Abstract
In recent years, P1 phage Cre-recombinase has become an indispensable tool for conditional activation and/or inactivation of genes in the mouse. By fusing Cre to a tamoxifen-sensitive form of the estrogen receptor (Cre-ER™) temporal regulation of gene expression can be achieved. Here, we report the initial characterization of the Cre-ER™ system for controlling gene expression in the lens of the mouse. Cre-ER™ mice were crossed with a reporter strain (Z/EG; lacZ/EGFP). Following IP injection of tamoxifen into Cre-ER™;Z/EG mice, GFP expression was observed in 1–5% of lens epithelial and fiber cells. GFP expression was maintained for at least six months although, in the fibers, GFP appeared to diffuse from the cells as they were internalized into the lens. The rate of elongation of the fiber cells was determined by measuring the length of GFP-expressing cells at intervals after tamoxifen injection. The results of this calculation suggested that tamoxifen treatment induced GFP expression predominantly in lens epithelial cells. The GFP-positive fibers were apparently derived from this cell population. The strong induced expression of GFP in a small proportion of lens cells allowed individual lens cells to be identified in the intact tissue and should serve as a useful lineage marker to track the fate of lens cells and their descendents.
Keywords: Lens, Cre recombinase, GFP, inducible expression
The use of transgenic and gene knockout technologies has greatly enhanced our understanding of the molecular mechanisms underlying lens development. However, the consequences of genetic manipulations often become apparent early in embyrogenesis and persist throughout the remainder of embryonic and adult development. This complicates and occasionally confounds the analysis of the phenotype. To circumvent this difficulty, inducible gene expression systems have been developed that allow the temporal regulation of gene expression in vivo. Here we report the initial characterization of one such system in the mouse lens.
Among several strategies for inducible gene expression are those that utilize the Cre-loxP recombination system. This approach takes advantage of the properties of P1 phage Cre-recombinase, a 38 kDa enzyme that recognizes a 34 bp DNA sequence called loxP. Introduction of loxP sites flanking a DNA target sequence enables binding by Cre-recombinase and either inversion or excision of the sequence, depending on the respective orientation of the loxP sites. Inducible Cre activity has been achieved by fusing Cre to a mutant form (ER™) of the estrogen receptor (Feil et al., 1997; Hayashi and McMahon, 2002; Metzger and Chambon, 2001). Cre-ER™ is insensitive to the natural ligand (17β-estradiol) but functions as a specific receptor for the synthetic ligand 4-hydroxy-tamoxifen (Danielian et al., 1993). In the absence of tamoxifen, Cre-ER™ is sequestered in the cytoplasm, complexed with hsp90 (Mattioni et al., 1994). Binding to tamoxifen disrupts the interaction with hsp90 and permits the translocation of Cre-ER™ to the nucleus wherein it catalyzes loxP-specific recombination events. Expression of the Cre-ER™ transgene is under the control of a chimeric promoter of the cytomegalovirus immediate-early enhancer and the chicken β-actin/promoter enhancer (CAGG) that has been shown to drive widespread expression of a number of genes in transgenic mice (Hayashi and McMahon, 2002).
For our studies, which involve cell lineage tracing, it is advantageous to induce GFP expression in a subset of lens cells. To this end we utilized two strains of transgenic mice: Cre-ER™ and a binary reporter strain (Z/EG; lacZ/EGFP). In the Z/EG strain, the lacZ gene is flanked by loxP recognition sites. In the absence of Cre activity, lacZ is expressed but, in the presence of Cre, lacZ is excised and the downstream gene, GFP, is expressed (Novak et al., 2000). Cre-ER™ mice were crossed with Z/EG mice to generate animals heterozygous for both transgenes. Three-week-old double transgenic animals were given two intraperitoneal (IP) injections of tamoxifen (Sigma-Aldrich) dissolved in corn oil (15 mg/ml) to a final dose of 0.15 mg tamoxifen/gram of body weight. The injections were given 8 hours apart. Mice were sacrificed by CO2 inhalation at intervals after tamoxifen injection. Lenses were removed from enucleated eyes via an incision in the posterior of the globe. The procedures described herein were approved by the Washington University Animal Studies committee.
