Abstract
The peroxisome biogenesis disorders (PBDs), including Zellweger syndrome (ZS) and neonatal adrenoleukodystrophy (NALD), are autosomal recessive diseases caused by defects in peroxisome assembly, for which at least 10 complementation groups have been reported. We have isolated a human PEX1 cDNA (HsPEX1) by functional complementation of peroxisome deficiency of a mutant Chinese hamster ovary (CHO) cell line, ZP107, transformed with peroxisome targeting signal type 1-tagged “enhanced” green fluorescent protein. This cDNA encodes a hydrophilic protein (Pex1p) comprising 1,283 amino acids, with high homology to the AAA-type ATPase family. A stable transformant of ZP107 with HsPEX1 was morphologically and biochemically restored for peroxisome biogenesis. HsPEX1 expression restored peroxisomal protein import in fibroblasts from three patients with ZS and NALD of complementation group I (CG-I), which is the highest-incidence PBD. A CG-I ZS patient (PBDE-04) possessed compound heterozygous, inactivating mutations: a missense point mutation resulting in Leu-664 → Pro and a deletion of the sequence from Gly-634 to His-690 presumably caused by missplicing (splice site mutation). Both PBDE-04 PEX1 cDNAs were defective in peroxisome-restoring activity when expressed in the patient fibroblasts as well as in ZP107 cells. These results demonstrate that PEX1 is the causative gene for CG-I peroxisomal disorders.
The peroxisome, a single membrane-bounded ubiquitous organelle, contains more than 50 different enzymes catalyzing various metabolic pathways, including β-oxidation of very long chain fatty acids and the synthesis of ether lipids, and bile acids (1). Peroxisomes are formed by division of preexisting peroxisomes after posttranslational import of newly synthesized proteins (2). The primary cause for the peroxisome deficiency in fatal genetic diseases such as Zellweger syndrome (ZS) was thought to be failure in peroxisome biogenesis (3–5). Genetic heterogeneities are seen in subjects with these peroxisome-deficiency disorders. Twelve complementation groups (CGs) have been identified in mammals: ten of these were defined by analysis of patient-derived fibroblasts (3, 6, 7) and peroxisome-deficient Chinese hamster ovary (CHO) cell mutants (3, 8–12), and two additional ones were defined only by the use of CHO mutant cell lines (13). Hence more than 12 genes are likely to be involved in mammalian peroxisome biogenesis.
To investigate molecular mechanisms involved in peroxisome biogenesis and the genetic cause of peroxisome biogenesis disorders (PBDs) such as ZS and neonatal adrenoleukodystrophy (NALD), we have to date isolated seven CGs of peroxisome-deficient CHO cell mutants, including Z24 and ZP107 (9, 11), Z65 (9), ZP92 (3), ZP105 and ZP139 (11, 12), ZP104 and ZP109 (11), ZP110 (13), and ZP114 (13) (see Table 2). All mutants resemble fibroblasts from patients with PBD, with regard to defects in biogenesis and functions of peroxisomes. We cloned PEX2, PEX6, and PEX12 cDNAs by genetic phenotype-complementation assay of CHO cell mutants Z65, ZP92, and ZP109, respectively (14–16). Several groups of investigators, including ours, demonstrated five PEX genes—i.e., PEX2 (formerly PAF-1) (14), PEX5 coding for peroxisome targeting signal type 1 (PTS1) receptor, PEX6 (formerly PAF-2) (15), PEX7 for peroxisome targeting signal type 2 (PTS2) receptor, and PEX12—to be responsible for the genetic events in patients with PBD (16–25). Thus, peroxisome biogenesis-defective CHO cell mutants are a useful mammalian somatic cell system for investigation of peroxisome assembly at the molecular and cellular levels, as well as for elucidation of genetic causes of PBD (5).
Table 2.
Complementation of CHO cell mutants and patient fibroblasts by HsPEX1
| CHO mutant | Peroxisome-positive clone | Patient fibroblasts from CG | Peroxisome-positive | Gene |
|---|---|---|---|---|
| ZP107 | 24/30 | E (PBDE-04) (I) | + | PEX1 |
| Z24 | 9/30 | E (PBDE-13) (I) | + | |
| E (PBDE-14) (I) | 6/30 | |||
| ZP139 | − | II | − | PEX5 |
| ZP109 | − | III | − | PEX12 |
| ZP92 | − | C (IV) | − | PEX6 |
| VI | − | |||
| B (VII) | − | |||
| A (VIII) | − | |||
| D (IX) | − | |||
| Z65 | − | F (X) | − | PEX2 |
| G | − | |||
| ZP110 | − | |||
| ZP114 | − |
Peroxisome-deficient CHO mutants (5, 13), including CG-I CHO cell mutants ZP107 (11) and Z24 (9), PEX5-impaired ZP139 (12), PEX12-defective ZP109 (11, 16), PEX6-defective ZP92 (3, 15), and PEX2-impaired Z65 (14, 17, 43), and patient fibroblasts of ten groups of peroxisomal diseases (5)—i.e., CGs A, B, C, D, E (three patients, PBDE-04, PBDE-13, and PBDE-14), F, and G of Gifu University, Japan, and CG-II, -III, and -VI of the Kennedy–Krieger Institute, Baltimore, MD, were transfected with pCMVSPORT⋅HsPEX1 and examined for peroxisome assembly by immunostaining with antisera to rat and human catalase, respectively, at 3 days after transfection. Parentheses indicate cells of CG not used in this experiment. In CG-I cells, ZP107, Z24, and PBDE-14 fibroblasts, peroxisome-positive colonies were counted in 30 colonies; in other cells: +, complemented; −, not complemented.
