Abstract
Premature termination of translation due to nonsense mutations is a frequent cause of inherited diseases. Therefore, many efforts were invested in the development of strategies or compounds to selectively suppress this default. Selenoproteins are interesting candidates considering the idiosyncrasy of the amino acid selenocysteine (Sec) insertion mechanism. Here, we focused our studies on SEPN1, a selenoprotein gene whose mutations entail genetic disorders resulting in different forms of muscular diseases. Selective correction of a nonsense mutation at the Sec codon (UGA to UAA) was undertaken with a corrector tRNASec that was engineered to harbor a compensatory mutation in the anticodon. We demonstrated that its expression restored synthesis of a full-length selenoprotein N both in HeLa cells and in skin fibroblasts from a patient carrying the mutated Sec codon. Readthrough of the UAA codon was effectively dependent on the Sec insertion machinery, therefore being highly selective for this gene and unlikely to generate off-target effects. In addition, we observed that expression of the corrector tRNASec stabilized the mutated SEPN1 transcript that was otherwise more subject to degradation. In conclusion, our data provide interesting evidence that premature termination of translation due to nonsense mutations is amenable to correction, in the context of the specialized selenoprotein synthesis mechanism.
INTRODUCTION
Selenoproteins belong to a family of proteins that contain a covalently bound atom of selenium as part of the amino acid selenocysteine. Selenocysteine (Sec) is co-translationally incorporated into proteins, and in all selenoenzymes, studied so far, it was shown that Sec is directly involved in the catalytic mechanism. The presence of the selenol group confers an important increase in reactivity versus a cysteine thiol (1,2). Insertion of the Sec residue into the polypeptide chain is a highly specific event which involves several exceptions to the canonical translational rules (3,4). First, Sec is encoded by UGA, a codon that would be recognized as a stop in a different context. In eukaryotes, reprogrammation of the UGA Sec is achieved by an RNA structure, the SECIS element (SElenoCysteine Insertion Sequence), located in the 3′UTR of selenoprotein mRNAs. This motif is specifically recognized by the RNA-binding protein SBP2, which in turn recruits the complex formed by the specialized elongation factor EFSec and the charged selenocysteine tRNASec (5). In prokaryotes, it was shown that the tRNASec harbors several determinants which prevent its recognition by the general translation elongation factor EF-Tu but allow specific binding of SelB, the elongation factor dedicated to Sec incorporation. In addition, synthesis of the Sec residue occurs directly on the tRNASec which is initially charged with serine by the seryl-tRNA synthetase. Conversion of the seryl- to selenocysteyl-residue is subject to multiple enzymatic steps in eukaryotes (6). How this complex machinery interacts with the ribosome to dictate specific incorporation of the selenocysteine residue in response to the UGA codon remains to be elucidated.
To date, 25 selenoproteins have been identified in humans. Among them is Selenoprotein N (SePN) (7), the first selenium-containing protein which was associated to a genetic disorder (8). Mutations in the SEPN1 gene cause different forms of autosomal recessive muscle disorders: congenital muscular dystrophy with spinal rigidity (RSMD1) (8); multiminicore myopathy (MmD) (9); desmin-related myopathy with Mallory body-like inclusions (MB-DRM) (10); and congenital fiber-type disproportion myopathy (CFTD) (11). Although the histopathological descriptions of these different disorders are distinct, clinical reevaluation of patients with these diagnoses showed that they share identical clinical features characterized by early weakness of axial muscles, development of spinal rigidity and scoliosis as well as severe respiratory insufficiency. These phenotypes are now grouped under the generic term of SEPN1-related myopathy. The molecular function of SePN is still unknown, but it was characterized as a glycoprotein of the endoplasmic reticulum. In addition, SePN was shown to be highly expressed in proliferative muscle precursor cells (12). In zebrafish, analysis of the SEPN1 gene-expression pattern during early development revealed high expression in the somites and the notochord, two tissue precursors for the differentiation of skeletal muscles. Moreover, knockdown of SEPN1 expression in developing zebrafish embryos causes disorganization of the muscle architecture and reduced motility (13).
Numerous mutations, scattered all over the SEPN1 gene, have now been identified in patients (14). Most of them are nonsense mutations and deletions, likely to induce a loss of function. Recently, we characterized a pathological mutation within the SECIS motif in the 3′UTR of SEPN1 mRNA, abolishing the binding of SBP2 and therefore preventing synthesis of a full-length active SePN protein (15). With the detailed knowledge of the molecular mechanisms and dysfunctions underlying genetic diseases, correction of genetic defects may now be envisioned. Over the last decade or so, a lot of efforts have been targeted towards the development of cell- or gene-based therapies. However, these approaches present a number of hurdles especially for muscular diseases, mainly due to the size of the genes considered and the selectivity of the target tissue (16). Here, we propose a mutation-specific correction strategy based on the use of a modified tRNASec. The presence of this tRNA will force recognition of a mutated Sec codon in a RSMD1 patient with a homozygous point mutation at the Sec codon (c.G1385A) (8), converting UGA to UAA. Indeed, it was previously reported that the UGA codon is not strictly required for Sec incorporation into proteins, and that other stop codons could be used as well, provided that the codon/anticodon complementarity is maintained (17). In addition, both the seryl-tRNA synthetase and the machinery which converts serine to selenocysteine were shown to act independently of the anticodon sequence (18,19). Therefore, it was a reasonable assumption that the expression of a corrector tRNASec would rescue expression of the mutated UAA SEPN1 gene. We engineered a mutant tRNASec gene carrying a point mutation in the anticodon, thereby restoring the base-pair complementarity with the SEPN1 mutated codon. We demonstrated, both in HeLa cells and in patient-derived primary fibroblasts, that the corrector tRNASec gene indeed allowed read-through of the UAA stop codon, thus enabling synthesis of the full-length SePN protein. In addition, the specificities of the molecular mechanisms of the selenoprotein synthesis machinery constitute an invaluable asset rendering this strategy feasible and avoiding cross-effects on the normal translation of other cellular proteins.
