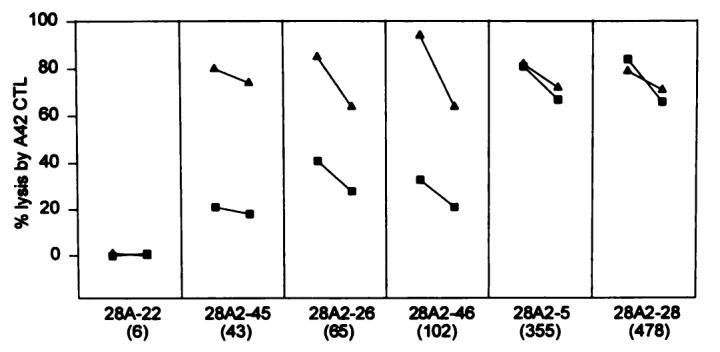
Enhancement of lysis of 624.28 clones by exogenous addition of the MART-127–35 peptide. 624.28 clones were incubated with the MART 127–35 peptide (▲) before testing. As controls unpulsed, clones were used as targets in the 51Cr release assay (■). In these experimental conditions, lysis is enhanced in the clones expressing an intermediate amount of HLA-A2 molecules to a plateau corresponding to the maximum lysis for the clones with the highest expression in the absence or presence of pulsing with peptide. Lysis of subclone 28A.22, which does not express HLA-A2, is not affected by pulsing with peptide. For all data points, lysis is expressed at 20:1 (left) and 5:1 (right) E:T ratio. Numbers in parentheses, MCN of HLA-A2 expression.
DISCUSSION
Although extensively investigated (4-16), expression of HLA antigens on tumor cells has almost uniformly been described as an all-or-none phenomenon, and little attention has been paid to the amount of expression and its relevance for in vitro as well as in vivo studies. Expression of MHC antigens is necessary for tumor recognition by CTL (1-3), the function of which is restricted in an allele-specific fashion. Most studies, however, have analyzed MHC expression on tumors using mAbs directed against monomorphic components of the HLA molecule (5). This leads to the underestimation of aberrations of expression of HLA, since all MHC class I alleles had to be lost before these antibodies could detect any abnormality. Recently, with the development of locus- and allele-specific mAbs as well as molecular techniques for the analysis of HLA, we and others could describe several mechanisms leading to aberrations of expression of HLA class I antigens (13-19), including total loss of MHC class I due to faulty β2-microglobulin (13-16) and allele-specific loss (17, 18, 20). Furthermore, expression of MHC antigens, when detectable, may be variable due to locus- or allele-specific down-regulation (18, 19). In this study, we show that, in cell lines expressing a determined HLA allele, the amount of surface expression of that allele significantly affects their lysability by HLA class I-restricted CTL.
Since most melanoma antigens, including MART-1/MelanA, are self molecules also expressed on the normal counterpart (25-30, 46), the relationship between tumor and immune system can be viewed as an autoimmune phenomenon in which a balance between recognition of self and tolerance may influence the final outcome in vivo. In natural conditions, endogenous peptides compete with more than 1000 other peptides for binding to specific HLA alleles (22). Therefore, not all proteins are expressed in sufficient amounts to give origin to peptides expressed on the cell surface with the density required for CTL recognition (23). Endogenous epitope/MHC complexes not expressed in a sufficient level may allow self tolerance in spite of the presence, in the same organism, of a CTL population specific for that epitope/MHC complex (24), as it may be for the MART-1 gene product.
It is often cited that a threshold of 200 MHC class I-peptide complexes is required to sensitize a target cell for lysis by CTLs (21). However, a much larger number of MHC and antigen molecules may be necessary to achieve this threshold. Indeed, this number is dependent on a number of factors influencing the binding of the peptide to a particular HLA allele. These include the amino acid sequence of the peptide in relation to the binding groove of the HLA allele (32, 47-51) and the relative amount of peptide available for binding (21-23). The theoretical question, therefore, arises as to whether the level of expression of a specific HLA class I allele may also influence tumor recognition by CTL and potentially offer a selective advantage in vivo to tumor cells expressing fewer MHC antigens.
