Abstract
The rewarding effects of nicotine contribute to the chronic use of tobacco products. The place conditioning task, a widely used preclinical model to study drug reward, has lead to mixed results in rats when nicotine was administered subcutaneously or intraperitoneally; intravenously administered nicotine has not been examined. Further, much of the research demonstrating a nicotine-conditioned place preference in rats has used a biased design making these results susceptible to non-reward interpretations. The present study assessed whether intravenous (IV) nicotine would condition a place preference in an unbiased design and evaluated important behavioral parameters: nicotine dose, number of conditioning trials, and infusion-to-placement interval. In adult male Sprague Dawley rats, IV nicotine (0.03 mg/kg) conditioned a place preference after 8 conditioning trials. This conditioned preference was observed whether nicotine was infused 10 min before or immediately after placement in the paired environment for 10 min; infusing nicotine immediately after removal from the paired environment did not condition a preference after 4 or 8 conditioning trials. Four conditioning trials were not sufficient to condition a preference regardless of the temporal relation between the paired environment and 0.03 mg/kg nicotine. A 0.01 mg/kg dose of nicotine did not condition a place preference after 4 or 8 trials when infused immediately upon placement in the paired environment. Intravenous nicotine (0.03 mg/kg) has rewarding effects in an unbiased design suggesting that the place conditioning protocol used in the present study might be an especially useful model for studying the processes underlying the conditioned rewarding effects of nicotine.
Keywords: conditioned place preference, nicotinic acetylcholine receptors, Pavlovian conditioning, reward, smoking, tobacco
Smoking has consistently been reported as the number one preventable cause of premature death in the United States (McGinnis & Foege 1993; Mokdad et al. 2004). Approximately 440,000 people die each year due to smoking-related diseases (CDC 2005), and more than $75 billion in annual medical costs are directly attributed to smoking. In spite of these facts, in the U.S., 21% of adults are considered current smokers (CDC 2005). Most smokers (ca. 70%) express a desire to quit (CDC 2005) and approximately 40% report attempting to quit at least once in the past 12 months (CDC 2005). Unfortunately, of those individuals that manage to quit, most relapse within the first few months of abstinence (NIDA 2006). Although the processes responsible for tobacco use and nicotine dependence are complex, there is general consensus that the rewarding effects of nicotine are likely involved (see Stolerman 1991; Stolerman & Jarvis 1995). As such, a better understanding of the factors mediating the chronic use of tobacco products will require a better understanding of the behavioral and neurobiological processes of nicotine reward.
Place conditioning is a widely used preclinical model to study the rewarding properties of drugs in rats and mice[for reviews see Bevins & Bardo (2000) and Tzschentke (1998)]. In a typical place conditioning experiment, one distinct context (environment) is paired with the drug of interest; the subject also receives equal exposure to a second distinct context in the absence of drug. Following this conditioning phase is a choice test in which the animal receives free access to both sets of contextual cues—usually in a drug-free state. The drug is considered rewarding if it produces an increase in the time spent in the environment paired with the drug compared to a control value [see Bevins & Cunningham (2006) for a more detailed discussion of methodological and measurement issues]. This increase in time in the drug-paired compartment is often referred to as a “conditioned place preference” and is thought to reflect a Pavlovian conditioned association between contextual stimuli and the rewarding effects of the drug (cf. Bardo & Bevins 2000; Carr et al. 1989; Panksepp et al. 2004).
Most drugs of abuse, such as amphetamine (Erb & Parker 1994; Lett 1989), cocaine (Bevins & Bardo 1999; Bevins 2005; Nomikos & Spyraki 1988; O’Dell et al. 1999), ethanol (Cunningham et al. 1997), methamphetamine (Cunningham and Noble, 1992; Gehrke et al, 2003), and morphine (Lett 1989; Randall et al. 1998), readily condition a place preference in rodents. Surprisingly, however, the results are less consistent with nicotine. Although the literature is mixed for rats and mice, the present research used rats and thus we will focus our discussion to the published research with rats [see Grabus et al. (2006) and Risinger & Oakes (1995) for research with mice]. Using rats some investigators have found that nicotine will condition an increase in time spent in the paired environment (Ashby et al. 2002; Calcagnetti & Schechter 1994; Dewey et al. 1999; Forget et al. 2005, 2006; Fudala et al. 1985; Fudala & Iwamoto 1986; Horan et al. 1997, 2001; Shoaib et al. 1994; Shram et al. 2006). In contrast, other researchers have reported either avoidance (i.e., an aversion) of the nicotine-paired environment (Fudala & Iwamoto 1987; Horan et al. 1997; Jorenby et al. 1990) or no place conditioning (Acquas et al. 1989; Carboni et al. 1989; Clarke & Fibiger 1987; Rogers et al. 2004; Shoaib et al. 1994; Shram et al. 2006). Some potential factors that might explain the inconsistent results include age and strain of the rat, pre-exposure to nicotine, and use of a biased versus unbiased procedure (see Le Foll and Goldberg (2005) for a more detailed review).
Importantly, a majority (ca. 70%) of the published reports of nicotine place preference have used a biased design (see LeFoll & Goldberg 2005). In a biased design, rats are initially given at least one free-choice test before conditioning as a screen for initial compartment (context) preference. During the conditioning phase, nicotine is then paired with the initially non-preferred compartment (i.e., often termed “conditioning against a preference”). An increase in time from the pre- to post-conditioning test is considered evidence for reward in a biased design. Of note, this biased design requires a control that never receives drug to determine how compartment preference would shift as a function of mere exposure to the environment. Further, unless a preference ratio (see later) or the time in the unpaired environment was reported, any increase in time from the pre- to post-conditioning test does not necessarily reflect a “preference” for the nicotine-paired compartment. That is, the animal might continue to spend more time on its initially preferred compartment, but still show an increase in time spent in the non-preferred (drug-paired) compartment (see Bevins & Cunningham 2006). Although this shift in preference may reflect the conditioned rewarding effects of the drug (cf. Cunningham et al. 2003), alternate explanations for the shift in preference exist, thus complicating interpretation of any place conditioning result using a biased design (e.g., Bardo & Bevins 2000; Carr et al. 1989). For example, the change in time spent in the initially non-preferred compartment might be measuring some anxiolytic or stress reduction property of the drug that decreases initial avoidance.
This discussion highlights the need to construct a balanced apparatus (i.e., no systematic preferences for either environment), as well as use an unbiased place conditioning design to facilitate interpretation of any results. In an unbiased place conditioning design, assignment of drug-paired environment is independent of any initial preference. Interestingly, there are very few published reports of nicotine conditioning a place preference in rats using an unbiased design. Indeed, LeFoll and Goldberg (2005) in a recent review of the literature only found 4 published papers, and these were all from the same laboratory (Ashby Jr.). Further, there have only been a few additional reports of a nicotine place preference using an unbiased design with rats since this review (e.g., Forget et al. 2005, 2006). The doses that produced a place preference in these studies (e.g., 0.06-0.21 mg/kg) are within the range of doses that others using the same route of administration (SC) have found no preference. With this discussion in mind, we used a balanced apparatus and an unbiased design in the present place conditioning experiments.