To examine tamoxifen-dependent recombination efficiency and the rate of spontaneous recombination in the absence of tamoxifen (i.e. the “leakiness” of the Cre-ER™ system) we used immunoblot analysis of tissues (heart, lung and lens) dissected from Cre-ER™;Z/EG mice one week after IP injection with tamoxifen or vehicle control (Figure 1). In corn-oil injected animals, GFP expression was undetectable by immunoblot in lung and lens tissue. In contrast, GFP was clearly expressed in the heart. No such expression was noted in heart tissue from Z/EG mice (data not shown), suggesting that recombination in heart tissue was due to tamoxifen-independent nuclear translocation of Cre. In all three tissues, treatment of Cre-ER™;Z/EG mice with tamoxifen induced a marked increase in GFP expression. GFP expression was evident in lens epithelial cells from tamoxifen-treated animals but undetectable in lens fiber cells. The latter observation may reflect the fact only a superficial shell of lens fiber cells contain the organelles necessary for de novo protein synthesis. GFP produced by this small contingent of cells may be diluted below the detection threshold by extant proteins from the synthetically inert cells in the lens core.
Figure 1.

Tamoxifen injection induces GFP expression in multiple tissues. Three-week-old Cre-ER™;Z/EG mice were injected with either tamoxifen (T) or corn oil vehicle (O). Animals were sacrificed one week after injection, and heart, lung and lens tissue was assayed by immunoblot, using an antibody to GFP. Note that heart tissue expresses GFP in the absence of tamoxifen. In contrast, in lung and lens tissue, GFP is undetectable in corn oil-injected animals. In the lenses of tamoxifen-treated mice, GFP expression is detectable in the epithelial cells but not the fiber cells.
Due to its inherent transparency, the lens lends itself to microscopic visualization of GFP expression patterns. In principle, even a single GFP-expressing lens cell can be detected by microscopy, sensitivity far exceeding that of the immunoblot technique. We used confocal microscopy to examine the effect of tamoxifen dose on recombination efficiency in the lens (Figure 2). Lenses were incubated in glass-bottomed Petri dishes on the heated (37°C) stage of an inverted confocal microscope (LSM510 META; Carl Zeiss Thornwood, NY) as described (Bassnett, 2005). GFP fluorescence was elicited and viewed using the 488 nm line of an argon laser and a 505 nm long-pass filter. Stacks of optical sections were collected through the anterior or posterior hemisphere of the lens and visualized as maximum intensity two dimensional projections. In agreement with observations made on other cell types (Hayashi and McMahon, 2002), the proportion of cells in which GFP expression was induced varied with the concentration of tamoxifen per IP injection and the number of injections. Single injections of tamoxifen rarely resulted in GFP expression. Tamoxifen doses of <0.1 mg/g body weight were ineffective and increased mortality was noted if the tamoxifen dose exceeded 0.3 mg/g body weight. We, therefore, adopted a regimen in which animals were given two IP injections of tamoxifen (0.15 mg/g body weight) spaced 8 hours apart.
Figure 2.

Effect of tamoxifen dosage on GFP expression level in the mouse lens. Three-week-old Cre-ER™;Z/EG mice were given either one or two injections of tamoxifen and GFP fluorescence was visualized by confocal microscopy one week after the first injection. A z-series of confocal optical sections was collected through the anterior hemisphere of the lens and then collapsed to a maximum intensity projection in which epithelial and fiber cells located at different depths in the tissue are in focus simultaneously. A double injection of 0.15 mg/g body weight tamoxifen induces GFP expression in epithelial cells and fiber cells. Single injections or lower doses of tamoxifen are less effective in inducing GFP expression.