MATERIALS AND METHODS
Cloning and plasmid constructs
The gene encoding the human tRNASec was PCR amplified from human genomic DNA. The resulting 678 bp fragment spans positions −420 to +257 relative to the 5′ end of the tRNASec and incorporates all the upstream promoter and downstream regions required for efficient transcription and maturation (20). A SalI restriction site was introduced at both extremities of the PCR fragment for cloning into the pSK(−) vector. This construct was named tRNASecwt. Mutation of the TCA anticodon sequence to TtA was engineered by site-directed mutagenesis. The cDNA-encoding SePN cloned into the eukaryotic expression vector pXJ41 and previously described as pXJSelN6 in Ref. (12) was used as the SePNwt construct. Mutations of the UGA selenocysteine codon to UaA stop or gGA glycine, were introduced into the SePNwt construct by site-directed mutagenesis. Deletion of the SECIS element in SePNwt, SePNuaa and SePNgga was obtained by removing 1609 bp of the 3′UTR sequence; downstream of the unique Nhe1 site located 200 bp upstream of the SECIS element.
Cell culture and transfection
HeLa cells (ATCC) were cultured in DMEM, supplemented with 10% fetal calf serum, 20 U/ml streptomycin and penicillin at 37°C with 5% CO2. At 70–80% confluence, cells were transfected by calcium phosphate precipitation with 1 µg of the different pXJ-SePN constructs and cotransfected with 1 µg of the different pSK-tRNASec variants, as described in the text. After 16 h incubation, the medium was changed and the cells were further grown for 20 h or defined periods as indicated in the figures. Primary fibroblasts were obtained from a skin biopsy of patient 14 961 (8) performed at the age of 13. Cells were grown as previously described (12). Following detachment using trypsin-EDTA, 5 × 105 cells (about 60–70% confluent) were transfected with 2 µg of tRNASecwt or tRNASecuua expression vectors, using the Normal Human Dermal Fibroblast Nucleofector™ kit (NHDF-Adult, Amaxa) according to the manufacturer recommendations (program U-23). Re-expression of SePN was investigated 24, 48, 72 and 96 h post-nucleofection. As a negative control, cells were nucleofected in the absence of plasmid DNA. As a control for transfection efficiency, a GFP reporter plasmid was co-transfected with the tRNASec constructs. GFP fluorescence was monitored 24–96 h post-transfection.
Protein extraction and immunodetection
Transfected HeLa cells were lyzed by the freeze–thaw procedure in 100 mM Tris–HCl, pH 8. The lysate was centrifuged at 13 000 r.p.m. for 20 min, and the SePN protein was extracted from the membranous pellet. The pellet was resuspended in 25–50 µl extraction buffer (50 mM Tris–HCl pH7.5, 200 mM NaCl, 1.25 mM CaCl2, 1 mM MgCl2, 1% Triton X-100) and incubated for 20 min at 4°C. After centrifugation for 15 min at 13 000 r.p.m., 5 µg of protein extract were subjected to SDS–PAGE and transferred to PVDF membranes. A 1/2000 dilution of the primary anti-SePN polyclonal antibody, Ac168, was used in a 3% milk, 0.1% Tween 20, PBS solution. Total proteins from confluent human fibroblasts were obtained as previously described (15). Twenty microgram of total proteins were fractionated and transferred to PVDF membranes which were hybridized with polyclonal antibody R139 against the C-terminus of the protein (dilution 1/100). For loading control, blots were subsequently stained with a monoclonal antibody against β-actin (Sigma, dilution 1/2000). HRP-conjugated secondary antibodies were used at a dilution of 1/1000.
Detection of the different tRNASec entities by chain termination primer extension
A total RNA fraction was prepared from transfected HeLa cells by Tri Reagent™ (Euromedex) extraction. A dideoxyoligonucleotide complementary to tRNASec sequence was 32P-labeled at its 5′ end using T4 polynucleotide kinase. Four microgram of total cellular RNAs were hybridized to 2.105 c.p.m. of the radioactive probe in a total volume of 8 µl, by heating at 90°C for 2 min, followed by immediate freezing in dry ice. Next, 2 µl of 5× RT buffer (250 mM Tris–HCl pH 8.3, 200 mM KCl, 40 mM MgCl2, 5 mM DTT) was added directly on top of the frozen reaction medium, and incubated at room temperature for 3 min. Primer extension was performed in a 15 µl reaction in 1× RT buffer, containing 2 U AMV Reverse Transcriptase (QBiogen). To monitor the tRNASecwt/tRNASecuua ratio, a nucleotide mix containing 0.5 mM dATP/dTTP and 0.25 mM ddGTP, was included. As a control, the total amount of tRNASec was measured by adding 0.5 mM dATP/dTTP/dGTP and 0.25 mM ddCTP. Extension was performed for 30 min at 42°C. The reaction mix was then precipitated, the pellet was resuspended in 6 µl TE plus 4 µl sequencing dye mix. The reaction product was heated for 3 min at 90°C and fractionated on a 10% denaturing polyacrylamide gel.
RNA extraction and quantitative RT-PCR
Total RNAs were extracted from cultured skin fibroblasts with Trizol as previously described (15). First strand cDNA was prepared in a 20 µl final volume reaction from 1 µg of total RNA using oligo(dT)18 and Superscript II RT as recommended by the manufacturer (InVitrogen). Quantitative real-time PCR was carried out by amplifying a 290 bp fragment spanning exons 9–11. The housekeeping gene HPRT was used as a reference on the Light Cycler real-time PCR machine using SyBR Green.