To our knowledge, there are no reports of place conditioning using intravenous (IV) administration of nicotine. This is somewhat surprising given the inconsistent findings using subcutaneous and intraperitoneal injections of nicotine (see LeFoll & Goldberg 2005). Further, self-administration studies with rats consistently report that IV nicotine maintains instrumental responding over a range of doses (e.g., Corrigall & Coen 1989; DeNoble & Mele 2006; Donny et al. 1995; Rauhut et al. 2003; Shoaib et al. 1996) indicating that IV nicotine has some reinforcing properties. Additionally, IV nicotine maintains behavior in a runway model of self-administration which combines the approach behavior of the place conditioning model and instrumental response requirement of self-administration (Cohen & Ettenberg 2007). Thus, one goal of the present research was to examine the ability of IV administered nicotine to condition a place preference using an unbiased design with rats. We also sought to begin examining some of the parameters important for acquisition of this nicotine-conditioned place preference: nicotine dose, number of conditioning trials, and temporal relation between chamber exposure and nicotine administration. Number of conditioning trials was expected to be important given that Pavlovian conditioned associations (Pavlov 1927; Wilkinson et al. 2006), including place conditioning (Brabant et al. 2005; Risinger & Oakes 1996), vary as a function of number of stimulus pairings. We also expected the temporal arrangement between context (end compartment) exposure and nicotine administration to be an important determinant of conditioning [for research and discussion of this variable (often termed “interstimulus interval”) see Bevins et al. (2005), Burgos & Bevins (1997), Gibbon et al. (1977), and Pavlov 1927]. The interstimulus interval can have especially pronounced effects in the place conditioning task. In mice, for example, alcohol produces a place preference when administered before placement, but a place aversion when it is administered immediately after exposure to the context [(Cunningham et al. (1997, 2002); for a comparable effect with cocaine see Ettenberg et al. (1999)].
Materials and Methods
Animals
Forty-five adult male Sprague-Dawley rats (329±2.4 g) from Harlan (Indianapolis, IN) were housed separately in polycarbonate tubs lined with wood shavings in a temperature- and humidity-controlled colony. Rat chow and water were continuously available in the home cage. All sessions were conducted during the light portion of a 12:12 h light/dark cycle. Experimental protocols were approved by the University of Nebraska-Lincoln IACUC and followed the “Guide for the Care and Use of Laboratory Animals” (National Research Council 1996).
Surgery
Rats were anesthetized with 1 ml/kg ketamine hydrochloride (100 mg/ml, IP) followed by 0.6 ml/kg xylazine hydrochloride (20 mg/ml, IP) (Midwestern Veterinary Supply, Des Moines, IA). One end of a silastic catheter was implanted into the left external jugular vein. The other end of the catheter was fed subcutaneously around the shoulder and exited via a backmount just below the scapula. The backmount allowed access to the catheter through a metal cannula. Buprenorphine hydrochloride (0.1 mg/kg) was injected SC immediately following surgery. For the evening and day following surgery, buprenorphine (0.5 mg/kg) was available in the drinking water to mange post-surgical pain. For the evening of surgery and the following 2 days (AM and PM), the catheter was flushed with 0.1 ml of streptokinase (ca. 8000 Units/ml) dissolved in sterile saline mixed with heparin (30 Units/ml; Midwest Veterinary Supply, Des Moines, IA). The catheter was flushed once to twice a day for the remaining duration of the experiment with 0.2 ml of 30 Units/ml of heparinized saline. Rats were allowed 5 days of recovery before the start of an experiment. Catheter patency was assessed with a 0.05 ml IV infusion of xylazine (20 mg/ml) at pre-established points in the study. This concentration produces clear motor ataxia within 5 sec if the catheter is patent (cf. Bevins & Bardo 1999; Bevins 2005). The 37 rats with patent catheters were included in analyses. The ‘n’ reported in the following sections reflect the number of patent rats in each experiment.
Apparatus
Place conditioning was assessed in one of two chambers with Plexiglas ceiling, front and back walls; the side walls were aluminum. Each chamber had two distinct end compartments [40 × 16 × 20 cm (l×w×h)] separated by a smaller center placement area [6.5 × 15.5 × 19.5 cm (l×w×h)]. Interchangeable floors were used to create the distinct environments. One floor had approximately 340 holes (1.3-cm diameter) drilled into a 16-gauge aluminum sheet. The other floor was made of 1-cm stainless steel rods. Two rods were mounted side-by-side on an acrylic base with the following adjacent rod pair separated from the next pair by 1 cm. During conditioning, a solid aluminum floor the same length as that used in the center compartment (6.5 cm) was placed in each end chamber nearest the wall blocking access to the center compartment. This maneuver reduced the novelty of this floor on post-conditioning choice tests. The experimental room was separate from the colony and was illuminated by a red light (40 W).
Drug
(−)Nicotine tartrate (Sigma, St Louis, MO) was dissolved in sterile saline and the pH was adjusted to 7.0 ± 0.2 with a dilute NaOH solution. Nicotine infusions were 0.5 ml/kg and all nicotine doses are reported as base.
Experiment 1A: Place conditioning with 0.03 mg/kg nicotine
Habituation
Rats (n=8) were attached to PE50 tubing connected to a syringe and then placed in the center compartment of the place conditioning chamber. The prescribed volume of saline was infused manually over 1 sec and then the syringe was replaced with another syringe of sterile saline and the tubing was cleared of solution from the first syringe with 0.1 ml of sterile saline. The tubing was then disconnected from the cannula and the rats were allowed to freely explore the entire apparatus for 10 min.
Conditioning & Testing (4 trials)
Conditioning occurred across 8 consecutive days with one session per day. Half of the rats received 0.03 mg/kg nicotine on days 1, 3, 5, and 7, and saline on opposite days; the order of nicotine and saline was reversed for the remaining rats. During a nicotine session, the rat was placed in the paired compartment where it received an infusion of nicotine followed by 0.1 ml of saline (see Habituation). Confinement to the paired compartment was 10 min once the tubing was detached from the cannula and the chamber ceiling closed. Saline sessions were similar to nicotine sessions except saline was infused instead of nicotine. Assignment to floor location (i.e., rod floor on left or right) and paired floor (i.e., nicotine paired with rod or hole flooring) was counterbalanced and irrespective of performance on the habituation session. Approximately 24 h after the last conditioning session was a drug-free (saline) choice test. Rats were placed in the center compartment and infused with saline as in the habituation session. The tubing was removed from the cannula and the rats were allowed to freely explore the entire chamber for 10 min.
