Abstract
Assessing malignant tumors for expression of multiple biomarkers provides data that are critical for patient management. Quantum dot-conjugated probes to specific biomarkers are powerful tools that can be applied in a multiplex manner to single tissue sections of biopsies to measure expression levels of multiple biomarkers.
There is increasingly compelling evidence that cancer varies both genetically and phenotypically between ethnically and demographically similar patients who have histologically identical types and stages of cancer. Each cancer appears to be as unique as a fingerprint. A consequent challenge is how to efficiently and accurately analyze a cancer using a panel of biomarkers and plot a specific “molecular portrait” of each patient’s tumor. This uniqueness helps explain the variable and often unpredictable responses of tumors in individual patients to the same therapies.
Current diagnostic and prognostic classifications of tumors are based on histopathology. However, the histological patterns of cancer cells are not directly correlated with the underlying molecular events that drive cancer development and progression. With new molecular profiling technologies, it should be possible to read the molecular signatures of an individual patient’s tumor and correlate a panel of tissue biomarkers with clinical outcome and personalized therapy. Indeed, molecular profiles of many different tumors are being developed. Using gene expression microarrays, Paik and colleagues1 identified 21 genes that predict 10-year metastasis-free survival in patients with estrogen receptor (ER)-positive, node-negative tumors. More recently, this 21-gene panel has been validated using reverse transcription-polymerase chain reaction and has demonstrated that two-thirds of patients with ER-positive, node-negative tumors get no benefit from chemotherapy and can be treated with Tamoxifen alone.1 Similar approaches have identified gene models for a range of other tumors including prostate carcinoma.2 Knowing the molecular profile of a cancer also raises the prospect of developing therapies targeted to specific molecules. Targeted therapy has been successful in tumors such as breast and lung carcinoma, chronic myelogenous leukemia, and gastrointestinal stromal tumor.3
Analysis of Tissue Biomarkers
For the purpose of this discussion, we will focus on the two basic categories of molecular biomarkers, mRNA and protein. The analysis of nucleic acids has the advantage that very small samples can be analyzed because RNA and DNA can be amplified.4 In contrast, to analyze protein content requires more protein than can be obtained from solid organs by needle biopsy, because protein cannot be amplified.
The first commercial assay (Oncotype DX; Genomic Health, Inc., Redwood City, CA) of a tumor based on molecular profiles analyzes tissue samples of breast cancer for expression of the genes that were found by Paik et al to be of prognostic value.1 However, this type of assay and other technologies that use digested tissue samples typically do not correct for cell composition of tissue because these methods require destructive preparation of tissue specimens into homogeneous solutions. Consequently, there is both loss of the ability to determine cell composition and loss of tissue architecture. Because solid tumors are highly heterogeneous, being composed of a mixture of benign, cancerous, inflammatory, and stromal cells, the results may be misleading. Tissue-based technologies, exemplified by in situ hybridization and immunohistochemistry, provide results that take into account cell composition and tissue architecture because tumor cells are being directly assayed.
In this issue of The Journal of Molecular Diagnostics, Massimo Loda and his colleagues report simultaneous localization of transcripts in formalin-fixed, paraffin-embedded tissue by in situ hybridization using quantum dot-conjugated probes.5 Although proteins and not mRNA are for the most part the functional products of genes, the lack of availability of antibodies specific to many genes means that expression analysis can only be done using probes to mRNA, as pointed out by the authors. Because this article is one of the first of what we expect to become a growing literature of gene product localization studies in tissue using quantum dots, we encourage the readership to learn more about the potential of this new nanotechnology application.
