Abstract
Objective
The workup of hypoglycemia requires frequent glucose sampling. We designed these studies to determine if the Continuous Glucose Monitoring System (CGMS) and the GlucoWatch G2® Biographer (GW2B) are sufficiently accurate to use in nondiabetic children.
Study Design
15 healthy children (9–17 years, 11 boys) wore a GW2B and a CGMS during 24-h, and reference serum glucose was measured hourly during the day and half-hourly overnight.
Results
Compared with the reference glucose, the median absolute difference in concentrations measured by the GW2B (487 pairs) was 13 mg/dL and by the CGMS was 17 mg/dL (668 pairs), with 30% and 42% of values using the GW2B and CGMS, respectively, deviating >20 mg/dL from the reference value. The GW2B reported values <60 mg/dL in 73% of subjects, the CGMS in 60%. In none of these episodes was serum glucose truly low. Spurious high glucose concentrations were also observed with the sensors. The mean reference glucose was lowest at 5AM (89 mg/dL) and highest at 11:30PM (106 mg/dL) during the 24-h.
Conclusions
Neither the CGMS nor GW2B is accurate enough to establish population standards of the glycemic profile of healthy children and cannot be recommended in the workup of hypoglycemia in nondiabetic youth.
Keywords: Normal Children, Carbohydrate Metabolism, Hypoglycemia
Introduction
Continuous glucose sensors have been developed to provide frequent glucose determinations throughout the day and night in individuals with diabetes. In the last few years, an explosion of data have been gathered on the use of continuous glucose sensors, and the application of this technology to the management of patients with diabetes has been the subject of intense investigation1–17. Two continuous glucose monitors, the GlucoWatch® G2™ Biographer (“GW2B”; Cygnus, Inc., Redwood City, CA), and the Continuous Glucose Monitoring System, CGMS™ (“CGMS”; Medtronic Minimed, Northridge, CA) are FDA-approved for use in children with diabetes. Both instruments measure interstitial fluid glucose concentrations, which are normalized for serum values using internal algorithms. Our group, the Diabetes Research in Children Network (DirecNet), has conducted studies assessing the accuracy of these sensors in diabetic children and have found relatively large differences between sensor data and reference serum glucose levels18, 19. The detection of false hypoglycemic values has been particularly common in diabetic children7. Although developed for use in diabetes, continuous glucose sensors have also been used in the management of children with hypoglycemia due to glycogen storage diseases16, and the sensors, if accurate, have a strong theoretical application in the diagnosis of hypoglycemic conditions in childhood or in the glucose monitoring of acutely ill neonates or children in the intensive care unit. To date, however, the accuracy of these devices has not been thoroughly studied and compared against frequently sampled serum reference blood glucose concentrations in nondiabetic youth.
The characterization of glucose values in healthy children during day and nighttime is critical to the interpretation of glucose profiles in pathological states, such as diabetes and hypoglycemic conditions; yet the glucose profile of normal healthy children has not been well described. In nondiabetic children, serum glucose concentrations have been characterized in a fasting state and during oral glucose tolerance tests20, but data are limited on the normal range of serum glucose concentrations over a 24-hour period. Studies in normal children of different ages report average blood glucose concentrations in the 80–110mg/dL range21–23.
In view of the important potential uses of the continuous glucose sensors in non-diabetics also, determination of their accuracy and limitations is of paramount importance. We hence designed a study in healthy, nondiabetic children to determine if either of the two commercially available continuous glucose sensors (CGMS and/or GW2B) is sufficiently accurate to use as a tool to establish norms for the glycemic profile of nondiabetic children. The study also provided data on the degree of physiological fluctuations in serum glucose concentrations during the day and night in these children.
Methods
This study was conducted by the Diabetes Research in Children Network (DirecNet) at five academic centers in the United States. The protocol and informed consent forms were approved by each center’s institutional review board. Informed consent was obtained from the parents/guardians and, when age appropriate, assent was obtained from the subjects.