Additional Conditioning & Testing (4 more trials)
Beginning the following day, conditioning was continued exactly as described above for an additional 4 conditioning trials (i.e., resulting in a total of 8 saline and 8 nicotine sessions). The drug-free test was 24 h after the last confinement and was identical to the previous drug-free test.
Experiment 1B: Place conditioning with 0.01 mg/kg nicotine
After establishing that 0.03 mg/kg nicotine administered IV conditioned a place preference, we sought to test a lower dose of nicotine (0.01 mg/kg, IV). A separate and experimentally naive set of rats (n=7) was conditioned and tested as described for Experiment 1A except 0.01 mg/kg nicotine was used instead of 0.03 mg/kg nicotine. All factors were counterbalanced as much as allowed by the sample size.
Experiment 2: Role of interstimulus interval
Habituation
Habituation was similar to Experiments 1A and 1B. Rats were randomly assigned to the -10, 0, or +10 min group. The group name denotes the time between the intravenous infusion and placement in the chamber. Thus for habituation, the -10 min group (n=7) was infused with saline and returned to the home cage for 10 min before placement in the center compartment of the place conditioning chamber. Rats in the 0 min group (n=8) were infused immediately after placement in the chamber. This group served as a replication of Experiment 1A. The +10 min group (n=7) was infused 10 min after placement (i.e., immediately after removal from the apparatus).
Conditioning & Testing (4 trials)
Conditioning proceeded in a manner similar to Experiment 1A. Each infusion (saline and 0.03 mg/kg nicotine) was administered at the time point denoted by group assignment (i.e., -10, 0, or +10 min). The drug-free choice test was identical to the previous experiment.
Additional Conditioning & Testing (4 more trials)
As in Experiment 1A, conditioning was continued for an additional 4 conditioning trials before conducting another drug-free test.
Dependent Measures
For each choice test, we calculated a preference ratio using the following formula: time spent in the nicotine paired compartment ÷ (time spent in the nicotine paired compartment + time spent in the unpaired compartment). A preference ratio of 0.5 indicates no preference for either end compartment; a preference ratio greater than 0.5 indicates a preference for the paired compartment. Time in each compartment was scored during the test sessions. A rat was considered in a specific compartment when its front paws, head, and shoulders were in that compartment. Table 1 shows the mean time spent in the paired, unpaired (saline), and center compartments across the three experiments. Horizontal activity in each end compartment was also scored during each of the test sessions by counting the number of times the head and shoulders of the rat crossed a line that bisected each end compartment. Interobserver reliabilities for each measure was conducted from video by an observer naïve to the experimental conditions. The Pearson-product moment correlations were high for the 66 observations made by both observers for time spent in each compartment, r=0.93, p<0.001, and for the 60 observations in common for line crosses, r=0.97, p<0.001.
Table 1.
Mean time (seconds) in each compartment of the chamber during each drug-free test (±1 SEM)
| Habituation | 4 Conditioning Trials | 8 Conditioning Trials | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Paired | Unpaired | Center | Paired | Unpaired | Center | Paired | Unpaired | Center | |
| Experiment 1A | |||||||||
| 0.03 mg/kg | 249.6 (8.7) | 255.4 (8.4) | 95.06 (10.8) | 223.4 (24.0) | 230.3 (22.0) | 146.3 (13.2) | 298.6 (23.5) | 172.7 (23.2) | 128.6 (15.8) |
| Experiment 1B | |||||||||
| 0.01 mg/kg | 280.0 (11.4) | 255.3 (7.5) | 64.8 (7.2) | 240.5 (15.6) | 235.5 (19.0) | 124.0 (9.5) | 221.3 (39.9) | 280.4 (48.8) | 98.3 (17.5) |
| Experiment 2 | |||||||||
| -10 min | 248.1 (7.0) | 249.8 (7.5) | 102.1 (5.2) | 258.4 (30.8) | 241.6 (25.7) | 100.0 (14.4) | 336.7 (30.3) | 173.8 (26.8) | 89.5 (13.4) |
| 0 min | 258.7 (10.9) | 257.7 (12.9) | 83.6 (8.7) | 277.7 (21.0) | 217.8 (13.8) | 104.5 (11.5) | 315.7 (23.1) | 160.9 (18.1) | 123.5 (16.3) |
| +10 min | 216.8 (30.6) | 292.9 (42.3) | 90.2 (13.5) | 228.9 (17.8) | 250.5 (17.1) | 120.6 (7.6) | 251.7 (27.4) | 240.4 (29.3) | 108.0 (10.5) |
Data Analyses
One-way repeated measures ANOVAs were used to examine preference ratios across the 3 test sessions (habituation, 4 conditioning trials, and 8 conditioning trials) for Experiment 1A and 1B. A mixed two-way ANOVA with Session as the within-subject repeated factor and Interstimulus Interval as the between-subjects factor was used to analyze preference ratios for Experiment 2. Post-hoc analyses prompted by a significant F-value utilized one-sample t-tests to compare each preference ratio to a hypothetical value of 0.5 (i.e., the value indicating no preference). For analyses, activity counts were converted to a rate measure by dividing the number of line crosses in an end compartment by the time in seconds spent in that end compartment. A two-way ANOVA with Compartment and Session as the within-subject repeated measures factors was used to analyze activity data in Experiment 1A and 1B. Activity from Experiment 2 was analyzed using a mixed three-way ANOVA with Compartment and Session as repeated within-subject factors and Interstimulus Interval as the between-subjects factor. A significant interaction for activity data prompted post-hoc Fisher’s Least Significance Difference (LSD) tests. Comparisons were limited to those relevant for the significant interaction. Statistical significance was declared using a two-tailed rejection region of 0.05.
Results
Experiment 1A: Place conditioning with 0.03 mg/kg nicotine
Preference scores on each of the drug-free tests are shown in Figure 1A. There was a main effect of Session, F(2,14)=4.07, p=0.04. The preference ratios for habituation and 4 conditioning trials were not different from 0.5, ts<1. However, 0.03 mg/kg nicotine administered IV was able to condition a place preference after 8 conditioning trials as indicated by a preference ratio significantly above 0.5, t(7)=2.93, p=0.022. Activity scores are shown in Table 2. Although the main effect of Compartment and Session for activity were not significant, Fs≤2.32, ps≥0.17, there was a Compartment × Session interaction, F(2,14)=4.80, p=0.026. None of the follow-up Fisher’s LSD comparisons were significant (LSD=0.08).
Figure 1.