As currently practiced, tissues are analyzed for biomarker antigen expression by immunohistochemical stains. Immunoperoxidase stains have advantages over immunofluorescence stains in that 1) analysis can be done by transmission light microscopy, thereby enabling the pathologist to accurately identify the cells expressing the localized gene product; 2) tissue autofluorescence does not confound interpretation; and 3) the marker is durable and does not quench, as do organic dyes. Thus, the stain is durable and can be reviewed at a later time. However, immunoperoxidase stains have a number of limitations: there is a limited range of color substrates, and, realistically, only one stain can be done on a section. These constraints limit the number of antigens that can be localized in a biopsy. Furthermore, the optical density of the immunoperoxidase stains, as is currently practiced, is not stoichiometric and does not truly quantify antigen content in a tissue specimen.6
A further limitation of the way that most cancers of solid organs are currently assessed lies in the nature of the samples. For diagnosis, many tissue samples are obtained by needle biopsies, which yield ∼1.5-cm long, <0.5-mm diameter cylinders of tissue from which, in general, only a small number of sections (typically a maximum of four) can be obtained for biomarker analysis after sections for diagnosis are cut. Because only one biomarker-based immunoperoxidase stain can be done on a section, the number of biomarkers that can be analyzed in a biopsy is limited. Each marker has to be stained one section at a time. However, accurate molecular profiling of tumors requires analyzing many more markers. For example, there are at least 10 biomarkers that can potentially be used to assess prostate cancer for prognosis and response to targeted therapy, ie, androgen receptor, CD10, Ki-67, p27, c-met, EZH2, her2-neu, insulin-like growth factor-1 receptor, MTA1, and survivin. These selected biomarkers are of both prognostic significance and therapeutic value, predicting response to molecular therapies, such as Herceptin and monoclonal antibody A12. Because more precise characterization of a tumor requires analysis of many proteins, we need a technology that enables us to analyze multiple biomarkers on the same tissue section.
Imaging Probe Development
A handful of technologies are currently available for in situ tissue-based biomarker analysis. Of these technologies, quantum dots (QDs) are particularly appealing due to their very high multiplexing and quantification capabilities (Table 1). Based on recent research,7,8,9,10,11,12,13,14,15 we expect that molecular pathology could become the first clinical application of QDs because the toxicity of nanomaterials is not a major concern for laboratory-based, in vitro studies.
Table 1.
Comparison of Current Technologies That Allow in Situ Analysis of Cancer Biomarkers
| Technologies | Tissue penetration depth | Detection sensitivity | Multiplexing capability | Quantification capability |
|---|---|---|---|---|
| Organic fluorophores | High | Low | Medium | Low |
| Quantum dots | Medium | High | High | High |
| Immunoenzyme assays | High | High | Single parameter | Low |
| Plasmon resonant nanoparticles | Very low | High | High | High |
| Nanogold with silver enhancement | High | High | Single parameter | Low |
Quantum dots are semiconductor nanocrystals with physical dimensions smaller than their exciton Bohr radius (see Table 2 for a glossary of common quantom dot terms). They are often composed of atoms from groups II–VI or III–V elements in the chemical periodic table, such as CdSe, CdTe, InP, and InAs. For the most studied spherical CdSe particles, their exciton Bohr radius is approximately 10 nm, and thus the particle sizes generally range from 2 to 6 nm. To better confine the excitons, remove surface traps, and increase the absorption cross-section, the CdSe core is often capped with a shell of another semiconductor, such as CdS and ZnS, which could lead to a substantial size increase.16 The optical properties of QDs arise from interactions between confined electrons and holes. QDs absorb photons when the excitation energy exceeds the bandgap. During this process, electrons are promoted from the valence band to the conduction band. Light emission arises from the recombination of the charge carriers.
Table 2.