Eligibility Criteria and Assessment
Subjects were recruited by each center’s diabetes clinic and the majority of the subjects had a friend or knew someone with diabetes. Eligibility criteria included (1) age 7.0 to less than 18.0 years; (2) no history of diabetes, no history of positive islet cell antibody testing, a normal HbA1c (<6.0% measured with the DCA2000+, Bayer Diagnostics, Tarrytown, NY), and no family history of type 1 or type 2 diabetes in a sibling or parent; (3) body mass index (BMI) between the 10th to 90th percentile for age and sex24, (4) no medication use of any type in the prior 7 days, (5) a normal hematocrit (according to normal range at the clinical center’s laboratory) and weight ≥16.0 kg (because of blood volume considerations), and (6) no skin abnormalities contraindicating sensor use.
Study Procedures
Prior to the start of the study, the study staff involved in the placement and management of the sensors underwent a formal training and certification procedure. The subjects were admitted to each center’s clinical research center (CRC) for approximately 26 hours. Upon CRC admission, one GW2B was placed and, after two hours, calibrated by trained study staff. A second GW2B was placed either immediately following the calibration of the first GW2B or if deferred, no more than nine hours later. Additional sensors were placed when indicated so that at least one GW2B would be functioning for the 24 hours of the study. A CGMS sensor was inserted in the abdomen or upper buttocks by study staff. Simultaneous use of a second CGMS sensor was optional. The instruments were calibrated using glucose values from either capillary or venous blood measured with One Touch® Ultra® meters (Life Scan, Milpitas, CA). Approximately two-thirds of the Ultra glucose measurements used to calibrate the sensors were from venous blood, which we found to be more accurate than Ultra measurements from capillary blood.25 Since in home sensor use, capillary blood is used for calibration, our results might be slightly overestimating accuracy of the sensors.
Blood samples were obtained through an intravenous catheter hourly during the day (7:00 AM to 9:00 PM) and every half hour overnight (9:30 PM to 6:30 AM) for serum glucose determinations. The first hourly measurement was made at least one hour following insertion of the CGMS. Blood was withdrawn through the catheter, allowed to clot, then separated and frozen until shipped. Analysis was done at the DirecNet Central Biochemistry Laboratory at the University of Minnesota. Glucose levels were measured using a hexokinase enzymatic method26, 27. There was no fixed dietary plan provided and subjects ate the meals selected from the CRC menus ad lib.
Statistical Methods
The underlying principle in the sample size estimation was to determine the number of subjects required for a pre-specified width of a two-sided 95% confidence interval for each measure of accuracy comparing the sensor glucose values with the reference glucose values. With a sample size of 15 subjects and 20 sensor-reference glucose pairs per subject (total of 300 pairs), the half-width of a 95% confidence interval for the mean absolute deviation is approximately 5 mg/dL.
The procedure for matching sensor and reference glucose measurements has been reported previously18, 19. For each matched pair, the absolute difference between the reference glucose value and the sensor glucose value was computed (in mg/dL) and the percentages of sensor values within 10 mg/dL and within 20 mg/dL of the paired reference value were determined.
During the course of the study, Medtronic MiniMed modified the sensor fabrication process that had been in place since 1999. Thirteen subjects used “original” sensors and two subjects used “modified” sensors. Since there were only two subjects who used the modified sensor, formal statistical comparisons were not made between the original and modified sensors.
Variability of reference glucose levels in individual subjects was assessed by calculating the standard deviation (SD) of the reference glucose values around each subject’s 24-h mean.
Results
The 15 subjects who participated in the study had a median age of 12.4 (range 9–17 years); 11 were male and 14 Caucasian. Mean (SD) body mass index was 20.9 (3.6) kg/m2. Ten subjects completed the full 24-hour protocol, four subjects completed 12 to 23 hours, and one subject completed less than 12 hours. Three of the five early study discontinuations were related to difficulties maintaining the intravenous line; the other two were at the subject’s request. None of the subjects experienced more than minimal skin irritation using either the GW2B or the CGMS sensors. Figure 1 shows representative profiles of the reference and the sensor glucose values for two subjects over the course of the hospitalization.
Figure 1 A &B.
Representative plots of the sensor and reference glucose values over a 24-hour period. In green is the GWB tracing and in blue the CGMS tracing. Solid points represent the reference laboratory glucose. Two tracings of the same color represent simultaneous use of two given monitors.