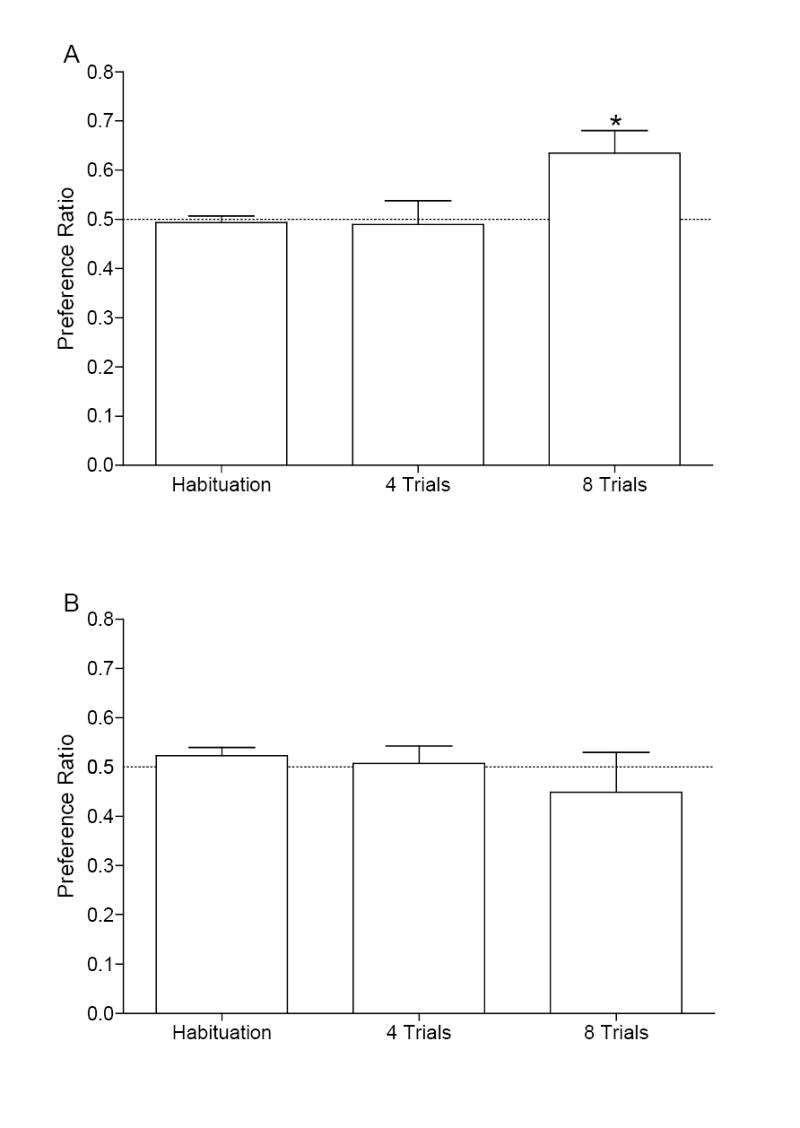
Panel A shows for each test session the mean preference ratios (+1 SEM) for rats (n=8) in Experiment 1A that were conditioned with 0.03 mg/kg nicotine administered IV. Panel B shows the mean preference ratios (+1 SEM) for rats (n=7) in Experiment 2A that were conditioned with 0.01 mg/kg nicotine administered IV. * indicates significant difference (p<0.05) compared to hypothetical value of 0.5 (i.e., no preference).
Table 2.
Mean activity counts per second in each end compartment during each drug-free test (±1 SEM)
| Habituation | 4 Conditioning Trials | 8 Conditioning Trials | ||||
|---|---|---|---|---|---|---|
| Paired | Unpaired | Paired | Unpaired | Paired | Unpaired | |
| Experiment 1A | ||||||
| 0.03 mg/kg | 0.12 (0.03) | 0.11 (0.02) | 0.14 (0.02) | 0.12 (0.04) | 0.08 (0.04) | 0.13 (0.05) |
| Experiment 1B | ||||||
| 0.01 mg/kg | 0.11 (0.01) | 0.11(0.02) | 0.16 (0.03) | 0.16 (0.04) | 0.16 (0.07) | 0.13 (0.07) |
| Experiment 2 | ||||||
| -10 min | 0.12 (0.03) | 0.12 (0.02) | 0.13 (0.05) | 0.13 (0.05) | 0.13 (0.06) | 0.20 (0.10) |
| 0 min | 0.13 (0.03) | 0.13 (0.02) | 0.14 (0.06) | 0.18 (0.08) | 0.13 (0.06) | 0.22 (0.11) |
| +10 min | 0.12 (0.04) | 0.11 (0.05) | 0.11 (0.06) | 0.10 (0.08) | 0.13 (0.06) | 0.12 (0.05) |
Experiment 1B: Place conditioning with 0.01 mg/kg nicotine
Preference scores for rats conditioned with 0.01 mg/kg nicotine are shown in Figure 1B. There was no main effect of Session, Fs<1, indicating that 0.01 mg/kg nicotine administered IV did not produce a place preference after 4 or 8 conditioning trials. None of the F-values for activity were significant, Fs≤2.71, ps≥0.11, (data shown in Table 2).
Experiment 2: Role of interstimulus interval
Preference scores across the test sessions are shown in Figure 2. A mixed ANOVA on the preference scores revealed a main effect of Session, F(2,38)=5.98, p=0.006, and Group, F(1,19)=6.05, p=0.009; the Session × Group interaction was not significant, F<1. Follow-up analysis indicated that preference ratios were significantly above 0.5 after 8 conditioning trials for the -10 min group, t(6)=2.84, p=0.029, and the 0 min group, t(7)=4.73, p=0.003, denoting that these temporal relations produced a place preference after 8 conditioning trials. No other preference ratio differed from the hypothetical value of 0.5, ts≤1.67, ps≥0.14. For activity, the Compartment × Session interaction was significant, F(2,36)=5.45, p=0.01; the main effects and remaining interactions for activity were not significant, Fs≤3.06, ps≥0.08, (see Table 2). None of the follow-up Fisher’s LSD comparisons were significant (LSD=0.15).
Figure 2.