From images such as those shown in Figure 2, it is evident that tamoxifen only induced GFP expression in a small proportion of lens cells, even when administered at relatively high doses or using multiple injections. The explanation for the relatively low induction efficiency appears to lie with the expression pattern of the Z/EG transgene. Beta galactosidase immunohistochemistry performed on isolated lens epithelia from Cre-ER™;Z/EG mice revealed that <10% of the cells expressed the Z/EG transgene (Figure 3A). GFP expression was induced in a high proportion of these cells following tamoxifen treatment (Figure 3B). In contrast to the mosaic expression pattern of the Z/EG transgene, the Cre-ER™ transgene is expressed uniformly in most tissues ((Hayashi and McMahon, 2002). Thus, it appears that the mosaicism of the Z/EG transgene places an upper limit on the number of cells that express GFP following tamoxifen treatment. Mosaic expression of transgenes in the lens is not uncommon (Shestopalov and Bassnett, 2003) and limits the utility of the Cre-ER™;Z/EG system in this tissue. However, for our purposes, which involve labeling individual cells within the intact lens at specific stages of development, the relatively low induction efficiency was advantageous.
Figure 3.

Mosaic expression of the Z/EG transgene in the lens of Cre-ER™;Z/EG mice. (A). The lens epithelium was treated with X-gal staining solution to visualize β-galactosidase activity in the epithelial cells. Less than 10% of the epithelial cells express the LacZ gene. (B). GFP expression is induced in a similar proportion (i.e. <10%) of epithelial cells following tamoxifen treatment of a Cre-ER™;Z/EG littermate.
To study the time course of GFP induction in the lens, three-week-old animals were given a double injection of tamoxifen (0.15 mg/g body weight) and sacrificed at intervals after the injection (Figure 4). In corn oil-injected animals no lens fluorescence was observed. In contrast, in tamoxifen-injected animals, GFP expression was observed in scattered lens epithelial and fiber cells 1 day after injection. By six days post-injection, elongated fluorescent fiber cells were readily visualized, especially from the posterior aspect. GFP expression was sustained in epithelial cells at least up to 24 weeks post-tamoxifen injection (Figure 5), the oldest stage examined. Fiber cell GFP expression was also evident at later stages, but the fluorescence was diffuse in fibers located deep within the lens. This may reflect the assimilation of those cells into the lens syncytium, a region of the lens core in which proteins are thought to diffuse between cells, perhaps as a result of the partial fusion of neighboring fibers (Shestopalov and Bassnett, 2003).
Figure 4.

GFP expression in the anterior hemisphere (upper panel) or posterior hemisphere (lower panel) of the lens 0–6 days after tamoxifen injection. Images are representative of at least 3 lenses at each age.
Figure 5.
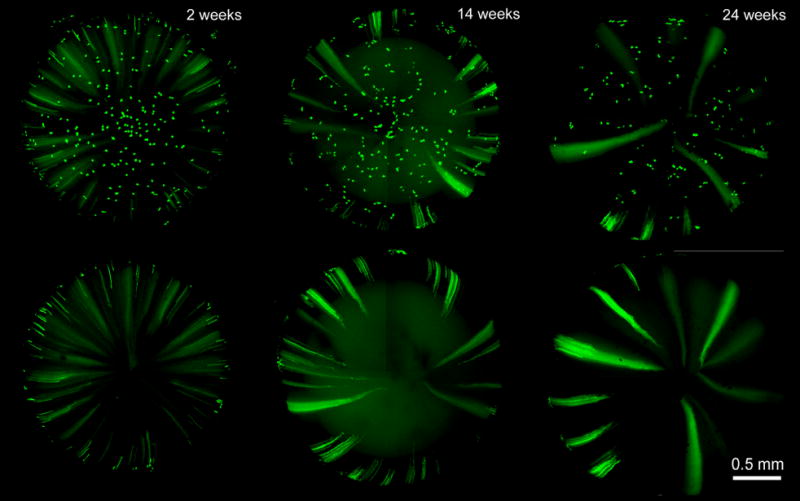
GFP expression in the anterior hemisphere (upper panel) or posterior hemisphere of the lens (lower panel) 2–24 weeks after tamoxifen injection. Images are representative of at least 3 lenses at each age.