GW2B Accuracy
The 15 subjects used 39 sensors during the study. There were 487 GW2B-reference paired glucose values, with 286 of the pairs from overnight sampling (11:00 PM to 6:00 AM) and 201 of the pairs from 6:30 AM to 10:30 PM. The median difference between the GW2B and reference glucose values was −3 mg/dL (25th, 75th percentiles: −17, 11 mg/dL). The absolute value of the differences between the GW2B and reference glucose values ranged from 0 to 65 mg/dL with a median of 13 mg/dL (25th and 75th percentiles: 6, 23). The GW2B values were within 10 mg/dL of the reference values 40% of the time and within 20 mg/dL 70% of the time. Results were similar for daytime and nighttime sampling (Table 1).
Table 1.
GW2B and CGMS Accuracy Summary Statistics Overall and According to Time of Day
| Total | 11:00 PM – 6:00 AM | 6:30 AM – 10:30 PM | |
|---|---|---|---|
| GW2B | |||
| # of paired data points | 487 | 286 | 201 |
|
Absolute difference* mg/dL
median (25th, 75th percentiles) |
13
(6, 23) |
13
(6, 24) |
13
(7, 21) |
| Values within 10 mg/dL percentage | 40% | 41% | 40% |
| Values within 20 mg/dL percentage | 70% | 66% | 74% |
| CGMS | |||
| # of paired data points | 668 | 307 | 361 |
|
Absolute difference* mg/dL
median (25th, 75th percentiles) |
17
(8, 29) |
18
(8, 29) |
16
(7, 29) |
| Values within 10 mg/dL percentage | 34% | 32% | 36% |
| Values within 20 mg/dL percentage | 58% | 56% | 60% |
Absolute difference defined as the absolute value of the sensor glucose value minus the reference glucose value (always positive).
During the 24 hours of sampling, there were 108 GW2B values <60 mg/dL, with 11 of the 15 subjects having at least one such value. Seven of the 108 values were single isolated low readings (i.e., prior and subsequent values ≥70 mg/dL) and 101 had at least one contiguous value <70 mg/dL. Of these 108 sensor values <60 mg/dL, 33 coincided with a reference glucose measurement. For all 33, the paired reference value was >70 mg/dL, ranging from 74 to 107 mg/dL.
We analyzed not only the frequency of lows but also the occurrence of high glucose values during sampling. There were 22 GW2B values >150 mg/dL, with 6 of the 15 subjects having at least one such value. Twenty of 22 values had at least one contiguous value >140 mg/dL. Of these 22 sensor values >150 mg/dL, 7 coincided with a reference glucose measurement. For all 7, the paired reference value was ≤140 mg/dL, ranging from 101 to 140 mg/dL. Minimum and maximum reference and sensor glucose values are given for each subject in Table 2.
Table 2.
Maximum and Minimum Glucose Values by Subject.
| Maximum Glucose (mg/dL) | Minimum Glucose (mg/dL) | |||||
|---|---|---|---|---|---|---|
| Sensor
|
Sensor
|
|||||
| Subject* | Reference | GWB | CGMS | Reference | GWB | CGMS |
| 1 | 143 | 176 | 194 | 77 | 64 | 71 |
| 2 | 140 | 192 | 159 | 67 | 53 | 71 |
| 3 | 137 | 145 | 240 | 84 | 52 | 53 |
| 4 | 136 | 163 | 189 | 89 | 55 | 72 |
| 5 | 133 | 137 | 154 | 80 | 65 | 68 |
| 6 | 132 | 171 | 140 | 78 | 61 | 58 |
| 7 | 128 | 130 | 176 | 78 | 40 | 50 |
| 8 | 125 | 147 | 153 | 74 | 50 | 55 |
| 9 | 124 | 157 | 177 | 74 | 43 | 56 |
| 10 | 119 | 179 | 172 | 60 | 57 | 47 |
| 11 | 114 | 150 | 156 | 63 | 51 | 61 |
| 12 | 110 | 116 | 122 | 71 | 58 | 55 |
| 13 | 109 | 147 | 114 | 36 | 69 | 71 |
| 14 | 107 | 113 | 181 | 76 | 43 | 62 |
| 15 | 99 | 142 | 150 | 77 | 40 | 58 |
Subjects sorted by maximum reference glucose value.
Restricted to sensor readings between the first and last reference blood glucose draws.
During periods of simultaneous use of two GW2B sensors, there were 445 paired sensor glucose values. The two paired values were within 10 mg/dL of each other 36% of the time and within 20 mg/dL 62% of the time.