Quantum Dot Glossary
| Bandgap (or band gap) is an intrinsic property of semiconductors and insulators and refers to the energy difference between the valence band and the conduction band. For quantum dot nanocrystals of the same chemical composition, their bandgap can be tuned in a certain range by varying the particle size. As a consequence, particles of different sizes produce multicolor fluorescence when excited with an external light source. |
| Exciton Bohr radius. An exciton refers to the electron-hole pair created in a semiconductor when an electron is promoted from the valence band to the conduction band and leaves a hole behind. Exciton Bohr radius is the physical distance between the separated electron and hole. It varies from substance to substance. |
| Stokes shift. For organic fluorophores, Stokes shift is measured by the distance between the excitation peak and emission peak maxima. In contrast, QDs absorb energy across a broad range of spectrum, and their molar extinction coefficient gradually increases towards shorter wavelengths. Therefore, the Stokes shift of QDs often refers to the wavelength difference of the excitation light and QD emission maximum. |
| Molar extinction coefficient (or molar absorptivity, ε) is a measure of how strongly a compound absorbs light at a certain wavelength. It is often used to quickly estimate the concentration of QDs based on Beer’s law. |
| Fluorescence excited state lifetime is a characteristic property of fluorescent molecules. If the excitation source is turned off instantaneously, the fluorescence signals decay exponentially. Lifetime is measured as the time lapse in which the fluorescence intensity decays to 1/e of the initial value. |
| Quantum yield. The quantum yield of a fluorescent molecule is defined as the ratio of the number of photons emitted to the number absorbed. |
What Are the Advantages of Quantum Dots for in Situ Biomarker Analysis?
Compared with organic fluorophores, QDs have several key spectral properties that render them the best fluorophores for sensitive, multicolor, and quantitative imaging of histopathological sections.17 QDs have very large molar extinction coefficients—on the order of 0.5 to 5 × 106 M−1 cm−1.18 This makes them sensitive probes for detecting low-abundance targets. Individual QDs are 20 to 50 times brighter than organic dyes (depending on quantum yield, molar extinction coefficient, and the fluorescence excited state lifetime).
1. The large Stokes shifts of QDs provide a means for separating the QD fluorescence from background autofluorescence. This factor becomes especially important for imaging tissue due to the high background autofluorescence typically seen in formalin-fixed, paraffin-embedded tissue specimens.
2. The longer excited state lifetimes of QDs can be used to further improve detection sensitivity. For example, the image contrast (measured by signal-to-noise or signal-to-background ratios) can be dramatically improved by time-gated data acquisition.19
3. Another advantage of QDs is that multicolor QD probes can be used to image and analyze multiple molecular targets simultaneously. Not only will this decrease the number of sections of tissue that need to be cut for biomarker analysis, but individual cells can be assessed for coexpression of molecules. The ability to localize gene products in the same cell provides the basis for diagnosing some leukemias. QD probes are particularly attractive because their broad absorption profiles allow simultaneous excitation of multiple colors, and their emission wavelengths can be continuously tuned by varying particle size and chemical composition. The emission peak width of QDs in solution is typically about 20 to 25 nm, although it can be as narrow as 14 nm (full width at half-maximum or FWHM) at room temperature.20 Such a narrow emission light results in minimum spectral overlap between adjacent colors and allows fitting three to four colors into the visible spectrum.
4. It is worth mentioning that QDs are 1000s of times more stable against photobleaching than organic fluorophores. Researchers have taken advantage of this property not only to accurately quantify emitted fluorescence but also to reduce (although not completely eliminate) tissue autofluorescence.7
Imaging Instrumentation
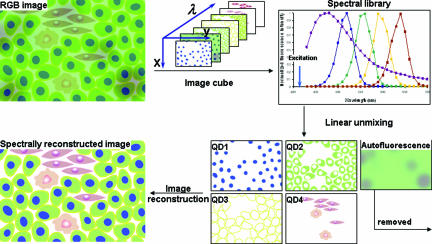
Despite the unique optical properties of QDs cited above, truly multiplexed and quantitative cellular imaging is still hindered by conventional RGB (red, green, and blue) cameras. For example, RGB cameras are incapable of 1) distinguishing pure yellow from co-localized mixture of green and red, 2) removing tissue autofluorescence, 3) resolving fluorescent components that spectrally overlap, and 4) providing a numerical readout. Recent research show that these problems could be solved by using a hyperspectral imaging (also known as spectral imaging) approach.21 Hyperspectral imaging has been widely used in remote sensing and is a relatively new technique for tumor marker fingerprinting. It provides spectral information for each pixel of a scene (eg, cells, tissue sections, and whole animal).22 A standard spectral imaging setup includes two major components, a passband controlling device (such as liquid crystal tunable filter and diffraction grating) and a scientific-grade monochrome CCD camera. Controlled by data acquisition software, the filter or grating automatically steps in a certain wavelength while the camera captures a series of images (image cube) of the sample at each wavelength with constant exposure (Figure 1). This process is repeated for each pure spectral component (fluorophores and autofluorescent tissue) to generate a spectrum library, which is then used to deconvolute mixed colors into separate channels (a mathematical process known as principle component analysis). Thus, spectral imaging allows rapid detection and analysis of small but meaningful spectral (and hence biomolecular) differences and removal of autofluorescence background.23,24,25 It is important to note that highly multiplexed analysis of cancer biomarkers is limited if hyperspectral imaging or QDs are used separately. The combination of the nanoprobe and spectral imaging enhances each other’s capability.