RESULTS
Incidence of two pathological mutations in the selenocysteine codon on SEPN1 expression
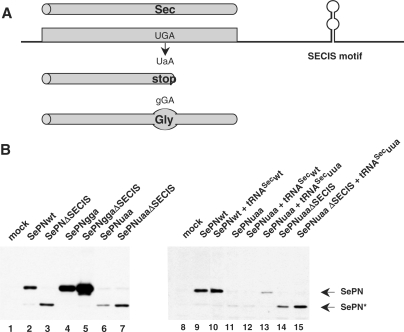
Several mutations were identified in the SEPN1 gene in patients with SEPN1-related myopathy. Many of them are clustered in the vicinity of the putative catalytic site, as deduced from the position of the selenocysteine (Sec) residue. Among them, two different point mutations affect the Sec codon directly. The first one consists in a UGA to UaA change [c.G1385A, p.U462X; (8)], introducing a stop codon which cannot be decoded by the Sec insertion machinery (Figure 1A). The second one converts the UGA Sec to a gGA glycine. To test the effects of these two mutations on SEPN1 expression, both nucleotide changes were introduced into the SEPN1 construct and the cDNAs were transiently expressed into HeLa cells. The translation products were compared to the wild-type selenoprotein N, SePNwt (Figure 1B, lane 2) or to that arising from a construct lacking the SECIS element, SePNΔSECIS. In the absence of the SECIS element, the UGA Sec codon was not decoded, yielding to the synthesis of a truncated SePN protein (Figure 1B, lane 3). As predicted from the coding capacity of the two mutations, expression of SePNgga produced a full-length protein whereas the mutated codon in SePNuaa was recognized as a stop (Figure 1B, lanes 4 and 6). In addition, translation of the full-length protein from SePNgga was no longer dependent on the presence of the SECIS element (Figure 1B, lane 5). The SePNgga product accumulated at a higher level than SePNwt, which is representative of the low efficiency of selenocysteine insertion compared to other amino acids. Since the SePNgga protein is highly expressed, and because the UGA to gGA mutation leads to a muscle phenotype in patients, one should deduce that substitution of glycine for selenocysteine is highly deleterious to SePN catalytic activity. As this activity is presently unknown, it could not be assayed in vitro. On the other hand, the expression level of the short SePNuaa product was lower than that obtained with the UGA-containing SePNΔSECIS construct. This may indicate that the UGA Sec to UaA mutation destabilized the SePN mRNA. Finally, it is interesting to note that, in both SePNggaΔSECIS and SePNuaaΔSECIS, removal of the 3′ UTR domain which contains the SECIS motif, led to increased expression levels (Figure 1B, compare lanes 4, 5 and 6, 7). Therefore, it is likely that in addition to the SECIS, other RNA regulatory motifs such as RNA destabilizing elements or translation inhibitory sequences, were removed.
Figure 1.
Expression of two SEPN1 mutations causing rigid spine muscular dystrophy. Rescue of a UGA Sec to UAA nonsense mutation by a corrector tRNASec in HeLa cells. (A) The presence of a SECIS RNA motif in the 3′UTR of selenoprotein mRNAs promotes insertion of a selenocysteine (Sec) residue in response to a UGA codon. Mutation of this triplet to UAA prevents recognition by the selenocysteine insertion machinery, inducing a premature stop of translation and synthesis of a truncated polypeptide. Proteins arising from translation of the wild-type UGA or mutant UAA mRNAs are schematized as cylinders. (B) SePN expression from transfected HeLa cells was analyzed by western blot using a SePN polyclonal antibody (Ac168). Lanes 1–7: Constructs carrying the UGA Sec to glycine (GGA, SePNgga) or nonsense (UAA, SePNuaa) mutations were transfected and the expression profiles were compared to the wild type (SePNwt). SECIS lacking constructs (ΔSECIS) were also tested. The upper band corresponds to the full-size SePN protein (SePN). The lower band, which is obtained with SePNuaa or with SECIS-lacking constructs, corresponds to the premature stop of translation at the UGA/UAA Sec position (Lanes 2, 3, 6, 7, 9–15; SePN*). Co-expression of the SePNuaa mutant with the corrector tRNASecuua (lane 13), but not with tRNASecwt (lane 12), restored synthesis of the full-size SePN protein. Rescue of the SePNuaa mutant is specific of the selenocysteine insertion machinery since it was dependent on the presence of the SECIS element (lane 15).
A corrector tRNASec transgene rescues expression of a SEPN1 mutant
Next, we investigated the possibility of correcting the SePNuaa mutation by expressing a tRNASec gene harboring a compensatory mutation in the anticodon sequence, tRNASecuua. In HeLa cells, co-expression of the wild-type tRNASec, tRNASecwt, had no major impact either on SePNwt or SePNuaa synthesis (Figure 1, compare lanes 9, 10 and 11, 12). However, co-expression of the SePNuaa mutant with the cognate corrector tRNASecuua produced a band of the size expected for the full-length SePN protein (Figure 1, lane 13). Interestingly, correction of the SePNuaa mutant by tRNASecuua was obtained only in the presence of the SECIS element (Figure 1, compare lanes 13 and 15), demonstrating that the read-through of the UAA codon by tRNASecuua was dependent on the selenocysteine insertion machinery. This observation strongly suggests that a selenocysteine residue was incorporated at the expected position. Additional experiments showed that expression of tRNASecuua did not alter synthesis of either SePNwt or SePNgga, verifying the specificity of the correction for the mutated UAA selenocysteine codon (Supplementary Figure 1).