CGMS Accuracy
Six of the 15 subjects used two CGMSs simultaneously. There were 668 CGMS-reference paired glucose values, with 307 of the pairs being from overnight sampling (11:00 PM to 6:00 AM) and 361 of the pairs being from 6:30 AM to 10:30 PM. The median difference between the CGMS and reference glucose values was 6 mg/dL (25th and 75th percentiles: −9, 23.5 mg/dL). The absolute value of the differences between the CGMS and reference glucose values ranged from 0 to 127 mg/dL with a median of 17 mg/dL (25th and 75th percentiles: 8, 29). The median remained 17 mg/dL when the analysis was limited to the 16 sensors that met Medtronic Minimed’s criteria for an optimal day18. The CGMS values were within 10 mg/dL of the reference values 34% of the time and within 20 mg/dL of target 58% of the time. Results were similar for daytime and nighttime sampling (Table 1).
Accuracy appeared to be better for the 71 paired values from the 3 modified sensors compared with the 597 values from the 18 original sensors. For the original sensors the median absolute difference was 17 mg/dL (25th and 75th percentiles: 8, 30) compared with 11 mg/dL (25th and 75th percentiles: 4, 23) for the modified sensors. The glucose values of the original sensors were within 10 mg/dL of the reference values 32% of the time and within 20 mg/dL of the reference value 57% of the time compared with 48% and 72% respectively with the modified sensors.
During the 24 hours of sampling, there were 134 CGMS values <60 mg/dL, with 8 of the 15 subjects having at least one such value. None of the 134 values was a single isolated low reading. Of these 134 sensor values <60 mg/dL, 14 coincided with a reference glucose draw. For all 14, the paired reference value was >70 mg/dL, ranging from 82 to 105 mg/dL.
There were 363 CGMS values >150 mg/dL, with 11 of the 15 subjects having at least one such value. None of the 363 values was a single isolated high reading. Of these 363 sensor values >150 mg/dL, 52 coincided with a reference glucose draw. For all 52, the paired reference value was <140 mg/dL, ranging from 74 to 135 mg/dL.
During periods of simultaneous use of two CGMS sensors, there were 1,476 paired sensor glucose values. The two paired values were within 10 mg/dL 24% of the time and within 20 mg/dL 44% of the time.
Reference Serum Glucose Concentrations
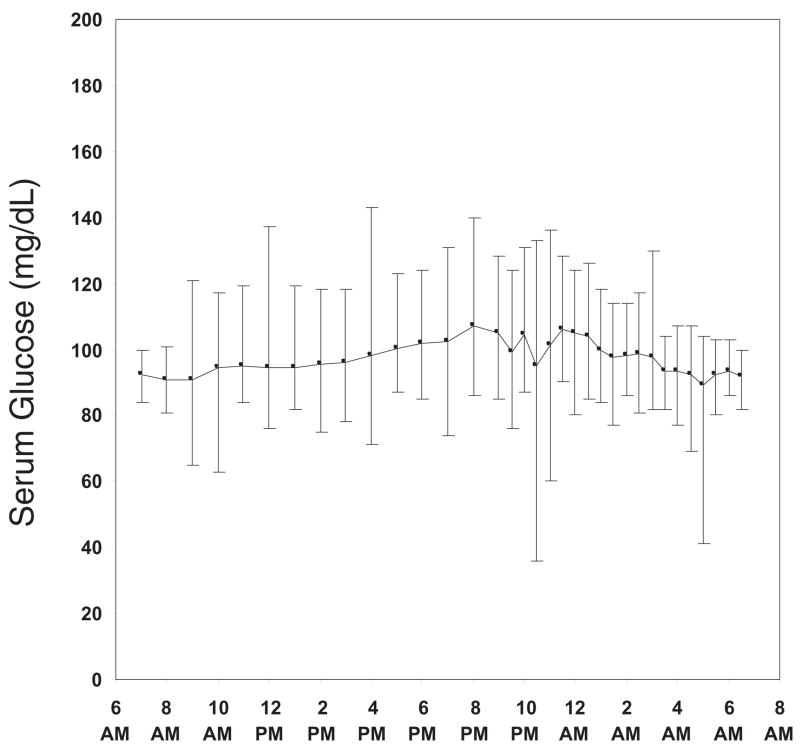
When examining only the 523 reference glucose values, the mean serum glucose concentrations were narrow during the 24 hours of sampling with a mean ± SD of 98 ± 13 mg/dL from 9:30 PM to 6:30 AM and 97 ± 14 mg/dL from 7:00 AM to 9:00 PM. The highest and lowest hourly or half-hourly mean values were 107 mg/dL and 89 mg/dL, respectively (Figure 2). On an individual subject level, the median high-low range was 51 mg/dL, with the widest range being 73 mg/dL and the narrowest range being 22 mg/dL. The median SD value was 12 mg/dL ranging from 4 to 19 mg/dL.