The lag time between tamoxifen injection and the first observation of lens fiber cell fluorescence made it difficult to identify the cell population in which tamoxifen-induced recombination originally occurred. It was possible that GFP expression was induced directly in lens fiber cells. Alternatively, recombination might have been induced in epithelial progenitors which differentiated subsequently into GFP-expressing fiber cells. To distinguish between these possibilities we imaged the equatorial region of the lens two- and three-days after tamoxifen injection. The full equatorial circumference of the lens was imaged and reconstructed in three dimensions (Figure 6). The arc length of each differentiating fiber cell was measured and the average length of the 20 longest GFP expressing cells in each lens at each age was computed. Measurements were made on lenses from three animals at each age (Table 1). It is believed that fiber cells elongate at a relatively constant rate (Bassnett and Winzenburger, 2003). Between the second and third day after injection the average length of the 20 longest fiber cells increased by 359 ± 115 μm. Three days after tamoxifen injection the longest 20 fiber cells had an average length of 802 ± 197 μm. It seems likely, therefore, that at the time of tamoxifen injection, those cells had yet to differentiate into fiber cells. Thus, the elongation rate measurements suggested that tamoxifen injection induced GFP expression primarily in lens epithelial cells rather than in lens fiber cells directly.
Figure 6.
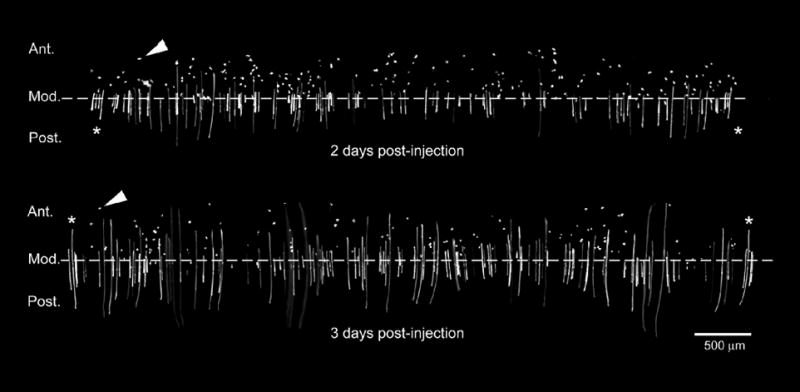
Reconstruction of the equatorial region of a Cre-ER™;Z/EG mouse lens two or three days after tamoxifen administration. Note that, on average, the fiber cell length three days after injection is greater than at two days after injection. Scattered GFP-positive epithelial cells (arrowheads) are present anterior to the modiolus (Zampighi et al., 2000). The reconstruction includes the entire lens equator as indicated by the presence of the same cells (marked *) at both the beginning and end of the equatorial strip. Images are representative of lenses from three animals at each age. Ant. = anterior; Post. = posterior, Mod. = modiolus.
Table 1.
Rate of elongation of GFP-expressing fiber cells. Three pairs of Cre-ER™;Z/EG littermates (A–C) were injected with tamoxifen. Animals were sacrificed two or three days after the injection and the lens equator was reconstructed in its entirety (see Figure 6). The average length of the 20 longest GFP-expressing fiber cells was measured in each lens. The mean cell length at two days was subtracted from that at three days to compute the fiber cell elongation rate.