Accumulation of the corrector tRNASecuua in HeLa cells allows high expression of the full-length mutant SePN protein
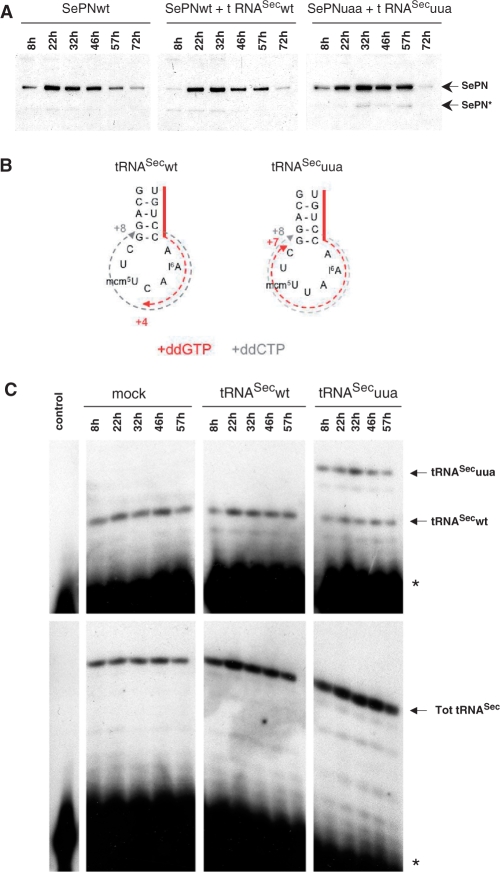
In the previous experiments, the amount of full-length SePN recovered by co-expressing the SePNuaa mutant and the corrector tRNASecuua was rather low compared to the wild-type protein. We hypothesized that this might reflect a delay in the accumulation of tRNASecuua within the intracellular tRNASec pool, therefore affecting the translation capacity of the reprogrammed UAA codon. To test this, we performed a time-course experiment of the expression of SePNwt or SePNuaa in the presence of the corresponding tRNASecwt or tRNASecuua (Figure 2A). Both in the presence or absence of tRNASecwt, SePNwt expression was already maximal 22 h post-transfection. In contrast, the highest expression level of SePNuaa with tRNASecuua was reached only 10 h later, at 32 h. At 72 h, the SePN level decreased in all cases, likely corresponding to the loss of the expression plasmids.
Figure 2.
Time-course expression of the various SePN or tRNASec constructs in transfected HeLa cells. (A) The accumulation of the wild-type SePN (SePNwt) or its mutated form (SePNuaa) co-expressed with the cognate tRNAsSec (tRNASecwt or tRNASecuua) were analyzed by western blot analysis at different time points (shown above the panels). SePNwt saturated at 22 h, whereas the SePNuaa mutant accumulated up to 32 h. Migration of the full size and truncated forms of SEPN are indicated (SePN and SePN*, respectively). (B and C) In parallel, the level of the different tRNAsSec was analyzed by chain termination primer extension. Because of the absence of a C residue in the anticodon sequence of the tRNASecuua, primer extension performed in the presence of ddGTP proceeded up to position +7 for tRNASecuua (numbering starting from the 3′ end of the primer), while it stopped at position +4 for tRNASecwt (B and top C panels). The total amount of tRNASec (Tot tRNASec) was estimated using ddCTP as the termination nucleotide, giving rise to a specific band at +8 with both the tRNASecwt and tRNASecuua (B and lower C panels). Accumulation of both tRNASecwt and tRNASecuua was analyzed at different time points and compared to the endogenous pool of cellular tRNASec (mock transfected). In the control (left columns), primer extension was performed under similar conditions, but omitting cellular RNAs. This control was used to visualize the migration of the primer (*) and to ascertain the absence of unspecific extension products. The solid red line (panel B) represents the 3′ end of the primer. The dotted red and gray lines show the extension products obtained in ddGTP and ddCTP-containing primer extension experiments, respectively.
In parallel, the level of the different tRNASec species in the transfected cells was evaluated by chain termination primer extension. An oligonucleotide, complementary to the variable arm and part of the anticodon stem of both tRNASecwt and tRNASecuua (Figure 2B, solid red line), was used as the primer for hybridization on total cellular RNAs. Primer extension was performed in the presence of ddGTP. In this case, extension will stop at the first C residue, which is encountered in the anticodon for tRNASecwt or 3 nt toward 5′ in tRNASecuua (red dotted line in Figure 2B). Hence, the differential tRNA variant-specific extension products were used to monitor the level of both tRNASecwt and tRNASecuua in the cells at different time points (Figure 2C). As an internal control, the total tRNASec level was measured by performing primer extension under conditions in which the extension proceeds up to the first G residue corresponding to the last G–C base pair closing the anticodon loop, a position common to both tRNASecwt and tRNASecuua. Transfection of the tRNASecwt expression vector in HeLa cells did not lead to an increase in the tRNASec level which remained stable over time (Figure 2C, compare left and central panels). This might reflect the saturation of the tRNA-processing machinery which regulates the intracellular pool of each tRNA species. Similar results were previously reported by Buvoli et al. (21). In contrast, the corrector tRNASecuua clearly accumulated within the tRNASec pool in a manner paralleling the accumulation of the SePN protein arising from the SePNuaa construct (Figure 2A and C, compare right panels). This observation further reinforced the criteria of a specific correction of SePNuaa by the recombinant tRNASecuaa. Because the primer extension was conducted in the presence of an excess of primer, the profiles obtained were representative of each tRNA entity level. Noteworthy, the tRNASecuua mutant was efficiently expressed and accumulated in the transfected cells to a level equivalent to the endogenous tRNASec.