Figure 2.
Mean and range for reference serum glucose values over a 24-hour period.
The lowest reference glucose was from a subject (a 12 year old white male) who had two reference values below 60 mg/dL (36 mg/dL at 10:30 PM and 41 mg/dL at 5:00 AM). The subject’s 10:00 PM and 11:00 PM values were 96 mg/dL and 91 mg/dL respectively, and the 4:30 AM value was 69 mg/dL. The CRC staff had difficulties with the IV line, which was removed shortly after the 5:00 AM blood draw and not replaced. At the time of the 36 mg/dL value, the CGMS glucose was 90 mg/dL and the GW2B value was 97 mg/dL. At the time of the 41 mg/dL reference value, the CGMS glucose was 88 mg/dL and the two GW2B values were 88 mg/dL and 91 mg/dL.
Discussion
This is the first study to evaluate the accuracy of the GW2B and the CGMS in healthy, nondiabetic children compared against reference venous serum samples. The GW2B and CGMS sensor glucose values differed from the reference values by >20 mg/dL 30% and 42% of the time, respectively. The recently modified CGMS sensor was evaluated in two subjects and accuracy appeared better, but the sensor values continued to differ from the reference values by >20 mg/dL 28% of the time.
In order to be used to establish population norms for glucose levels in nondiabetic children, the accuracy of the glucose measurements must approach that of laboratory-measured blood glucose, a level that neither the CGMS nor the GW2B currently approach. Hence these sensors are unable to provide conclusive normative data in nondiabetic individuals. This is particularly important as the use of these sensors has been used by some to evaluate glycemic profiles of children with hypoglycemic conditions such as glycogen storage disease16. If accurate, they could be ideal for the initial assessment of children with complaints suggestive of hypoglycemia, instead of using frequently sampled blood during a hospitalization. They also could be considered in monitoring glycemic levels in neonates and critically ill children. Unfortunately, this generation of sensors is not accurate enough.
We do recognize that these sensors were not developed to assess glucose levels in nondiabetic individuals and that the accuracy required to establish population norms is greater than that required to monitor the glucose levels in diabetic patients. However, in separate recent publications, we reported our experience on the accuracy of these sensors in 91 diabetic children, similarly studied in an inpatient CRC setting18, 19, and we found that the degree of sensor error was similar to what we found in this study of nondiabetic children. The absolute difference for the GW2B was 16% and for the CGMS was 18%. The recently modified CGMS sensors performed better than the original sensors (median absolute difference 11% versus 19%). Neither the GW2B nor the CGMS was accurate in the detection of hypoglycemia in diabetic children28.
Anecdotal information abounds of healthy subjects wearing the continuous glucose sensor having frank hypoglycemic values, particularly at night. Whether these decrements in glucose concentrations are real or represent an inherent analytical/accuracy issue was not previously clear, as these sensors had not been extensively used in healthy children. One study of CGMS use in 25 normal children in the home environment reported 8.2 ± 7.9% of serum glucose values below 70 mg/dL overall, and 17.9 ± 18.3% during the nocturnal period (12:00 AM to 6:00 AM), but reference laboratory glucose values were not obtained to validate the reliability of these measurements29. In our study, none of the sensor values <60 mg/dL were confirmed with a reference glucose measurement. These data also indicate that the current generation of glucose sensors has limited usefulness in the diagnosis and management of hypoglycemic disorders in children. Our results also indicate that sensor glucose values in the hyperglycemic range are likely to be spurious.
Interestingly, the sensors used also detected multiple hyperglycemic values (>150mg/dL) in these nondiabetic children, and in all of those in which there was a paired reference glucose concentration available the glucose concentration was <140mg/dL. Collectively, these data indicate that both spurious lows and high readings can be obtained with both sensors.