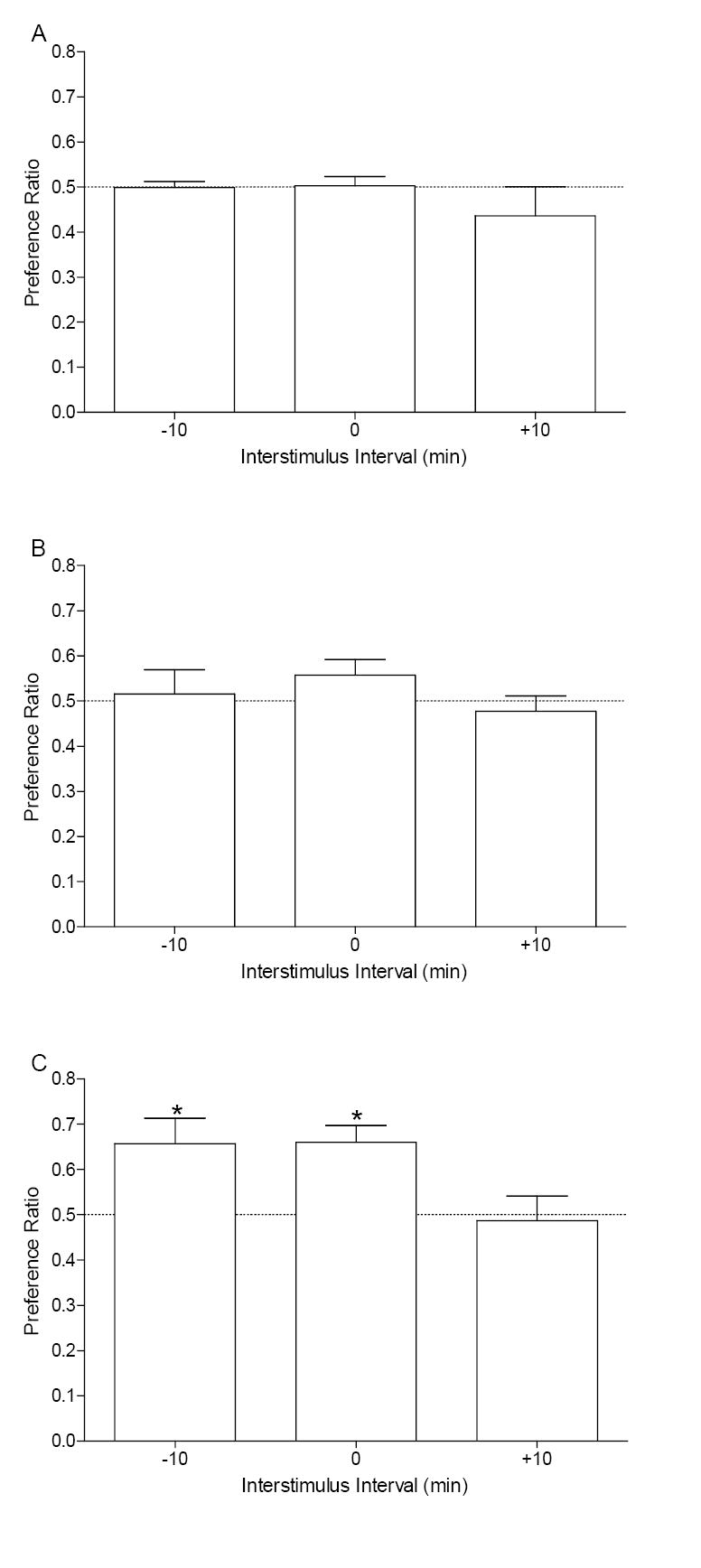
Panel A shows the mean preference ratio (+1 SEM) for the habituation phase of Experiment 2 for rats that were assigned to groups-10 min (n=7), 0 min (n=8), and +10 min (n=7). Panel B shows the mean preference ratio (+1 SEM) after 4 conditioning trials for each group in Experiment 2. Panel C shows the mean preference ratio (+1 SEM) for each group after 8 conditioning trials. * indicates significant difference (p<0.05) compared to hypothetical value of 0.5 (i.e., no preference).
Discussion
We found that intravenously administered nicotine (0.03 mg/kg) conditioned a place preference after 8 conditioning trials. This conditioned preference was observed whether nicotine was infused 10 min or immediately before placement in the paired context for 10 min. Infusing nicotine immediately after removal from the paired context did not produce place conditioning. At the 0.03 mg/kg dose of nicotine, 4 conditioning trials were not sufficient to condition a preference regardless of the interstimulus interval. Finally, the 0.01 mg/kg dose of nicotine when infused immediately upon placement in the environment did not condition a place preference after 4 or 8 conditioning trials.
For our initial attempt (i.e., Experiment 1A) we selected a dose of nicotine (0.03 mg/kg) that has been shown to maintain self-administration in rats across many laboratories (e.g., Bevins in press; Corrigall & Coen 1989; DeNoble & Mele 2006; Donny et al. 1995; Rauhut et al. 2003; Shoaib et al. 1996; see also Cohen & Ettenberg 2007). Although there are some notable differences between what processes might be under investigation in place conditioning versus self-administration, there is also significant overlap in the list of drugs that will condition approach behavior and maintain instrumental responding (see Bardo & Bevins 2000). Of note, this self-administered dose of nicotine required 8 conditioning trials to condition a place preference—4 trials was not sufficient. This result is in concordance with those recently reported by Cohen and Ettenberg (2007). A conditioned increase in run speed down a straight alley was observed with 0.03 mg/kg nicotine IV and this increase appeared after more than 6 conditioning trials.
The lack of a nicotine-conditioned place preference after 4 trials was predicted by a casual observation made during the experiment. That is, rats were consistently defecating on the first few trials, with most stopping by the third conditioning trial. This observation was highly salient to us given that rats in our laboratory do not defecate to this extent in this apparatus when given cocaine or amphetamine. The defecation might be a result of the peripheral actions of nicotine which has been shown to stimulate intestinal smooth muscle and increase fecal pellets in rats (Aikawa & Ohmori 2000). Alternatively, defecation has been used as a measure of fear and aversion (cf. Bevins et al. 1997; Fanselow 1986; Hunt & Otis 1953) and suggested to us that the earlier exposures to nicotine might have some of these qualities (cf. Parker & Carvell 1986). Such qualities could compete with any early rewarding effect of nicotine thus preventing acquisition of a conditioned place preference. Although we understand the possible difficulties with deriving conclusions from such observation, we felt that it was important to report this observation since it provided part of the impetus for conducting an additional four conditioning trials.
This observation also provided the impetus for assessing the lower dose of nicotine (0.01 mg/kg) in Experiment 1B. This dose of nicotine is on the lower end of the dose-effect curve that can maintain self-administration (e.g., Rauhut et al. 2003). Thus, we were looking for a dose that might not evoke early defecation, but have some rewarding effects. The 0.01 mg/kg dose of nicotine did not produce the early defecation nor did it condition a place preference. Notably, this dose of IV nicotine did not condition an increase in running speed in the Cohen and Ettenberg (2007) study even after 21 trials. Thus, under the present set of experimental parameters we found no evidence for reward at the 0.01 mg/kg dose. Additional manipulations such as more conditioning trials and briefer chamber exposure with this lower dose of nicotine will be of interest in future studies.