Figure 1.
Schematic illustration of hyperspectral imaging of multicolor QD-labeled tissue specimens. Conventional RGB cameras display images in three color channels (red, green, and blue) and thus cannot distinguish spectrally overlapping fluorophores. In contrast, hyperspectral imaging works in a way similar to spectroscopy in that it samples the emission spectra of every pixel at a series of wavelengths (thus creating an image cube). For example, blue QDs only appear in the images taken at shorter wavelength, whereas red QDs appear at longer wavelengths. Based on the spectral information obtained for each pixel, the fluorescent components (multicolor QDs and autofluorescence) can be unmixed into separate images for quantification or merged together with the autofluorescence removed. The use of QDs and hyperspectral imaging enhances the ability of each technique in multicolor imaging.
Potential Limitations
Despite the promise of this nanotechnology, there are potential concerns and limitations of using quantum dot-conjugated RNA probes:
1. Because quantum dots are complexes of heavy metals, they are potentially toxic to organisms. For example, cadmium is both hepatotoxic and nephrotoxic. Heavy metals can poison normal enzymes by competing with cofactors. At sufficiently high levels they cause cell death. Although toxicity must be addressed before quantum dots are used in humans, their toxicity is not so great that they cannot be used in laboratory assays or in experimental animals (given the slow rate of degradation of QDs), as long as appropriate precautions are taken by lab personnel.
2. The transmission light microscopic image must be readily correlated with the fluorescent image for the pathologist to identify the cells of interest. Inability to efficiently make these correlations has precluded the use of organic dyes in anatomical pathology labs. For example, distinction of cell types in fluorescent images can be quite challenging, ie, lymphocytes versus a very infiltrative tumor such as Gleason pattern 5 prostate adenocarcinoma and invasive lobular breast carcinoma. This disadvantage might be readily overcome using a microscope with combination transmission and fluorescent light, producing images that can be analyzed and displayed after deconvolution with a spectral imaging application.21
Potential Future Developments
The technology of QD-based analysis of tissue biomarkers can be further improved from its present state. Although spectral imaging is currently limited to 10 colors, faster computers and improved software could allow the analysis of hundreds of cancer biomarkers in a single tissue section. Although QDs have become a new and better biological label, the “perfect” nanoparticle bioconjugate still does not exist. Nanoparticles can be improved in several ways. Current commercial QD-antibody conjugates have hydrodynamic radii of ∼15 nm.16 This relatively large size limits the penetration of QDs into tissue specimens, especially for nuclear targets (Z. Yang, L.D. True, X. Gao, unpublished results). Compact bioconjugates, in which the number of biomolecules per nanoparticle and the orientation of the biomolecules are precisely controlled, would allow more uniform labeling of subcellular targets. Furthermore, simplified bioconjugation methods would make QDs more readily and widely accessible.