Our study adds to the limited data in the literature on the range of glucose values in healthy, nondiabetic children. The range in serum glucose concentrations measured by the central laboratory was narrow during the 24 hours of sampling. In our healthy children the median standard deviation of the reference glucose values was only 12 mg/dL. In contrast, in the children with type 1 diabetes who were studied under similar conditions, the median SD score was 69 mg/dL. One subject had two reference glucose values in the hypoglycemic range, but there was difficulty maintaining the intravenous line. In view of the fact that the reference glucose levels at the contiguous half-hour time points were not low and the fact that the subject’s sensor values at these times were not low, we believe that it is likely that these low values represented erroneous measurements.
In conclusion, our data support that: 1) neither the GW2B nor CGMS is accurate enough to provide normative glycemia profiles for nondiabetic children, 2) previous reports of low sensor glucose values in nondiabetic children may represent inaccurate glucose measurements, and 3) serum glucose concentrations in nondiabetic children are tightly controlled.
Acknowledgments
Appreciation is expressed for the work also performed by the CRC Nurses at the five clinical centers. These data were presented at the 2003 Pediatric Academic Societies’ Meeting in Seattle, WA, May 3–6.
This research has been supported by the following NIH/NICHD Grants: HD041919-0; HD041915; HD041890; HD041918-01; HD041908-01; and HD041906-01. Clinical Centers also received funding through the following GCRC Grant Numbers M01 RR00069; RR00059; RR 06022 and RR00070-41. LifeScan, Milpitas, CA, provided the One Touch® Ultra† Blood Glucose Monitoring Systems and the blood glucose test strips. The GlucoWatch® G2™ Biographer were purchased from Cygnus, Inc., at a discounted price.
Appendix
Writing Committee
Nelly Mauras, MD (chair); Roy W. Beck, MD, PhD; Katrina J. Ruedy, MSPH; Craig Kollman, PhD; William V. Tamborlane, MD; H. Peter Chase, MD; Bruce A. Buckingham, MD; Eva Tsalikian, MD; Stuart A. Weinzimer, MD; Andrea D. Booth, MS; Dongyuan Xing, MPH
The DirecNet Study Group: Clinical Centers
Listed in alphabetical order with clinical center name, city, and state. Personnel are listed as (PI) for Principal Investigator, (I) for co-Investigator and (C) for Coordinators. 1. Barbara Davis Center for Childhood Diabetes, University of Colorado, Denver, CO: H. Peter Chase, MD (PI); Rosanna Fiallo-Scharer, MD (I); Jennifer H. Fisher, ND, RN (C); 2. Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, IA: Eva Tsalikian, MD (PI); Michael J. Tansey, MD (I); Linda F. Larson, RN (C); 3. Nemours Children’s Clinic, Jacksonville, FL: Tim Wysocki, PhD, ABPP (PI); Nelly Mauras, MD (I); Kristen M. Gagnon, MS, RD (C); Pauline Todd, RN (C); 4. Division of Pediatric Endocrinology and Diabetes, Stanford University, Stanford, CA: Bruce A. Buckingham, MD (PI); Darrell M. Wilson, MD (I); Jennifer M. Block, RN, CDE (C); Elizabeth L. Kunselman, RN, CDE (C); 5. Department of Pediatrics, Yale University School of Medicine, New Haven, CT: William V. Tamborlane, MD (PI); Stuart A. Weinzimer, MD (I); Elizabeth A. Doyle, MSN (C); Coordinating Center: Jaeb Center for Health Research, Tampa, FL: Roy W. Beck, MD, PhD; Katrina J. Ruedy, MSPH; Craig Kollman, PhD; Andrea D. Booth, MS; Dongyuan Xing, MPH; Data and Safety Monitoring Board: Dorothy M. Becker, MBBCh; Christopher Cox, PhD; Christopher M. Ryan, PhD; Neil H. White, MD, CDE; Perrin C. White, MD; University of Minnesota Central Laboratory: Michael W. Steffes, MD, PhD; Jean M. Bucksa, CLS; Maren L. Nowicki, CLS; National Institutes of Health: Gilman D. Grave, MD; Barbara Linder MD, PhD; Karen K. Winer, MD