In this study, an in vitro model was developed to answer this question by analyzing the ability of a CD8+ T-cell clone or CTL line specifically recognizing an immunodominant peptide of the melanoma antigen (MART-1) in the context of HLA-A2 restriction element (32). As a target, clones derived from a melanoma line expressing MART-1 but varying amounts of HLA-A2 molecules were used. Contrary to previously described mechanisms (14, 15, 17, 18, 52, 53), the “fading” of expression of HLA-A2 in 624-MEL represented a gradual event not due to defects of antigen presentation (54) or posttranslational (55) abnormalities since HLA-A2 expression and CTL recognition were reinstituted in 624.28 after transduction of the HLA-A2.1 gene. The data obtained with 624-MEL clones showed a strong correlation between expression of HLA-A2 antigens and efficiency of lysis by anti-MART-127–35 CTL, either at the bulk or clonal level. This association seemed causative, since the same correlation was noted in subclones of 624.28 (HLA-A2 negative) expressing different amounts of HLA-A2 after transduction with the HLA A2.1 gene.
The crucial role of the level of allele expression on tumor sensitivity to CTL lysis observed in this model suggests that only a limited percentage of MHC molecules is bearing the relevant peptide MART-127–35. These results are in agreement with previous data showing that endogenous peptides are not naturally recognized because insufficient binding to MHC can sensitize T cells if added exogenously (24). It is possible, therefore, that at some “borderline” levels of antigen expression, the amount of HLA allele available becomes the determining factor, particularly in situations of low or intermediate affinity between peptide and HLA molecule, as is the case for MART-127–35 (28). In fact, when we tested different 624.28 clones in saturating conditions of exogenous MART-127–35 peptide, lysis of clones with lower expression of HLA-A2 molecules was greatly enhanced by the exogenous addition of peptide and matched the lysis observed in the high-expressing clones. However, lysis of clones expressing higher levels of HLA-A2 was not affected by the addition of peptide.
The phenomenon described in this study may represent an additional mechanism potentially used by tumors to escape from immune recognition (56, 57). Adoptive therapy with melanoma-specific TIL can cause regression of metastases (3), and CD8+ MHC class I-restricted CTL represents a powerful component of this immune response (1-3, 25-30). Although many other mechanisms may play a significant role in the sensitivity of tumor cells to CTL lysis (56, 57), the correlation between the level of surface expression of HLA-peptide complexes and efficiency of lysis by tumor-specific CTLs could have important implications for studies of tumor immunology. Analysis of heterogeneity of expression of HLA molecules may help explain resistance of tumors to therapy with HLA-restricted CTL. In a purely theoretical model, this efficiency depends on the intratumoral ratio between effector and target cells and the rate in which tumor targets are lysed. Ideally, for a clinical perception of tumor shrinkage (response), CTL should recognize and kill target cells at a rate faster than the tumor cells divide. Since immunotherapy with TILs is an event of limited duration and the E:T ratio achieved in vivo may not be as favorable as in in vitro, the efficiency by which CTL can kill their targets may become extremely important in vivo. Modulation of expression of endogenous proteins with 5′ deoxyazacytidine (58) or of HLA molecules by cytokines such as the interferons may enhance the recognition of tumor cells in vitro (59) or in vivo (60) during the immunotherapy of cancer. Finally, it is possible that MHC molecules genetically attached to a single peptide may overcome the low expression of MHC-peptide complexes on the tumor cell surface (61) and provide a potent immunogen in protocols of vaccine therapy.
ACKNOWLEDGMENTS
We thank Dr. Giorgio Parmiani (Experimental Oncology D, National Tumor Institute, Milan Italy) for the useful discussion and revision of this paper, and Dr. Matthew A. Gonda for giving us anti-MART-1 rabbit polyclonal antibody.
Footnotes
The abbreviations used are: FACS, fluorescence-activated cell sorter; CM, complete medium; E:T, effector-to-target ratio; rIFN-γ, recombinant IFN-γ, MCN, mean channel number; TIL, tumor-infiltrating lymphocytes; MARF, fluorescein-conjugated mouse anti-rat IgG; ICAM-1, intracellular adhesion molecule-1.