There is a substantial Pavlovian conditioning literature indicating the importance of the temporal arrangement between the to-be-conditioned stimulus and the reinforcer (unconditioned stimulus) for acquisition of conditioned responding. The conditioning tasks demonstrating the importance of the interstimulus interval have been as varied as eye-blink conditioning in humans (McAllister 1953), aversive conditioning in goldfish (Bitterman 1964), key-peck autoshaping in pigeons (Gibbon et al. 1977), context fear conditioning in rats (Bevins & Ayres 1995), nicotine-conditioned hyperactivity in rats (Bevins et al. 2005), and ethanol place conditioning in mice (Cunningham et al. 1997). The present research extended this list to include place conditioning with IV administered nicotine. In brief, 0.03 mg/kg nicotine administered immediately or 10 min before confined exposure to the paired environment for 10 min conditioned a place preference after 8 conditioning trials. IV administration of nicotine immediately after removal from the paired compartment (i.e., -10 min group) had no apparent effect on choice behavior after 4 or 8 conditioning trials (i.e., no approach or avoidance tendencies). This data pattern suggests that the rewarding effects of IV nicotine extend long enough that there is sufficient temporal contiguity between the to-be-paired compartment and nicotine to condition an appetitive association.
Interestingly, under some experimental protocols the interstimulus interval can reveal different motivational properties of the same drug. For example, alcohol (2 g/kg, 20% v/v) given IP to mice conditioned a place preference when administered before placement in the paired context, but the same dose conditioned an aversion when administered immediately after exposure to the context [Cunningham et al. (1997); see also Ettenberg et al. (1999) for research with cocaine]. Although we did not find evidence for this dual property/opponent process for nicotine in the present study, it will be of interest to examine different doses on IV nicotine against different interstimulus intervals, context confinement durations, etceteras.
As noted in the Introduction, much of the nicotine place conditioning research demonstrating a place “preference” has used a biased design (i.e., nicotine paired with an initially identified non-preferred compartment). Unfortunately, using a biased design introduces alternative non-reward explanations for preference shifts such as stress reduction or anxiolytic effects of the drug (Bardo & Bevins 2000; Carr et al. 1989; Bevins & Cunningham 2006). To avoid such difficulties, the present research used an apparatus with balanced construction and an experimental design that was unbiased. As evidence of the balanced construction of our place conditioning apparatus, rats (n=37) averaged across the three experiments in the present study spent 260.9 ± 8.8 sec on the rod floor and 251.8 ± 7.4 sec on the hole floor during habituation. By assigning rats to paired versus unpaired environment irrespective of their performance on the habituation day, the shifts in preference for the paired compartment at the 0.03 mg/kg dose of nicotine are less susceptible to non-reward interpretations.
Related to the previous discussion, some researchers have suggested that differential patterns of locomotor activity between the drug-paired and unpaired environments on the test day could complicate interpretation of a place conditioning effect (e.g., Parker 1992; Swerdlow & Koob 1984). This potential interaction could be important for the present research given that an environment reliably paired with nicotine administered SC comes to evoke a conditioned increase in activity on a drug-free test (e.g., Bevins et al. 2001; Bevins et al. 2005; Walter & Kuschinsky 1989). To assess a possible role of motor activity, we scored line crosses in each end compartment across all free-choice test sessions. Although there was a Compartment × Session interaction in each experiment showing place conditioning, the post-hoc analyses did not reveal any significant differences in activity. Further, any trend seen in the mean activity scores was the opposite of that expected if conditioned hyperactivity was evident. That is, rats were slightly more active in the unpaired compartment relative to the paired compartment. Thus, an account of our nicotine place conditioning results with 0.03 mg/kg nicotine based on conditioned alterations in motor activity seems unlikely.
Given the discussion in the previous paragraphs, we suggest that 0.03 mg/kg IV nicotine has rewarding effects that are readily measured in a place conditioning task. Conditioned associations and reward processes involving nicotine likely contribute to tobacco use and the tenacity of nicotine dependence (e.g., Bevins & Palmatier 2004; Rose & Levin 1991; West & Schneider 1987). Accordingly, a better understanding of these processes will contribute to designing better intervention strategies for smoking cessation. With this goal in mind, we suggest that the IV nicotine place conditioning protocol used in the present study might be an especially useful model for studying the processes underlying the conditioned rewarding effects of nicotine. Of course, adoption of such a recommendation will require replication by other laboratories. This replication and hence adoption might be slowed by the added technical, temporal, and fiscal burden of catheter surgeries and maintenance.
Acknowledgments
We thank Jonathan Fullner and Jill Rosno for scoring the behaviors used to assess interobserver reliability and Jessica Linkugel, Jennifer Murray, Carmela Reichel, and Amanda Struthers for their thorough read of an earlier version of this manuscript. The research and R. A. Bevins were supported by United States Public Health Service grant DA018114 and DA017086. Jamie Wilkinson was supported by Nebraska Tobacco Settlement Biomedical Research Enhancement Funds while preparing this manuscript for publication.