Transient expression of the corrector tRNASecuua in patient fibroblasts restored expression of the full-length SePN protein
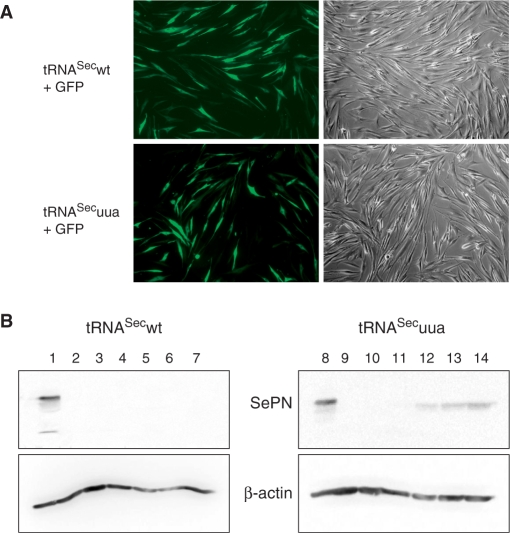
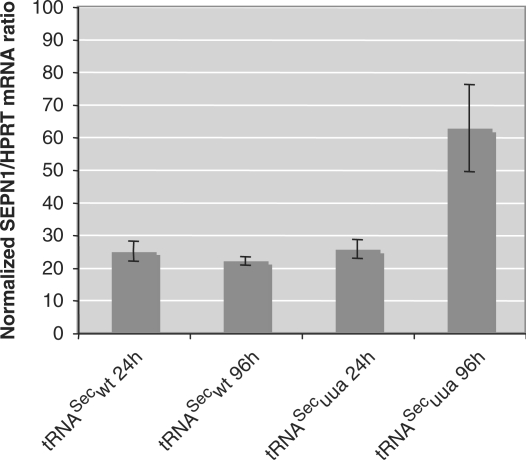
Another aspect of this study was to assess the ability of the corrector tRNASecuua to allow expression of the full-length SePN from the mutated SEPN1uaa gene in human primary fibroblasts derived from a RSMD1 patient. Western blot analysis using a C-terminal polyclonal antibody showed that no band could be detected in the total protein extract from the patient fibroblast, as opposed to the ∼70 kDa band obtained from control fibroblasts (Figure 3B, compare lanes 1 and 2). Interestingly, no shorter product could be detected in the patient fibroblasts. Next, the primary fibroblasts were transiently transfected with the plasmid expressing either the tRNASecwt or the tRNASecuua, using the Nucleofector (Amaxa). Transfection efficiency was monitored by co-transfection of a GFP reporter gene. Both tRNASecwt and tRNASecuua expression vectors were transfected with a similar efficiency of ∼30% (Figure 3A). Noticeably, a band corresponding to SePN started to appear in the patient fibroblasts transfected with the corrector tRNASecuua, as soon as 24 h post-transfection (Figure 3B, lanes 11–14). This correction was specific to tRNASecuua since no equivalent band was observed in the mock-transfected mutant fibroblasts (Figure 3B, lane 10) or upon transfection of the tRNASecwt (Figure 3B, lanes 4–7). The intensity of this band increased with time as expected from the previous experiments. In parallel, we analyzed the SEPN1 mRNA level by quantitative RT-PCR. In the RSMD1 mutant fibroblasts, this level was ∼25% of the control. This result demonstrated that mutation of the UGA Sec codon to UAA, which introduces a premature stop of translation, led to an increased turnover of the SEPN1 transcript. Strikingly, expression of the corrector tRNASecuua induced stabilization of the mutated transcript which rose up to ∼60% of a control individual, 96 h after transfection. Expression of tRNASecwt had no influence under similar conditions (Figure 4). Therefore, expression of the corrector tRNASecuua had a dual role at the post-transcriptional level on the expression of the mutated SEPN1 gene, (i) by stabilizing the transcript and (ii) by allowing insertion of the selenocysteine residue.
Figure 3.
Expression of the corrector, but not wild-type tRNASec, rescues SePN expression in fibroblasts from a RSMD1 patient carrying the SEPN1uaa mutation. Both the wild-type and corrector tRNASec expression vectors were transfected into cultured fibroblasts from a patient carrying the SEPN1uaa mutation. (A) The efficiency of transfection was assayed by co-expression of a GFP reporter protein. Transfected cells were visualized using fluorescence microscopy (left panels). The transfection efficiency of both tRNAs was similar. (B) Expression of SePN was determined by western blot analysis in transfected fibroblasts at 24 h (lanes 4 and 11), 48 h (lanes 5 and 12), 72 h (lanes 6 and 13) or 96 h (lanes 7 and 14) and compared to mock transfected at 96 h (lanes 3 and 10), untransfected cells (lanes 2 and 8) or control fibroblasts (lanes 1 and 7). As shown above each panel, either the wild-type (lanes 1–7) or corrector tRNAsSec constructs (lanes 8–14) were transfected. β-Actin was used as a control for equivalent loading (lower panels).
Figure 4.
Stability of the SEPN1uaa transcript under pathological or corrected conditions. SEPN1 mRNA levels were measured by quantitative PCR from nucleofected fibroblasts of a RSMD1 patient transfected either with tRNASecwt or tRNASecuua expression vectors. Scores were normalized to a HPRT control gene and levels were expressed as a percentage of the wild-type SEPN1 mRNA in fibroblasts from a control individual. The SEPN1uaa transcript was destablized, but transfection of the corrector tRNASecuua restored ∼60% of the SEPN1 wt mRNA level.