A final point to make concerns the stoichiometry of tissue localization assays and the possibility that QDs may be reagents that can truly quantify gene product levels in tissue. Immunoperoxidase stains are routinely reported in a manner that implies that they are quantifying antigens, ie, 0, 1+, 2+, 3+, etc. However, the multistep, enzyme-based immunoperoxidase stain is inherently not stoichiometric. Attempts to demonstrate the degree to which immunoperoxidase stains can “measure” antigens in tissue using experimental systems have shown just how susceptible the optically dense reaction product is to the conditions of the method, ie, concentrations of reagents, temperature of reaction.6 One can imagine that the intensity of light emitted by direct QD-antibody conjugates bound to antigens in tissue might quantify antigens on a per cell basis. Even then, standard curves using cell lines expressing known levels of the sought protein must accompany each QD stain. In addition, other potential sources of variance, ie, number of antibodies per QD (mentioned above), thickness of tissue sections, nonuniform optics, must also be considered.26
Footnotes
This commentary relates to Byers et al, J Mol Diagn 2007, 20–29.
Related Article on page 20
References
- Paik S, Tang G, Shak S, Kim C, Baker J, Kim W. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, Rubin MA, Chinnaiyan AM. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther. 2005;315:971–979. doi: 10.1124/jpet.105.084145. [DOI] [PubMed] [Google Scholar]
- Eberwine J, Yeh H, Miyashiro K, Cao Y, Nair S, Finnell R, Zettel M, Coleman P. Analysis of gene expression in single live neurons. Proc Natl Acad Sci USA. 1992;89:3010–3014. doi: 10.1073/pnas.89.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers R, Di Vizio D, O’Connell F, Tholouli E, Levenson R, Gossard K, Twomey D, Yang Y, Ostrom L, Benedettini E, Rose J, Ligon K, Finn S, Golub T, Loda M. Semi-automated multiplexed quantum dot based in-situ hybridization and spectral deconvolution. J Mol Diagn. 2007;9:20–29. doi: 10.2353/jmoldx.2007.060119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross DS, Rothfeld JM. Quantitative immunocytochemistry of hypothalamic and pituitary hormones: validation of an automated, computerized image analysis system. J Histochem Cytochem. 1985;33:11–20. doi: 10.1177/33.1.2578140. [DOI] [PubMed] [Google Scholar]
- Yezhelyev MV, Gao X, Xing Y, Al-Hajj A, Nie SM, O’Regan RM. Emerging use of nanoparticles in diagnosis and treatment of breast cancer. Lancet Oncol. 2006;7:657–667. doi: 10.1016/S1470-2045(06)70793-8. [DOI] [PubMed] [Google Scholar]
- Smith AM, Dave S, Nie S, True L, Gao X. Multicolor quantum dots for molecular diagnostics of cancer. Expert Rev Mol Diagn. 2006;6:231–244. doi: 10.1586/14737159.6.2.231. [DOI] [PubMed] [Google Scholar]
- Giepmans BNG, Deerinck TJ, Smarr BL, Jones YZ, Ellisman MH. Correlated light and electron microscopic imaging of multiple endogenous proteins using quantum dots. Nat Methods. 2005;2:743–749. doi: 10.1038/nmeth791. [DOI] [PubMed] [Google Scholar]
- Matsuno A, Itoh J, Takekoshi S, Nagashima T, Osamura RY. Three-dimensional imaging of the intracellular localization of growth hormone and prolactin and their mRNA using nanocrystal (quantum dot) and confocal laser scanning microscopy techniques. J Histochem Cytochem. 2005;53:833–838. doi: 10.1369/jhc.4A6577.2005. [DOI] [PubMed] [Google Scholar]