Acknowledgement of funds or grants: DA018114, DA017086, and Nebraska Tobacco Settlement Biomedical Research Enhancement Funds
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- Acquas E, Carboni PL, DiChiara G. SCH-23390 blocks drug-conditioned place-preference and place-aversion: anhedonia (lack of reward) or apathy (lack of motivation) after dopamine-receptor blockade. Psychopharmacology. 1989;99:151–155. doi: 10.1007/BF00442800. [DOI] [PubMed] [Google Scholar]
- Aikawa N, Ohmori K. Effect of zaldaride maleate, an antidiarrheal compound, on fecal pellet output induced by hyperpropulsion in gastrointestine of rats. Jpn J Pharmacol. 2000;82:350–352. doi: 10.1254/jjp.82.350. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Jr, Paul M, Gardner EL, Gerasimov MR, Dewey SL, Lennon IC, Taylor SJC. Systemic administration of 1R,4s-4amino-cyclopent-2-ene-carboxylic acid, a reversible inhibitor of GABA transaminase, blocks expression of conditioned place preference to cocaine and nicotine in rats. Synapse. 2002;44:61–63. doi: 10.1002/syn.10052. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Valone JM, Bevins RA. Locomotion and conditioned place preference produced by acute intravenous amphetamine: Role of dopamine receptors and individual differences in amphetamine self-administration. Psychopharmacology. 1999;143:39–46. doi: 10.1007/s002130050917. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bevins RA. Altering the motivational function of nicotine through conditioning processes. In: Bevins RA, Caggiula AR, editors. The motivational impact of nicotine and its role in tobacco use: The 55th Nebraska Symposium on Motivation. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Cunningham CL. Place conditioning: A methodological analysis. In: Anderson M, editor. Tasks and Techniques: A Sampling of Methodologies for the Investigation of Animal Learning, Behavior, and Cognition. Hauppauge NY: Nova Science Publisher; 2006. pp. 99–110. [Google Scholar]
- Bevins RA. The reference-dose place conditioning procedure yields a graded dose-effect function. Internat J Compar Psychol. 2005;18:101–111. [Google Scholar]
- Bevins RA, Eurek S, Besheer J. Timing of conditioned response in a nicotine locomotor conditioning preparation: Manipulations of the temporal arrangement between context cues and drug administration. Behav Brain Res. 2005;59:135–143. doi: 10.1016/j.bbr.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Palmatier MI. Extending the role of associative learning processes in nicotine addiction. Behav Cogn Neurosci Rev. 2004;3:143–158. doi: 10.1177/1534582304272005. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J, Pickett KS. Nicotine-conditioned locomotor activity in rats: Dopaminergic and GABAergic influences on conditioned expression. Pharmacol Biochem Behav. 2001;68:135–145. doi: 10.1016/s0091-3057(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Bardo MT. Conditioned increase in place preference by access to novel objects: Antagonism by MK-801. Behav Brain Res. 1999;99:53–60. doi: 10.1016/s0166-4328(98)00069-2. [DOI] [PubMed] [Google Scholar]
- Bevins RA, McPhee JE, Rauhut AS, Ayres JJB. Converging evidence for one-trial context fear conditioning with an immediate shock: Importance of shock potency. J Exp Psychol Anim Behav Process. 1997;23:312–324. doi: 10.1037//0097-7403.23.3.312. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Ayres JJB. One-trial context fear conditioning as a function of the interstimulus interval. Anim Learn Behav. 1995;23:400–410. [Google Scholar]
- Bitterman ME. Classical conditioning in the goldfish as a function of the CS-US interval. J Comp Physio. 1964;58:359–366. doi: 10.1037/h0046793. [DOI] [PubMed] [Google Scholar]
- Brabant C, Quertemont E, Tirelli E. Influence of the dose and the number of drug-context pairings on the magnitude and the long-lasting retention of cocaine-induced conditioned place preference in C57BL/6J mice. Psychopharmacology. 2005;180:33–40. doi: 10.1007/s00213-004-2138-6. [DOI] [PubMed] [Google Scholar]
- Burgos JE, Bevins RA. The P-system: A scheme for organizing Pavlovian procedures. Behav Res Meth Inst Comp. 1997;29:473–483. [Google Scholar]
- Calcagnetti DJ, Schechter MD. Nicotine place preference using the biased method of conditioning. Prog Neuro-Psychopharmacol & Biol Psychiat. 1994;18:925–933. doi: 10.1016/0278-5846(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Carboni E, Acquas E, Leone P, DiChiara G. 5HT3 receptor antagonists block morphine- and nicotine- but not amphetamine-induced reward. Psychopharmacology. 1989;97:175–178. doi: 10.1007/BF00442245. [DOI] [PubMed] [Google Scholar]
- Carr GD, Fibiger HC, Phillips AG. Conditioned place preference as a measure of drug reward. In: Liebman JM, Cooper SJ, editors. The neuropharmacological basis of reward. Clarendon Press; Oxford: 1989. pp. 264–319. [Google Scholar]
- Center for Disease Control. Cigarette Smoking Among Adults- United States, 2004. MMWR Weekly. 2005;54:1121–1124. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5444a2.htm. [PubMed]
- Clarke PB, Fibiger HC. Apparent absence of nicotine-induced conditioned place preference in rats. Psychopharmacology. 1987;92:84–88. doi: 10.1007/BF00215484. [DOI] [PubMed] [Google Scholar]
- Cohen A, Ettenberg A. Motivational effects of nicotine as measured in a runway model of drug self-administration. Behav Pharm. 2007;18:265–271. doi: 10.1097/FBP.0b013e3281f19b3c. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited access schedule. Psychopharmacology. 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1:1662–70. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology. 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Clemens JM, Fidler TL. Injection timing determines whether intragastric ethanol produces conditioned place preference or aversion in mice. Pharmacol Biochem Behav. 2002;72:659–68. doi: 10.1016/s0091-3057(02)00734-7. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Okorn DM, Howard CE. Interstimulus interval determines whether ethanol produces conditioned place preference or aversion in mice. Anim Learn Behav. 1997;25:31–42. [Google Scholar]
- Cunningham C, Noble D. Methamphetamine-induced conditioned place preference or aversion depending on dose and presence of drug. Ann N Y Acad Sci. 1992;654:431–433. doi: 10.1111/j.1749-6632.1992.tb25989.x. [DOI] [PubMed] [Google Scholar]
- DeNoble VJ, Mele PC. Intravenous nicotine self-administration in rats: effects of mecamylamine, hexamethonium and naloxone. Psychopharmacology. 2006;184:266–272. doi: 10.1007/s00213-005-0054-z. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Brodi JD, Gerasimov MR, Horan B, Gardner EL, Ashby CR., Jr A pharmacological strategy for the treatment of nicotine addiction. Synapse. 1999;31:76–86. doi: 10.1002/(SICI)1098-2396(199901)31:1<76::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Donny EC, Cagguila AR, Knopf A, Brown C. Nicotine self-administration in rats. Psychopharmacology. 1995;122:390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Erb SM, Parker LA. Individual differences in novelty-induced activity do not predict strength of amphetamine-induced place conditioning. Pharmacol Biochem Behav. 1994;48:581–586. doi: 10.1016/0091-3057(94)90317-4. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Raven MA, Danluck DA, Necessary BD. Evidence for opponent-process actions of intravenous cocaine. Pharmacol Biochem Behav. 1999;64:507–512. doi: 10.1016/s0091-3057(99)00109-4. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Associative vs. topographical accounts of the immediate shock-freezing deficit in rats: Implications for the response selection roles governing species--Specific defensive reactions. Learning Motiv. 1986;17:16–39. [Google Scholar]