References
- 1.Metzger M, Leibowitz G, Wainstein J, Glaser B, Raz I. Reproducibility of glucose measurements using the glucose sensor. Diabetes Care. 2002;25:1185–91. doi: 10.2337/diacare.25.7.1185. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman FR, Austin J, Neinstein A, Jeng L, Halvorson M, Devoe DJ, et al. Nocturnal hypoglycemia detected with the Continuous Glucose Monitoring System in pediatric patients with type 1 diabetes. J Pediatr. 2002;141:625–30. doi: 10.1067/mpd.2002.129175. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman FR, Halvorson GLCM, Carpenter S, Fisher LK, Pitukcheewanont P. A pilot study of the continuous glucose monitoring system:clinical decisions and glycemic control after its use in pediatric type 1 diabetic subjects. Diabetes Care. 2001;24:2030–4. doi: 10.2337/diacare.24.12.2030. [DOI] [PubMed] [Google Scholar]
- 4.Boland E, Monsod T, Delucia M, Brandt CA, Fernando S, Tamborlane WV. Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care. 2001;24:1858–62. doi: 10.2337/diacare.24.11.1858. [DOI] [PubMed] [Google Scholar]
- 5.Chase HP, Kim LM, Owen SL, Mackenzie TA, Klingensmith GJ, Murtfeldts R, et al. Continuous subcutaneous glucose monitoring in children with type 1 diabetes. Pediatrics. 2001;107:222–6. doi: 10.1542/peds.107.2.222. [DOI] [PubMed] [Google Scholar]
- 6.Maran A, Crepaldi C, Tiengo A, Grassi G, Vitali E, Pagano G, et al. Continuous subcutaneous glucose monitoring in diabetic patients. Diabetes Care. 2002;25:347–52. doi: 10.2337/diacare.25.2.347. [DOI] [PubMed] [Google Scholar]
- 7.McGowan K, Thomas W, Moran A. Spurious reporting of nocturnal hypoglycemia by CGMS in patients with tightly controlled type 1 diabetes. Diabetes Care. 2002;25:1499–1503. doi: 10.2337/diacare.25.9.1499. [DOI] [PubMed] [Google Scholar]
- 8.Monsod TP, Flanagan DE, Rife F, Saenz R, Caprio S, Sherwin RS, et al. Do sensor glucose levels accurately predict plasma glucose concentrations during hypoglycemia and hyperinsulinemia. Diabetes Care. 2002;25:889–93. doi: 10.2337/diacare.25.5.889. [DOI] [PubMed] [Google Scholar]
- 9.Cheyne EH, Cavan DA, Kerr D. Performance of a continuous glucose monitoring system during controlled hypoglycemia in healthy volunteers. Diabetes Technol Ther. 2002;4:607–13. doi: 10.1089/152091502320798222. [DOI] [PubMed] [Google Scholar]
- 10.Potts RO, Tamada JA, Tierney MJ. Glucose monitoring by reverse iontophoresis. Diabetes Metab Res. 2002;18:S49–53. doi: 10.1002/dmrr.210. [DOI] [PubMed] [Google Scholar]
- 11.Tierney MJ, Tamada JA, Potts RO, Jovanovic L, Garg S Cygnus Research Team. Clinical evaluation of the GlucoWatch biographer: a continual, non-invasive glucose monitor for patients with diabetes. Biosens Biolectron. 2001;16:621–9. doi: 10.1016/s0956-5663(01)00189-0. [DOI] [PubMed] [Google Scholar]
- 12.Pitzer KR, Desai S, Dunn T, Edelman SV, Jayalakshmi Y, Kennedy J, et al. Detection of hypoglycemia with the GlucoWatch Biographer. Diabetes Care. 2001;24:881–5. doi: 10.2337/diacare.24.5.881. [DOI] [PubMed] [Google Scholar]
- 13.Tierney MJ, Tamada JA, Potts RO, Eastman RC, Pitzer KR, Ackerman NR, et al. The GlucoWatch Biographer: a frequent, automatic and noninvasive glucose monitor. Ann Med. 2000;32:632–41. doi: 10.3109/07853890009002034. [DOI] [PubMed] [Google Scholar]
- 14.Tamada JA, Garg SK, Jovanovic L, Pitzer KR, Fermi SJ, Potts RO, et al. Noninvasive glucose monitoring: comprehensive clinical results. JAMA. 1999;282:1839–44. doi: 10.1001/jama.282.19.1839. [DOI] [PubMed] [Google Scholar]
- 15.Garg SK, Potts RO, Ackerman NR, Fermi SJ, Tamada JA, Chase HP. Correlation of fingerstick blood glucose measurements with GlucoWatch Biographer glucose results in young subjects with type 1 diabetes. Diabetes Care. 1999;22:1708–14. doi: 10.2337/diacare.22.10.1708. [DOI] [PubMed] [Google Scholar]