DISCUSSION
In this article we report the correction, by a recombinant tRNASec gene, of a nonsense UAA mutation in the selenoprotein N gene. This private mutation arose at the position of the selenocysteine UGA codon and caused at the homozygous state a congenital muscular dystrophy, RSMD1, in a patient affected by early axial weakness, rigid spine and respiratory insufficiency (8). We showed that correction of this mutation can be efficiently achieved provided that codon/anticodon complementarity is restored. First, we demonstrated in HeLa cells that the corrector tRNASecuaa was readily expressed and accumulated to levels comparable to that of the endogenous wild-type tRNASec. The corrector tRNASecuua was active in the read-through of the nonsense mutation since it allowed specific expression of a full-length SePN protein in a SECIS-dependent manner. Second, expression of the corrector tRNASecuaa in skin fibroblasts obtained from the patient with the mutant UAA codon, restored synthesis of the full-length SePN protein by providing both recoding of the mutated codon and stabilization of the transcript. Actually, mutation of the UGA selenocysteine to a UAA stop codon resulted in a lower accumulation of the SEPN1 mRNA in the patient fibroblasts. We previously reported a similar observation for a mutation in the central core of the SECIS element. This mutation, observed in a patient with the RSMD1 phenotype, prevented SBP2 from binding to the SECIS element. As a result, the UGA Sec codon was recognized as a premature stop codon, inducing destabilization of the transcript (15). The presence of nonsense mutations within mRNA-coding regions was demonstrated to promote mRNA degradation by nonsense-mediated mRNA decay (NMD), a mechanism dedicated to mRNA quality control (22). Our results suggest that read-through of the nonsense codon following expression of the corrector tRNASec, allowed the mutated SEPN1 mRNA to escape the NMD pathway, increasing the level of this mRNA in the patient fibroblasts and therefore stimulating expression of the full-length SePN protein. Phylogenetic analysis by others, validated by mutagenesis experiments, identified the presence of a conserved cis-acting selenocysteine codon redefinition element, also termed SRE, downstream of the UGA Sec codon in SEPN1 (23,24). This element, consisting in a stem-loop structure, was shown to stimulate read-through of both UGA and UAG codons. Here, our data reinforce the notion that the activity of the SRE in the SEPN1 mRNA is not dependent on the identity of the triplet recoding selenocysteine, since UAA was efficiently decoded by the corrector tRNASecuua.
Forced read-through of premature termination codons through tRNA suppression or treatment by aminoglycosides has been reported as a potential therapeutic approach to restore translation of mRNAs containing nonsense mutations, which cause a large number of human diseases (21,25–27). Recently, a new drug issued from high-throughput screens, PTC124, was characterized for its ability to selectively induce read-through of premature termination codons (28). The success of a therapeutic suppression strategy relies on the possibility of removing the translation blockage caused by nonsense mutations without affecting termination at genuine stop codons. Indeed, over-expression of suppressor tRNA genes was shown to have toxic effects on cell metabolism in different systems (29–31). Our correction strategy presents several advantages derived from the specificity of the selenocysteine insertion mechanism. First, the tRNASec is not recognized by the general translation elongation factor EF1A. Rather, it is recruited only and specifically by the specialized elongation factor EFSec to decode UGA Sec codons in selenoprotein mRNAs. Therefore, the corrector tRNASec will not suppress the genuine UAA stop codons in other cellular mRNAs and is expected to have less off-target effects than a traditional tRNA suppressor strategy. Generally, efficient suppression of nonsense mutations using tRNA suppressors requires high expression from multicopy genes (21). In contrast to other tRNAs, tRNASec is transcribed from a single copy gene (tsrp, 32), using a peculiar promoter organization corresponding to a combination of internal and external RNA polymerase III promoter and activator elements yielding high transcription levels (33,34). Therefore, high representativity of the corrector tRNASecuua within the pool of wild-type tRNASec can easily be obtained in transfected cells, as verified in our experiments.
One of the major hurdles in gene therapy is the ability to efficiently and safely deliver the therapeutic gene via a systemic route (16). For this purpose, both viral and non-viral vectors have been developed. A major limitation of viral vectors such as adeno-associated viruses (AAV), is their relatively low cloning capacity. For non-viral vectors, increasing plasmid size was shown to have a dramatic effect on transfection efficiency as well (35). In this respect, our strategy presents another asset in that the tRNASec transcription unit was extensively characterized and shown to harbor a compact organization with the entire gene residing in a 680 bp DNA fragment (20). Because of this small size, tRNASec is readily amenable to all vectors. Of paramount consideration for gene therapy is the need to obtain high expression of the recombinant gene in the target tissue and to avoid ectopic expression. Because tRNA genes are ubiquitously expressed and their product efficiently matured, high levels of corrector tRNASec can be obtained without any accessory factors in all cell lines or terminally differentiated tissues, including muscle cells. This is not necessarily the case for a selenoprotein-coding gene whose expression might be regulated both at the transcriptional and post-transcriptional levels in a tissue-specific manner (36). Consequently, the correction strategy by a recombinant tRNASec presents several advantages versus a more direct expression of a wild-type SEPN1 gene: first the size of the SEPN1 gene makes it much more difficult to handle with currently available vectors, and second its expression is more complicated to control because of sophisticated regulations. In addition, over-expression through plasmid or viral vectors carrying a normal copy of the SEPN1 gene is likely to result in the production of truncated proteins, due to partial recognition of the UGA Sec codon as a stop. Indeed, the efficiency of Sec insertion into selenoproteins is rather low (37,38) and the components of this machinery may become rapidly saturated upon transfection of high amounts of the SEPN1 gene.
Taken together, our results demonstrate the efficiency and advantages of a corrector tRNASec-based strategy to restore incorporation of a selenocysteine residue and therefore to promote translation of the full-length selenoprotein N. This mutation-specific approach constitutes an interesting alternative to conventional gene-therapy strategies in the highly specific context of selenoproteins.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
ACKNOWLEDGEMENTS
This work was supported by the Association Française contre les Myopathies. We are thankful to the Plate-forme Post-génomique de la Pitié-Salpêtrière (IFR14). We are grateful to Karine Parain for purification of antibody R139, Valérie Goldschmitt and Magali Frugier for helpful advice with the primer extension termination technique and critical reading of the manuscript. Christine Loegler and Anne Schweigert are acknowledged for excellent technical assistance. Funding to pay the Open Access publication charges for this article was provided by CNRS.