REFERENCES
- 1.Wolfel T, Klehmann E, Muller C, Schutt KH, Meyer zum Buschenfelde KH, Knuth A. Lysis of human melanoma cells by autologous cytolytic T cell clones: identification of human histocompatibility leukocyte antigen A2 as a restriction element for three different antigens. J. Exp. Med. 1989;170:797–810. doi: 10.1084/jem.170.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crowley NJ, Darrow TL, Quinn-Allen MA, Seigler HF. MHC-restricted recognition of autologous melanoma by tumor-specific cytotoxic T cells: evidence for restriction by a dominant HLA-A allele. J. Immunol. 1991;146:1692–1699. [PubMed] [Google Scholar]
- 3.Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma: a preliminary report [see comments] N. Engl. J. Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 4.Pellegrino MA, Ferrone S, Reisfeld RA, Irie RF, Golub SH. Expression of histocompatibility (HLA) antigens on tumor cells and normal cells from patients with melanoma. Cancer (Phila.) 1977;40:36–41. doi: 10.1002/1097-0142(197707)40:1<36::aid-cncr2820400108>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Ruiter DJ, Mattijssen V, Broecker EB, Ferrone S. MHC antigens in human melanomas. Semin. Cancer Biol. 1991;2(Suppl 1):35–45. [PubMed] [Google Scholar]
- 6.Natali PG, Nicotra MR, Bigotti A, Venturo I, Marcenaro L, Giacomini P, Russo C. Selective changes in expression of HLA class I polymorphic determinants in human solid tumors. Proc. Natl. Acad. Sci. USA. 1989;86:6719–6723. doi: 10.1073/pnas.86.17.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Momburg F, Ziegler A, Harpprecht J, Moller P, Moldenhauer G, Hammerling GJ. Selective loss of HLA-A or HLA-B antigen expression in colon carcinoma. J. Immunol. 1989;142:352–358. [PubMed] [Google Scholar]
- 8.Momburg F, Degener T, Bacchus E, Moldenhauer G, Hammerling GJ, Moller P. Loss of HLA-A, B, C and de novo expression of HLA-D in colorectal cancer. Int. J. Cancer. 1986;37:179–184. doi: 10.1002/ijc.2910370203. [DOI] [PubMed] [Google Scholar]
- 9.Holzmann B, Brocker EB, Lehmann JM, Ruiter DJ, Sorg C, Riethmuller G, Johnson JP. Tumor progression in human malignant melanoma: five stages defined by their antigenic phenotypes. Int. J. Cancer. 1987;39:466–471. doi: 10.1002/ijc.2910390410. [DOI] [PubMed] [Google Scholar]
- 10.D'Alessandro G, Zardawi I, Grace J, McCarthy WH, Hersey P. Immunohistological evaluation of MHC class I and II antigen expression on nevi and melanoma: relation to biology of melanoma. Pathology. 1987;19:339–346. doi: 10.3109/00313028709103880. [DOI] [PubMed] [Google Scholar]
- 11.Cordon-Cardo C, Fuks Z, Drobnjak M, Moreno C, Eisenbach L, Feldman M. Expression of HLA-A, B, C antigens on primary and metastatic tumor cell populations of human carcinomas. Cancer Res. 1991;51:6372–6380. [PubMed] [Google Scholar]
- 12.Mechtersheimer G, Staudter M, Majdic O, Dorken B, Moldenhauer G, Moller P. Expression of HLA-A, B, C, β2-microglobulin (β2m). HLA-DR, -DP, -DQ and of HLA-D-associated invariant chain (Ii) in soft-tissue tumors. Int. J. Cancer. 1990;46:813–823. doi: 10.1002/ijc.2910460512. [DOI] [PubMed] [Google Scholar]
- 13.Gattoni-Celli S, Kirsch K, Timpane R, Isselbacher KJ. β2-Microglobulin gene is mutated in a human colon cancer cell line (HCT) deficient in the expression of HLA class I antigens on the cell surface. Cancer Res. 1992;52:1201–1204. [PubMed] [Google Scholar]
- 14.D'Urso CM, Wang ZG, Cao Y, Tatake R, Zeff RA, Ferrane S. Lack of HLA class I antigen expression by cultured melanoma cells FO-1 due to a defect in β2m gene expression. J. Clin. Invest. 1991;87:284–292. doi: 10.1172/JCI114984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Cao Y, Albino AP, Zeff RA, Houghton A, Ferrone S. Lack of HLA class I antigen expression by melanoma cells SK-MEL-33 caused by a reading frameshift in β2-microglobulin messenger RNA. J. Clin. Invest. 1993;91:684–692. doi: 10.1172/JCI116249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz-Cabello F, Perez-Ayala M, Gomez O, Redondo M, Concha A, Cabrera T, Garrido F. Molecular analysis of MHC-class-I alterations in human tumor cell lines. Int. J. Cancer Suppl. 1991;6:123–130. doi: 10.1002/ijc.2910470723. [DOI] [PubMed] [Google Scholar]