- 16.Hershkovitz E, Rachmel A, Ben-Zaken H, Phillip M. Continuous glucose monitoring in children with glycogen storage disease type 1. J Inherit Metab Dis. 2001;24:863–9. doi: 10.1023/a:1013996325720. [DOI] [PubMed] [Google Scholar]
- 17.Delucia M, Boland E, Brandt CA, Vasconcelos M, Tamborlane WV. How low (and high) can you go? Nighttime sensor glucose levels in healthy children (Abstract) Diabetes. 2002;51:A120–A121. [Google Scholar]
- 18.The Diabetes Research in Children Network (DirecNet) Study Group. The accuracy of the CGMS in children with type 1 diabetes: results of the Diabetes Research in Children Network (DirecNet) accuracy study. Diabetes Technol Ther. 2003;5:781–789. doi: 10.1089/152091503322526987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Diabetes Research in Children Network (DirecNet) Study Group. The accuracy of the GlucoWatch Biographer in children with type 1 diabetes: results of the Diabetes Research in Children Network (DirecNet) accuracy study. Diabetes Technol Ther. 2003;5:791–800. doi: 10.1089/152091503322526996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenbloom AL. Insulin responses of children with chemical diabetes mellitus. N Eng J Med. 1970;282:1228–31. doi: 10.1056/NEJM197005282822203. [DOI] [PubMed] [Google Scholar]
- 21.Arslanian S, Ohki Y, Becker DJ, Drash AL. Demonstration of a dawn phenomenon in normal adolescents. Horm Res. 1990;34:27–32. doi: 10.1159/000181791. [DOI] [PubMed] [Google Scholar]
- 22.Marin G, Rose SR, Kibarian M, Barnes K, Cassorla F. Absence of dawn phenomenon in normal children and adolescents. Diabetes Care. 1988;11:393–6. doi: 10.2337/diacare.11.5.393. [DOI] [PubMed] [Google Scholar]
- 23.Heptulla R, Smitten A, Teague B, Tamborlane WV, Yong-Zhan MA, Caprio S. Temporal patterns of circulating leptin levels in lean and obese adolescents: relationships to insulin, growth hormone, and free fatty acids rhythmicity. J Clin Endocrinol Metab. 2001;86:90–96. doi: 10.1210/jcem.86.1.7136. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. BMI-for-age charts, 2 to 20 years, LMS parameters and selected smoothed BMI percentiles, by sex and age, 2000. [Accessed March 2003]; Available from http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/datafiles.htm.
- 25.The Diabetes Research in Children Network (DirecNet) Study Group. A Multicenter Study of the Accuracy of the OneTouch Ultra Home Glucose Meter in Children with Type 1 Diabetes. Diabetes Technol Ther. 2003;5:933–941. doi: 10.1089/152091503322640971. [DOI] [PubMed] [Google Scholar]
- 26.Neese J, Duncan P, Bayse D, Robinson M, Cooper T, Stewart C. Development and evaluation of a hexokinase/glucose-6-phosphate dehydrogenase procedure for use as a national reference method. HEW Publication No. (CDC) 77–8330. Atlanta: Centers for Disease Control; 1976. [Google Scholar]
- 27.Passey RB, Gillum RL, Fuller JB, Urry FM, Giles ML. Evaluation and comparison of 10 glucose methods and the reference method recommended in the proposed product class standard (1974) Clin Chem. 1977;23:131–9. [PubMed] [Google Scholar]
- 28.The Diabetes Research in Children Network (DirecNet) Study Group. Accuracy of the GlucoWatch G2 Biographer and the Continuous Glucose Monitoring System During Hypoglycemia. Experience of the Diabetes Research in Children Network. Diabetes Care. doi: 10.2337/diacare.27.3.722. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinzimer SA, Delucia M, Boland E, Steffen A, Tamborlane WV. Analysis of Continuous Glucose Monitoring Data from Non-Diabetic and Diabetic Children: A Tale of Two Algorithms. Diabetes Technol Ther. 2003;5:375–379. doi: 10.1089/152091503765691866. [DOI] [PubMed] [Google Scholar]