Conflict of interest statement. None declared.
REFERENCES
- 1.Johansson L, Gafvelin G, Arner ES. Selenocysteine in proteins-properties and biotechnological use. Biochim. Biophys. Acta. 2005;1726:1–13. doi: 10.1016/j.bbagen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Kim HY, Fomenko DE, Yoon YE, Gladyshev VN. Catalytic advantages provided by selenocysteine in methionine-S-sulfoxide reductases. Biochemistry. 2006;45:13697–13704. doi: 10.1021/bi0611614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allmang C, Krol A. Selenoprotein synthesis: UGA does not end the story. Biochimie. 2006;88:1561–1571. doi: 10.1016/j.biochi.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Hatfield DL, Carlson BA, Xu XM, Mix H, Gladyshev VN. Selenocysteine incorporation machinery and the role of selenoproteins in development and health. Prog. Nucleic Acid Res. Mol. Biol. 2006;81:97–142. doi: 10.1016/S0079-6603(06)81003-2. [DOI] [PubMed] [Google Scholar]
- 5.Zavacki AM, Mansell JB, Chung M, Klimovitsky B, Harney JW, Berry MJ. Coupled tRNA(Sec)-dependent assembly of the selenocysteine decoding apparatus. Mol. Cell. 2003;11:773–781. doi: 10.1016/s1097-2765(03)00064-9. [DOI] [PubMed] [Google Scholar]
- 6.Xu XM, Carlson BA, Mix H, Zhang Y, Saira K, Glass RS, Berry MJ, Gladyshev VN, Hatfield DL. Biosynthesis of selenocysteine on its tRNA in eukaryotes. PLoS Biol. 2006;5:e4. doi: 10.1371/journal.pbio.0050004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lescure A, Gautheret D, Carbon P, Krol A. Novel selenoproteins identified in silico and in vivo by using a conserved RNA structural motif. J. Biol. Chem. 1999;274:38147–38154. doi: 10.1074/jbc.274.53.38147. [DOI] [PubMed] [Google Scholar]
- 8.Moghadaszadeh B, Petit N, Jaillard C, Brockington M, Roy SQ, Merlini L, Romero N, Estournet B, Desguerre I, et al. Mutations in SEPN1 cause congenital muscular dystrophy with spinal rigidity and restrictive respiratory syndrome. Nat. Genet. 2001;29:17–18. doi: 10.1038/ng713. [DOI] [PubMed] [Google Scholar]
- 9.Ferreiro A, Quijano-Roy S, Pichereau C, Moghadaszadeh B, Goemans N, Bonnemann C, Jungbluth H, Straub V, Villanova M, et al. Mutations of the selenoprotein N gene, which is implicated in rigid spine muscular dystrophy, cause the classical phenotype of multiminicore disease: reassessing the nosology of early-onset myopathies. Am. J. Hum. Genet. 2002;71:739–749. doi: 10.1086/342719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreiro A, Ceuterick-de Groote C, Marks JJ, Goemans N, Schreiber G, Hanefeld F, Fardeau M, Martin JJ, Goebel HH, et al. Desmin-related myopathy with Mallory body-like inclusions is caused by mutations of the selenoprotein N gene. Ann. Neurol. 2004;55:676–686. doi: 10.1002/ana.20077. [DOI] [PubMed] [Google Scholar]
- 11.Clarke NF, Kidson W, Quijano-Roy S, Estournet B, Ferreiro A, Guicheney P, Manson JI, Kornberg AJ, Shield LK, et al. SEPN1: associated with congenital fiber-type disproportion and insulin resistance. Ann. Neurol. 2006;59:546–552. doi: 10.1002/ana.20761. [DOI] [PubMed] [Google Scholar]
- 12.Petit N, Lescure A, Rederstorff M, Krol A, Moghadaszadeh B, Wewer UM, Guicheney P. Selenoprotein N: an endoplasmic reticulum glycoprotein with an early developmental expression pattern. Hum. Mol. Genet. 2003;12:1045–1053. doi: 10.1093/hmg/ddg115. [DOI] [PubMed] [Google Scholar]
- 13.Deniziak M, Thisse C, Rederstorff M, Hindelang C, Thisse B, Lescure A. Loss of selenoprotein N function causes disruption of muscle architecture in the zebrafish embryo. Exp. Cell Res. 2007;313:156–167. doi: 10.1016/j.yexcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Rederstorff M, Krol A, Lescure A. Understanding the importance of selenium and selenoproteins in muscle function. Cell Mol. Life Sci. 2006;63:52–59. doi: 10.1007/s00018-005-5313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allamand V, Richard P, Lescure A, Ledeuil C, Desjardin D, Petit N, Gartioux C, Ferreiro A, Krol A, et al. A single homozygous point mutation in a 3′ untranslated region motif of selenoprotein N mRNA causes SEPN1-related myopathy. EMBO Rep. 2006;7:450–454. doi: 10.1038/sj.embor.7400648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster K, Foster H, Dickson JG. Gene therapy progress and prospects: Duchenne muscular dystrophy. Gene Ther. 2006;13:1677–1685. doi: 10.1038/sj.gt.3302877. [DOI] [PubMed] [Google Scholar]
- 17.Berry MJ, Harney JW, Ohama T, Hatfield DL. Selenocysteine insertion or termination: factors affecting UGA codon fate and complementary anticodon:codon mutations. Nucleic Acids Res. 1994;22:3753–3759. doi: 10.1093/nar/22.18.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu XQ, Gross HJ. The long extra arms of human tRNA((Ser)Sec) and tRNA(Ser) function as major identify elements for serylation in an orientation-dependent, but not sequence-specific manner. Nucleic Acids Res. 1993;21:5589–5594. doi: 10.1093/nar/21.24.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohama T, Yang DC, Hatfield DL. Selenocysteine tRNA and serine tRNA are aminoacylated by the same synthetase, but may manifest different identities with respect to the long extra arm. Arch. Biochem. Biophys. 1994;315:293–301. doi: 10.1006/abbi.1994.1503. [DOI] [PubMed] [Google Scholar]