- 17.Browning MJ, Krausa P, Rowan A, Bicknell DC, Bodmer JG, Bodmer WF. Tissue typing the HLA-A locus from genomic DNA by sequence-specific PCR: comparison of HLA genotype and surface expression on colorectal tumor cell lines. Proc. Natl. Acad. Sci. USA. 1993;90:2842–2845. doi: 10.1073/pnas.90.7.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marincola FM, Shamamian P, Alexander RB, Gnarra JR, Turetskaya RL, Nedospasov SA, Simonis TB, Taubenberger JK, Yannelli J, Mixon A, Restifo NP, Herlyn M, Rosenberg SA. Loss of HLA haplotype and B locus down-regulation in melanoma cell lines. J. Immunol. 1994;153:1225–1237. [PubMed] [Google Scholar]
- 19.Marincola FM, Shamamian P, Simonis TB, Abati A, Hackett J, O'Dea T, Fetsch P, Yannelli J, Restifo NP, Mule JJ, Rosenberg SA. Locus-specific analysis of human leukocyte antigen class I expression in melanoma cell lines. J. Immunother. Emphasis Tumor Immunol. 1994;16:13–23. doi: 10.1097/00002371-199407000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kageshita T, Wang Z, Calorini L, Yoshii A, Kimura T, Ono T, Gattoni-Celli S, Ferrone S. Selective loss of human leukocyte class I allospecificities and staining of melanoma cells by monoclonal antibodies recognizing monomorphic determinants of class I human leukocyte antigens. Cancer Res. 1993;53:3349–3354. [PubMed] [Google Scholar]
- 21.Christinck ER, Luscher MA, Barber BH, Williams DB. Peptide binding to class I MHC on living cells and quantitation of complexes required for CTL lysis. Nature (Lond.) 1991;352:67–70. doi: 10.1038/352067a0. [DOI] [PubMed] [Google Scholar]
- 22.Hunt DF, Henderson RA, Shabanowitz J, Sakaguchi K, Michel H, Sevilir N, Cox AL, Appella E, Engelhard VH. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry [see comments] Science (Washington DC) 1992;255:1261–1263. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- 23.Carbone FR, Moore MW, Sheil JM, Bevan MJ. Induction of cytotoxic T lymphocytes by primary in vitro stimulation with peptides. J. Exp. Med. 1988;167:1767–1779. doi: 10.1084/jem.167.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schild H, Rotzschke O, Kaibacher H, Rammensee HG. Limit of T cell tolerance to self proteins by peptide presentation. Science (Washington DC) 1990;247:1587–1589. doi: 10.1126/science.2321019. [DOI] [PubMed] [Google Scholar]
- 25.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science (Washington DC) 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 26.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Rivoltini L, Topalian SL, Miki T, Rosenberg SA. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc. Natl. Acad. Sci. USA. 1994;91:3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawakami Y, Eliyahu S, Sakaguchi K, Robbins PF, Rivoltini L, Yannelli JR, Appella E, Rosenberg SA. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J. Exp. Med. 1994;180:347–352. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivoltini L, Kawakami Y, Sakaguchi K, Southwood S, Sette A, Robbins PF, Marincola FM, Salgaller M, Yannelli JR, Appella E, Rosenberg SA. Induction of tumor reactive CTL from peripheral blood and tumor-infiltrating lymphocytes of melanoma patients by in vitro stimulation with an immunodominant peptide of the human melanoma antigen MART-1. J. Immunol. 1995;154:2257–2265. [PubMed] [Google Scholar]