- Forget B, Barthelemy S, Saurini F, Hamon M, Thiebot MH. Differential involvement of the endocannabinoid system in short- and long-term expression of incentive learning supported by nicotine in rats. Psychopharmacology. 2006;189:59–69. doi: 10.1007/s00213-006-0525-x. [DOI] [PubMed] [Google Scholar]
- Forget B, Hamon M, Thiebot MH. Cannabinoid CB1 receptors are involved in motivational effects of nicotine in rats. Psychopharmacology. 2005;181:722–734. doi: 10.1007/s00213-005-0015-6. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Iwamoto ET. Conditioned aversion after delay place conditioning with nicotine. Psychopharmacology. 1987;92:376–381. doi: 10.1007/BF00210847. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Iwamoto ET. Further studies on nicotine-induced conditioned place preference in rats. Pharmcol Biochem Behav. 1986;25:1041–1049. doi: 10.1016/0091-3057(86)90083-3. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Teoh DW, Iwamoto ET. Pharmacologic characterization of nicotine-induced conditioned place preference. Pharmacol Biochem Behav. 1985;22:237–241. doi: 10.1016/0091-3057(85)90384-3. [DOI] [PubMed] [Google Scholar]
- Gehrke B, Harrod S, Cass W, Bardo M. The effect of neurotoxic doses of methamphetamine on methamphetamine-conditioned place preference in rats. Psychopharmacol. 2003;166:249–258. doi: 10.1007/s00213-002-1318-5. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Baldock MD, Locurto C, Gold L, Terrace HS. Trial and intertrial durations in autoshaping. J Exp Psychol Anim Behav Process. 1977;3:264–84. [Google Scholar]
- Grabus SD, Martin BR, Brown SE, Damaj MI. Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology. 2006;184:456–463. doi: 10.1007/s00213-006-0305-7. [DOI] [PubMed] [Google Scholar]
- Horan B, Gardner EL, Dewey SL, Brodie JL, Ashby CR., Jr The selective sigma(1) receptor agonist, 1-(3,4-dimethoxyphenethyl)-4-(phenylpropyl)piperazine (SA4503), blocks the acquisition of the conditioned place preference response to (-)-nicotine in rats. Eur J Pharmacol. 2001;426:R1–2. doi: 10.1016/s0014-2999(01)01229-8. [DOI] [PubMed] [Google Scholar]
- Horan B, Smith M, Gardner EL, Lepore M, Ashby CR., Jr (-)-nicotine produces conditioned place preference in Lewis but not Fischer 344 rats. Synapse. 1997;26:93–94. doi: 10.1002/(SICI)1098-2396(199705)26:1<93::AID-SYN10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Hunt HF, Otis LS. Conditioned and unconditioned emotional defecation in the rat. J Comp Physiol Psych. 1953;46:378–382. doi: 10.1037/h0063632. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Steinpreis RE, Sherman JE, Baker TB. Aversion instead of preference learning indicated by nicotine place conditioning in rats. Psychopharmacology. 1990;101:533–538. doi: 10.1007/BF02244233. [DOI] [PubMed] [Google Scholar]
- LeFoll B, Goldberg SR. Nicotine induces conditioned place preference over a large range of doses in rats. Psychopharmacology. 2005;178:481–492. doi: 10.1007/s00213-004-2021-5. [DOI] [PubMed] [Google Scholar]
- Lett BT. Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology. 1989;98:357–362. doi: 10.1007/BF00451687. [DOI] [PubMed] [Google Scholar]
- McAllister WR. Eyelid conditioning as a function of the CS-US interval. J Exp Psych. 1953;45:417–422. doi: 10.1037/h0059534. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. Cigarettes and other tobacco products. NIDA Info Facts. 2006 July; Available from: www.drugabuse.gov/pdf/infofacts/Tobacco06.
- National Research Council. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Nomikos GG, Spyraki C. Cocaine-induced place conditioning: importance of route of administration and other procedural variables. Psychopharmacology. 1988;94:119–125. doi: 10.1007/BF00735892. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Khroyan TV, Neisewander JL. Dose-dependent characterization of the rewarding and stimulant properties of cocaine following intraperitoneal and intravenous administration in rats. Psychopharmacology. 1996;123:144–153. doi: 10.1007/BF02246171. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Nocjar C, Burgdorf J, Panksepp JB, Huber R. The role of emotional systems in addiction: a neuroethological perspective. Nebr Symp Motiv. 2004;50:85–126. [PubMed] [Google Scholar]
- Parker LA. Place conditioning in a three- or four-choice apparatus: role of stimulus novelty in drug-induced place conditioning. Behav Neurosci. 1992;106:294–306. doi: 10.1037//0735-7044.106.2.294. [DOI] [PubMed] [Google Scholar]
- Parker LA, Carvell T. Orofacial and somatic responses elicited by lithium-, nicotine-, and amphetamine-paired sucrose solution. Pharmacol Biochem Behav. 1986;24:883–7. doi: 10.1016/0091-3057(86)90431-4. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes. New York: Dover: 1927. [Google Scholar]
- Randall CK, Kraemer PJ, Bardo MT. Morphine-induced conditioned place preference in preweanling and adult rats. Pharmacol Biochem Behav. 1998;60:217–222. doi: 10.1016/s0091-3057(97)00585-6. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT. Effect of bupropion on nicotine self-administration in rats. Psychopharmacology. 2003;169:1–9. doi: 10.1007/s00213-003-1450-x. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Oakes RA. Dose- and conditioning trial-dependent ethanol-induced conditioned place preference in Swiss Webster mice. Pharmacol Biochem Behav. 1996;55:117–123. doi: 10.1016/0091-3057(96)00069-x. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Oakes RA. Nicotine-induced conditioned place preference and conditioned aversion in mice. Pharmacol Biochem Behav. 1995;51:457–61. doi: 10.1016/0091-3057(95)00007-j. [DOI] [PubMed] [Google Scholar]
- Rogers DT, Barron S, Littleton JM. Neonatal ethanol exposure produces a hyperalgesia that extends into adolescence, and is associated with increased analgesic and rewarding properties of nicotine in rats. Psychopharmacology. 2004;171:204–211. doi: 10.1007/s00213-003-1574-z. [DOI] [PubMed] [Google Scholar]
- Rose JE, Levin ED. Inter-relationship between conditioned and primary reinforcement in the maintenance of cigarette smoking. Br J Addict. 1991;86:605–609. doi: 10.1111/j.1360-0443.1991.tb01816.x. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology. 1996;129:35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Stolerman IP, Kumar RC. Nicotine-induced place preferences following prior nicotine exposure in rats. Psychopharmacology. 1994;113:445–452. doi: 10.1007/BF02245221. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Zhaoxia L, Le AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology. 2006;186:201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Stolerman IP. Behavioral pharmacology of nicotine: multiple mechanisms. Br J Addict. 1991;86:533–536. doi: 10.1111/j.1360-0443.1991.tb01803.x. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology. 1995;117:2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Koob GF. Restrained rats learn amphetamine-conditioned locomotion, but not place preference. Psychopharmacology. 1984;84:163–166. doi: 10.1007/BF00427440. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- West R, Schneider N. Craving for cigarettes. Br J Addict. 1987;82:407–415. doi: 10.1111/j.1360-0443.1987.tb01496.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson JL, Murray JE, Li C, Wiltgen SM, Penrod RD, Berg SA, Bevins RA. Interoceptive Pavlovian conditioning with nicotine as the conditional stimulus varies as a function of number of conditioning trials and unpaired sucrose deliveries. Behav Pharmacol. 2006;17:161–172. doi: 10.1097/01.fbp.0000197456.63150.cd. [DOI] [PubMed] [Google Scholar]