| A | B | C | Mean ± S.D. | |
|---|---|---|---|---|
| Cell length 2 days post-injection | 647 | 159 | 521 | 442 ± 253 μm |
| Cell length 3 days post-injection | 1016 | 629 | 760 | 802 ± 197 μm |
| Elongation rate (μm/day) | 369 | 470 | 239 | 359 ± 115 μm |
Although only a small proportion of cells express GFP following tamoxifen treatment, this is ideal for certain applications. For example, it allows individual lens cells to be viewed in optical isolation and permits living cells to be imaged at high resolution within the intact lens (Figure 7). Such images confirm the complex three dimensional morphology of living lens epithelial cells, as recently described (Bassnett, 2005), and will potentially allow dynamic behaviors, such as cell elongation, to be studied directly. Furthermore, because tamoxifen treatment induces a permanent rearrangement of epithelial cell DNA, the subsequent expression of GFP represents a useful lineage tracer to identify cells and their descendents. This should facilitate studies of the control of mitosis in the lens epithelium and, ultimately, provide insights into the growth of the tissue. Most exciting is the possibility of crossing Cre-ER™ mice with strains in which important regulatory genes are flanked by loxP. By properly titrating the tamoxifen dose it should be possible to delete such genes in individual lens cells and thus study the effects of a gene knockout in the context of a surrounding cohort of wildtype cells. To unambiguously identify the cells in which genes had been knocked out would, however, require the use of reporter genes that are expressed uniformly in the lens, such as those cloned into the Rosa26 locus (Soriano, 1999).
Figure 7.

Three dimensional morphology of GFP- expressing cells in the lens of a tamoxifen-treated Cre-ER™;Z/EG mouse. A. an xy (en face) view of a living anterior epithelial cell imaged in the intact lens. Note the presence of complex lateral process. B. xy and xz images of a GFP-expressing fiber cell in the lens cortex.
In summary, we described the use of an inducible gene expression system in the lens. This system, which utilized cre-recombinase fused to a mutant form of the estrogen receptor, was used here to excise lacZ and thereby induce expression of GFP in a subset of lens epithelial cells. The Cre-ER™;Z/EG system was shown to be tightly regulated in the lens (i.e. gene induction did not occur in the absence of tamoxifen) and represents a useful method for manipulating gene expression in identifiable cohorts of lens cells.
Acknowledgments
The authors thank Seta Dikranian for her help with genotyping. This study was supported by NIH grants R01EY009852 and EY02687 (Core grant for vision research), and an unrestricted grant to the Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness (RPB). S.B. is a William and Mary Greve RPB scholar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bassnett S. Three-dimensional reconstruction of cells in the living lens: the relationship between cell length and volume. Exp Eye Res. 2005;81:716–23. doi: 10.1016/j.exer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Winzenburger PA. Morphometric analysis of fibre cell growth in the developing chicken lens. Exp Eye Res. 2003;76:291–302. doi: 10.1016/s0014-4835(02)00315-9. [DOI] [PubMed] [Google Scholar]
- Danielian PS, White R, Hoare SA, Fawell SE, Parker MG. Identification of residues in the estrogen receptor that confer differential sensitivity to estrogen and hydroxytamoxifen. Mol Endocrinol. 1993;7:232–40. doi: 10.1210/mend.7.2.8469236. [DOI] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–7. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–18. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- Mattioni T, Louvion JF, Picard D. Regulation of protein activities by fusion to steroid binding domains. Methods Cell Biol. 1994;43(Pt A):335–52. doi: 10.1016/s0091-679x(08)60611-1. [DOI] [PubMed] [Google Scholar]
- Metzger D, Chambon P. Site- and time-specific gene targeting in the mouse. Methods. 2001;24:71–80. doi: 10.1006/meth.2001.1159. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–55. [PubMed] [Google Scholar]
- Shestopalov VI, Bassnett S. Development of a macromolecular diffusion pathway in the lens. J Cell Sci. 2003;116:4191–9. doi: 10.1242/jcs.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Zampighi GA, Eskandari S, Kreman M. Epithelial organization of the mammalian lens. Exp Eye Res. 2000;71:415–35. doi: 10.1006/exer.2000.0895. [DOI] [PubMed] [Google Scholar]