- Ness JM, Akhtar RS, Latham CB, Roth KA. Combined tyramide signal amplification and quantum dots for sensitive and photostable immunofluorescence detection. J Histochem Cytochem. 2003;51:981–987. doi: 10.1177/002215540305100801. [DOI] [PubMed] [Google Scholar]
- Nisman R, Dellaire G, Ren Y, Li R, Bazett-Jones DP. Application of quantum dots as probes for correlative fluorescence, conventional, and energy-filtered transmission electron microscopy. J Histochem Cytochem. 2004;52:13–18. doi: 10.1177/002215540405200102. [DOI] [PubMed] [Google Scholar]
- Sukhanova A, Venteo L, Devy J, Artemyev M, Oleinikov V, Pluot M, Nabiev I. Highly stable fluorescent nanocrystals as a novel class of labels for immunohistochemical analysis of paraffin-embedded tissue sections. Lab Invest. 2002;82:1259–1261. doi: 10.1097/01.lab.0000027837.13582.e8. [DOI] [PubMed] [Google Scholar]
- Wu X, Liu H, Liu J, Haley K, Treadway JA, Larson JP, Ge NF, Peale F, Bruchez MP. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol. 2003;21:41–46. doi: 10.1038/nbt764. [DOI] [PubMed] [Google Scholar]
- Ghazani AA, Lee JA, Klostranec J, Xiang Q, Dacosta RS, Wilson BC, Tsao MS, Chan WC. High throughput quantification of protein expression of cancer antigens in tissue microarray using quantum dot nanocrystals. Nano Lett. 2006;6:2881–2886. doi: 10.1021/nl062111n. [DOI] [PubMed] [Google Scholar]
- Oh E, Hong MY, Lee D, Nam SH, Yoon HC, Kim HS. Inhibition assay of biomolecules based on fluorescence resonance energy transfer (FRET) between quantum dots and gold nanoparticles. J Am Chem Soc. 2005;127:3270–3271. doi: 10.1021/ja0433323. [DOI] [PubMed] [Google Scholar]
- Gao XH, Yang LL, Petros JA, Marshal FF, Simons JW, Nie SM. In vivo molecular and cellular imaging with quantum dots. Curr Opin Biotech. 2005;16:63–72. doi: 10.1016/j.copbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Leatherdale CA, Woo WK, Mikulec FV, Bawendi MG. On the absorption cross section of CdSe nanocrystal quantum dots. J Phys Chem B. 2002;106:7619–7622. [Google Scholar]
- Dahan M, Laurence T, Pinaud F, Chemla DS, Alivisatos AP, Sauer M, Weiss S. Time-gated biological imaging by use of colloidal quantum dots. Opt Lett. 2001;26:825–827. doi: 10.1364/ol.26.000825. [DOI] [PubMed] [Google Scholar]
- Zhong XH, Feng YY, Knoll W, Han MY. Alloyed ZnxCd1-xS nanocrystals with highly narrow luminescence spectral width. J Am Chem Soc. 2003;125:13559–13563. doi: 10.1021/ja036683a. [DOI] [PubMed] [Google Scholar]
- Levenson RM. Spectral imaging and pathology: seeing more. Lab Med. 2004;35:244–251. [Google Scholar]
- Hiraoka Y, Shimi T, Haraguchi T. Multispectral imaging fluorescence microscopy for living cells. Cell Struct Funct. 2002;27:367–374. doi: 10.1247/csf.27.367. [DOI] [PubMed] [Google Scholar]
- Gao XH, Cui YY, Levenson RM, Chung LWK, Nie SM. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- Voura EB, Jaiswal JK, Mattoussi H, Simon SM. Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nat Med. 2004;10:993–998. doi: 10.1038/nm1096. [DOI] [PubMed] [Google Scholar]
- Fountaine TJ, Wincovitch SM, Geho DH, Garfield SH, Pittaluga S. Multispectral imaging of clinically relevant cellular targets in tonsil and lymphoid tissue using semiconductor quantum dots). Mod Pathol. 2006;19:1181–1191. doi: 10.1038/modpathol.3800628. [DOI] [PubMed] [Google Scholar]
- True LD. Quantitative immunohistochemistry: a new tool for surgical pathology? Am J Clin Pathol. 1988;90:324–325. doi: 10.1093/ajcp/90.3.324. [DOI] [PubMed] [Google Scholar]