- 29.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Sakaguchi K, Appella E, Yannelli JR, Adema GJ, Miki T, Rosenberg SA. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc. Natl. Acad. Sci. USA. 1994;91:6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salgaller ML, Weber JS, Koenig S, Yannelli JR, Rosenberg SA. Generation of specific anti-melanoma reactivity by stimulation of human tumor-infiltrating lymphocytes with MAGE-1 synthetic peptide. Cancer Immunol. Immunother. 1994;39:105–116. doi: 10.1007/BF01525316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foa R, Guarini A, Gansbacher B. IL2 treatment for cancer: from biology to gene therapy. Br. J. Cancer. 1992;66:992–998. doi: 10.1038/bjc.1992.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature (Lond.) 1987;329:512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- 33.Hopkins KA, van Leeuwen A, Tardiff GN, LeFor WM. Lymphotoxicity testing. In: Zachary AA, Teresi GA, editors. ASHI Laboratory Manual. American Society for Histocompatibility and Immunogenetics; Lenexa, KS: 1990. pp. 195–199. [Google Scholar]
- 34.Adams SD, Marincola FM, Shamamian P, Simonis TB, Davey RJ. HLA class I typing of tumor cell lines using fluorescence activated cell sorting (FACS) and complement mediated cytotoxicity (CDC) Hum. Immunol. 1994;37(Suppl):149. [Google Scholar]
- 35.Brodsky FM, Parham P. Monomorphic anti-HLA-A, B, C monoclonal antibodies detecting molecular subunits and combinatorial determinants. J. Immunol. 1982;128:129–135. [PubMed] [Google Scholar]
- 36.Shaw S, Ziegler A, DeMars R. Specificity of monoclonal antibodies directed against human and murine class II histocompatibility antigens as analyzed by binding to HLA-deletion mutant cell lines. Hum. Immunol. 1985;12:191–211. doi: 10.1016/0198-8859(85)90336-2. [DOI] [PubMed] [Google Scholar]
- 37.McMichael AJ, Parham P, Rust N, Brodsky F. A monoclonal antibody that recognizes an antigenic determinant shared by HLA A2 and B17. Hum. Immunol. 1980;1:121–129. doi: 10.1016/0198-8859(80)90099-3. [DOI] [PubMed] [Google Scholar]
- 38.Berger AE, Davis JE, Cresswell P. Monoclonal antibody to HLA-A3. Hybridoma. 1982;1:87–90. doi: 10.1089/hyb.1.1982.1.87. [DOI] [PubMed] [Google Scholar]
- 39.Radka SF, Kostyu DD, Amos DB. A monoclonal antibody directed against the HLA-Bw6 epitope. J. Immunol. 1982;128:2804–2806. [PubMed] [Google Scholar]
- 40.Kawakami Y, Rosenberg SA, Lotze MT. Interleukin 4 promotes the growth of tumor-infiltrating lymphocytes cytotoxic for human autologous melanoma. J. Exp. Med. 1988;168:2183–2191. doi: 10.1084/jem.168.6.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawakami Y, Zakut R, Topalian SL, Stotter H, Rosenberg SA. Shared human melanoma antigens: recognition by tumor-infiltrating lymphocytes in HLA-A2.1-transfected melanomas. J. Immunol. 1992;148:638–643. [PubMed] [Google Scholar]
- 42.Sadler AM, Petronzelli F, Krausa P, Marsh SGE, Guttridge MG, Browning MJ, Bodmer JG. Low-resolution DNA typing for HLA-B using sequence-specific primers in allele- or group-specific ARMS/PCR. Tissue Antigens. 1994;44:148–154. doi: 10.1111/j.1399-0039.1994.tb02372.x. [DOI] [PubMed] [Google Scholar]
- 43.Rivoltini L, Cattoretti G, Arienti F, Mastroianni A, Parmiani G. CEA and NCA expressed by colon carcinoma cells affect their interaction with and lysability by activated lymphocytes. Int. J. Biol. Markers. 1992;7:143–147. doi: 10.1177/172460089200700304. [DOI] [PubMed] [Google Scholar]
- 44.Braakman E, Goedegebuure PS, Vreugdenhil RJ, Segal DM, Shaw S, Bolhuis RLH. ICAM-1 melanoma cells are relatively resistant to CD3-mediated T cell lysis. Int. J. Cancer. 1990;46:475–480. doi: 10.1002/ijc.2910460325. [DOI] [PubMed] [Google Scholar]
- 45.Cerundolo V, Alexander J, Anderson K, Lamb C, Cresswell P, McMichael A, Gotch F, Townsend A. Presentation of viral antigen controlled by a gene in the major histocompatibility complex. Nature (Lond.) 1990;345:449–452. doi: 10.1038/345449a0. [DOI] [PubMed] [Google Scholar]
