Full text
PDF


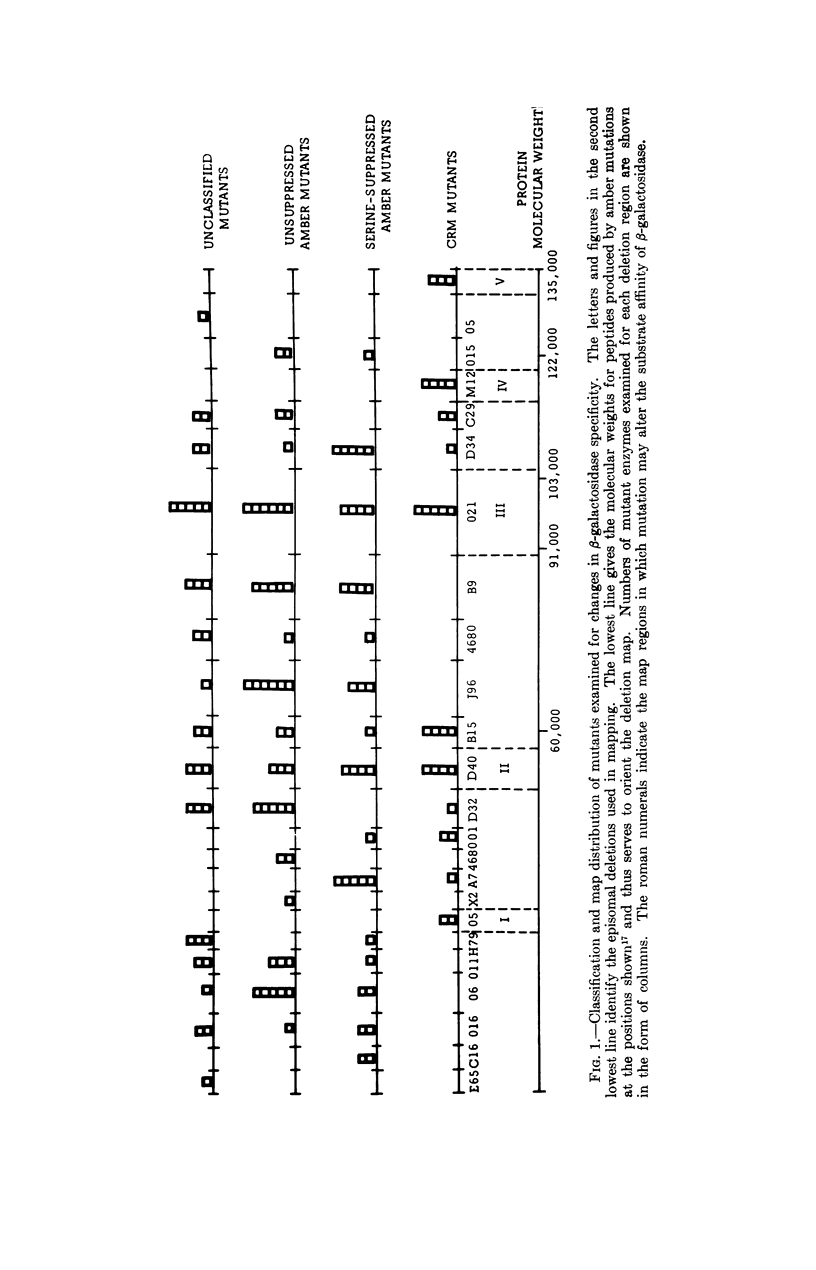




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKWITH J. R. A DELETION ANALYSIS OF THE LAC OPERATOR REGION IN ESCHERICHIA COLI. J Mol Biol. 1964 Mar;8:427–430. doi: 10.1016/s0022-2836(64)80206-0. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. Co-linearity of beta-galactosidase with its gene by immunological detection of incomplete polypeptide chains. Science. 1966 Nov 25;154(3752):1027–1029. doi: 10.1126/science.154.3752.1027. [DOI] [PubMed] [Google Scholar]
- HORIUCHI T., TOMIZAWA J. I., NOVICK A. Isolation and properties of bacteria capable of high rates of beta-galactosidase synthesis. Biochim Biophys Acta. 1962 Jan 22;55:152–163. doi: 10.1016/0006-3002(62)90941-1. [DOI] [PubMed] [Google Scholar]
- JACOB F., ULLMAN A., MONOD J. LE PROMOTEUR, 'EL'EMENT G'EN'ETIQUE N'ECESSAIRE 'A L'EXPRESSION D'UN OP'ERON. C R Hebd Seances Acad Sci. 1964 Mar 16;258:3125–3128. [PubMed] [Google Scholar]
- Johnson L. N., Phillips D. C. Structure of some crystalline lysozyme-inhibitor complexes determined by X-ray analysis at 6 Angstrom resolution. Nature. 1965 May 22;206(4986):761–763. doi: 10.1038/206761a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MUELLER-HILL B., RICKENBERG H. V., WALLENFELS K. SPECIFICITY OF THE INDUCTION OF THE ENZYMES OF THE LAC OPERON IN ESCHERICHIA COLI. J Mol Biol. 1964 Nov;10:303–318. doi: 10.1016/s0022-2836(64)80049-8. [DOI] [PubMed] [Google Scholar]
- Newton W. A., Beckwith J. R., Zipser D., Brenner S. Nonsense mutants and polarity in the lac operon of Escherichia coli. J Mol Biol. 1965 Nov;14(1):290–296. doi: 10.1016/s0022-2836(65)80250-9. [DOI] [PubMed] [Google Scholar]
- PERRIN D. Immunological studies with genetically altered beta-galactosidases. Ann N Y Acad Sci. 1963 May 8;103:1058–1066. doi: 10.1111/j.1749-6632.1963.tb53757.x. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Stadler J. THE ENZYMATIC ACTIVITY ASSOCIATED WITH THE PROTEIN IMMUNOLOGICALLY RELATED TO TRYPTOPHAN SYNTHETASE. Proc Natl Acad Sci U S A. 1958 Mar;44(3):245–253. doi: 10.1073/pnas.44.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAMENHOF S. Gene unstabilization induced by heat and by nitrous acid. J Bacteriol. 1961 Jan;81:111–117. doi: 10.1128/jb.81.1.111-117.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]