- 20.Myslinski E, Krol A, Carbon P. Optimal tRNA((Ser)Sec) gene activity requires an upstream SPH motif. Nucleic Acids Res. 1992;20:203–209. doi: 10.1093/nar/20.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buvoli M, Buvoli A, Leinwand LA. Suppression of nonsense mutations in cell culture and mice by multimerized suppressor tRNA genes. Mol. Cell Biol. 2000;20:3116–3124. doi: 10.1128/mcb.20.9.3116-3124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Behm-Ansmant I, Kashima I, Rehwinkel J, Sauliere J, Wittkopp N, Izaurralde E. mRNA quality control: an ancient machinery recognizes and degrades mRNAs with nonsense codons. FEBS Lett. 2007;581:2845–2853. doi: 10.1016/j.febslet.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 23.Howard MT, Aggarwal G, Anderson CB, Khatri S, Flanigan KM, Atkins JF. Recoding elements located adjacent to a subset of eukaryal selenocysteine-specifying UGA codons. EMBO J. 2005;24:1596–1607. doi: 10.1038/sj.emboj.7600642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard MT, Moyle MW, Aggarwal G, Carlson BA, Anderson CB. A recoding element that stimulates decoding of UGA codons by Sec tRNA[Ser]Sec. RNA. 2007;13:912–920. doi: 10.1261/rna.473907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Temple GF, Dozy AM, Roy KL, Kan YW. Construction of a functional human suppressor tRNA gene: an approach to gene therapy for beta-thalassaemia. Nature. 1982;296:537–540. doi: 10.1038/296537a0. [DOI] [PubMed] [Google Scholar]
- 26.Barton-Davis ER, Cordier L, Shoturma DI, Leland SE, Sweeney HL. Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. J. Clin. Invest. 1999;104:375–381. doi: 10.1172/JCI7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panchal RG, Wang S, McDermott J, Link C.J., Jr Partial functional correction of xeroderma pigmentosum group A cells by suppressor tRNA. Hum. Gene. Ther. 1999;10:2209–2219. doi: 10.1089/10430349950017194. [DOI] [PubMed] [Google Scholar]
- 28.Welch EM, Barton ER, Zhuo J, Tomizawa Y, Friesen WJ, Trifillis P, Paushkin S, Patel M, Trotta CR, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- 29.Hudziak RM, Laski FA, RajBhandary UL, Sharp PA, Capecchi MR. Establishment of mammalian cell lines containing multiple nonsense mutations and functional suppressor tRNA genes. Cell. 1982;31:137–146. doi: 10.1016/0092-8674(82)90413-5. [DOI] [PubMed] [Google Scholar]
- 30.Doerig RE, Suter B, Gray M, Kubli E. Identification of an amber nonsense mutation in the rosy516 gene by germline transformation of an amber suppressor tRNA gene. EMBO J. 1988;7:2579–2584. doi: 10.1002/j.1460-2075.1988.tb03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laski FA, Ganguly S, Sharp PA, RajBhandary UL, Rubin GM. Construction, stable transformation, and function of an amber suppressor tRNA gene in Drosophila melanogaster. Proc. Natl Acad. Sci. USA. 1989;86:6696–6698. doi: 10.1073/pnas.86.17.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee BJ, Rajagopalan M, Kim YS, You KH, Jacobson KB, Hatfield D. Selenocysteine tRNA[Ser]Sec gene is ubiquitous within the animal kingdom. Mol. Cell. Biol. 1990;10:1940–1949. doi: 10.1128/mcb.10.5.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carbon P, Krol A. Transcription of the Xenopus laevis selenocysteine tRNA(Ser)Sec gene: a system that combines an internal B box and upstream elements also found in U6 snRNA genes. EMBO J. 1991;10:599–606. doi: 10.1002/j.1460-2075.1991.tb07987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuster C, Myslinski E, Krol A, Carbon P. Staf, a novel zinc finger protein that activates the RNA polymerase III promoter of the selenocysteine tRNA gene. EMBO J. 1995;14:3777–3787. doi: 10.1002/j.1460-2075.1995.tb00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang G, Ludtke JJ, Thioudellet C, Kleinpeter P, Antoniou M, Herweijer H, Braun S, Wolff JA. Intraarterial delivery of naked plasmid DNA expressing full-length mouse dystrophin in the mdx mouse model of duchenne muscular dystrophy. Hum. Gene Ther. 2004;15:770–782. doi: 10.1089/1043034041648408. [DOI] [PubMed] [Google Scholar]
- 36.Sunde R. edn. In: Hatfield D, Berry MJ, Gladyshev VN, editors. Selenium: Its Molecular Biology and Role in Human Health. 2nd. Springer ed; 2006. pp. 149–160. [Google Scholar]
- 37.Nasim MT, Jaenecke S, Belduz A, Kollmus H, Flohe L, McCarthy JE. Eukaryotic selenocysteine incorporation follows a nonprocessive mechanism that competes with translational termination. J. Biol. Chem. 2000;275:14846–14852. doi: 10.1074/jbc.275.20.14846. [DOI] [PubMed] [Google Scholar]
- 38.Grundner-Culemann E, Martin G.W., III, Tujebajeva R, Harney JW, Berry MJ. Interplay between termination and translation machinery in eukaryotic selenoprotein synthesis. J. Mol. Biol. 2001;310:699–707. doi: 10.1006/jmbi.2001.4809. [DOI] [PubMed] [Google Scholar]