- 46.Coulie PG, Brichard V, Van Pel A, et al. A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas [see comments] J. Exp. Med. 1994;180:35–42. doi: 10.1084/jem.180.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barber LD, Parham P. Peptide binding to major histocompatibility complex molecules. In: Palade GE, Alberts BM, Spudich JA, editors. Annual Review of Cell Biology. Annual Reviews. Inc.; Palo Alto. CA: 1993. pp. 163–206. [DOI] [PubMed] [Google Scholar]
- 48.Falk K, Rotzschke O, Stevanovic S, Jung G, Rammensee HG. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature (Lond.) 1991;351:290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 49.Falk K, Rotzschke O, Rammensee HG. Cellular peptide composition governed by major histocompatibility complex class I molecules [see comments] Nature (Lond.) 1990;348:248–251. doi: 10.1038/348248a0. [DOI] [PubMed] [Google Scholar]
- 50.Madden DR, Garboczi DN, Wiley DC. The antigenic identity of peptide-MHC complexes: a comparison of the conformations of five viral peptides presented by HLA-A2. Cell. 1993;75:693–708. doi: 10.1016/0092-8674(93)90490-h. [DOI] [PubMed] [Google Scholar]
- 51.Ruppert J, Sidney J, Celis E, Kubo RT, Grey HM, Sette A. Prominent role of secondary anchor residues in peptide binding to HLA-A2.1 molecules. Cell. 1993;74:929–937. doi: 10.1016/0092-8674(93)90472-3. [DOI] [PubMed] [Google Scholar]
- 52.Spies T, DeMars R. Restored expression of major histocompatibility class I molecules by gene transfer of a putative peptide transporter [see comments) Nature (Lond.) 1991;351:323–324. doi: 10.1038/351323a0. [DOI] [PubMed] [Google Scholar]
- 53.Powis SJ, Townsend AR, Deverson EV, Bastin J, Butcher GW, Howard JC. Restoration of antigen presentation to the mutant cell line RMA-S by an MHC-linked transporter. Nature (Lond.) 1991;354:528–531. doi: 10.1038/354528a0. [DOI] [PubMed] [Google Scholar]
- 54.Restifo NP, Esquivel F, Kawakami Y, Yewdell JW, Mule JJ, Rosenberg SA, Bennink JR. Identification of human cancers deficient in antigen processing. J. Exp. Med. 1993;177:265–272. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greenwood R, Shimizu Y, Sekhon GS, DeMars R. Novel allele-specific, post-translational reduction in HLA class I surface expression in a mutant human B cell line. J. Immunol. 1994;153:5525–5536. [PubMed] [Google Scholar]
- 56.Runger TM, Klein CE, Becker JC, Brocker EB. The role of genetic instability, adhesion, cell motility, and immune escape mechanisms in melanoma progression. Curr. Opin. Oncol. 1994;6:188–196. doi: 10.1097/00001622-199403000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Bodmer WF, Browning MJ, Krausa P, Rowan A, Bicknell DC, Bodmer JG. Tumor escape from immune response by variation in HLA expression and other mechanisms. Ann. NY Acad. Sci. 1993;690:42–49. doi: 10.1111/j.1749-6632.1993.tb43994.x. [DOI] [PubMed] [Google Scholar]
- 58.Weber J, Salgaller M, Samid D, Johnson B, Herlyn M, Lassam N, Treisman J, Rosenberg SA. Expression of the MAGE-1 tumor antigen is up-regulated by the demethylating agent 5-aza-2′-deoxycytidine. Cancer Res. 1994;54:1766–1771. [PubMed] [Google Scholar]
- 59.Stötter H, Wiebke EA, Tomita S, Belldegrun A, Topalian S, Rosenberg SA, Lotze MT. Cytokines alter target cell susceptibility to lysis. II. Evaluation of tumor-infiltrating lymphocytes. J. Immunol. 1989;142:1767–1773. [PubMed] [Google Scholar]
- 60.Kirkwood JM, Ernstoff MS. Interferons—clinical applications: cutaneous melanoma. In: DeVita VT, Hellman S, Rosenberg SA, editors. Biologic Therapy of Cancer. J. B. Lippincott Co.; Philadelphia: 1991. pp. 311–333. [Google Scholar]
- 61.Mottez E, Langlade-Demoyen P, Gournier H, Martinon F, Maryanski J, Kourilsky P, Abastado J. Cells expressing a major histocompatibility complex class I molecule with a single covalently bound peptide are highly immunogenic. J. Exp. Med. 1995;181:493–502. doi: 10.1084/jem.181.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]