Herein we describe the isolation of human PEX1 cDNA (HsPEX1), which restored peroxisome biogenesis in the CHO cell mutant ZP107, by a genetic complementation cloning strategy (14, 15, 17, 20) using “enhanced” green fluorescent protein (EGFP) (26). Peroxisomes were also complemented in peroxisome-deficient fibroblasts from patients with CG-I PBD by transfection of HsPEX1. In a CG-I patient, we identified the mutation sites that inactivated PEX1.
MATERIALS AND METHODS
Cell Lines.
Skin fibroblast cell lines from patients were transformed with simian virus 40 and cultured in complete medium [Dulbecco’s modified Eagle’s medium (DMEM) high glucose supplemented with 10% fetal calf serum] as described (20). CHO cell mutants, including Z24 and ZP107, were cultured as described (9). ZP107EG1, ZP107 stably expressing EGFP tagged with Ala-Lys-Leu (AKL) peroxisome targeting signal type 1, PTS1 (EGFP-PTS1), was isolated by transfecting pUcD2Hyg⋅EGFP-PTS1 (see below) followed by selection with 200 μg/ml hygromycin B.
Construction and Screening of a cDNA Library.
Human liver poly(A)+ RNA was purchased from CLONTECH and used for cDNA synthesis. cDNA was synthesized by using a SuperScript plasmid system (GIBCO/BRL). cDNA of a larger size was ligated into the vector pCMVSPORT I (GIBCO/BRL), by using SalI and NotI sites to unidirectionally insert cDNA under the cytomegalovirus (CMV) promoter. Cloned cDNA in pCMVSPORT was electroporated into ElectroMAX DH10B cells (GIBCO/BRL). The mean length of cDNA inserts of this library was ≈3.0 kb. The library was divided into small pools, each containing about 4,000 clones.
For transfection of cDNA library, ZP107EG1 cells were plated on a coverslip at 1 × 105 cells per well of a 6-well plate, 1 day before the transfection. Two micrograms of plasmid DNA and 12 μg of Lipofectamine (GIBCO/BRL) were separately diluted with 100 μl of serum-free medium, Opti-MEM (GIBCO/BRL). The following steps were done as recommended by the manufacturer. EGFP-PTS1 in cells was observed without fixation, under a Carl Zeiss Axioskop FL microscope with a no. 17 filter. Among the cDNA pools examined, a positive one (F6-17) that restored peroxisomes in ZP107EG1 was further divided into subpools and screened. A single positive clone, F6-17-6-34, was isolated from the subpool F6-17. The nucleotide sequence of both strands was determined by the dideoxynucleotide chain-termination method using a Dye-terminator DNA sequence kit (Applied Biosystems). Sequences were aligned by using the genetyx-mac program (SDC, Tokyo).
Transfection of PEX1.
CHO cell mutants were transfected with the plasmid F6-17-6-34, termed pCMVSPORT⋅HsPEX1, as described above. Fibroblasts (1 × 106 cells) derived from peroxisome-deficient patients were transfected with 20 μg of pCMVSPORT⋅HsPEX1, by using a Gene Pulser II electroporator (Bio-Rad) with settings at 320 V and 500 μF. Plasmid pUcD2Hyg⋅HsPEX1 was generated by inserting SalI–ApaI fragment (−67–4330) of pCMVSPORT⋅HsPEX1 into the pUcD2SRαMCSHyg vector (15) (K.O. et al., unpublished work). A stable clone of the HsPEX1 transformant of the mutant ZP107, 107P1, was isolated by transfection of pUcD2Hyg⋅HsPEX1 followed by selection with hygromycin B and limiting dilution.
Morphological Analysis.
Peroxisomes in CHO cells and human fibroblasts were visualized by indirect immunofluorescence light microscopy, as described (3). We used rabbit antibodies to rat liver catalase (9), human catalase (3), the PTS1 peptide comprising the C-terminal 10 amino acid residues of rat acyl-CoA oxidase (12), 3-ketoacyl-CoA thiolase (9), and 70-kDa peroxisomal integral membrane protein (PMP70) (9, 27). Antigen–antibody complex was detected by fluorescein isothiocyanate-labeled sheep antibody to rabbit IgG (Cappel), under a Carl Zeiss Axioskop FL microscope.
Mutation Analysis.
Poly(A)+ RNA was obtained from cultured patients’ fibroblasts by using a QuickPrep mRNA purification kit (Pharmacia Biotech). Reverse transcription (RT)-PCR using poly(A)+ RNA was done with a pair of human PEX1-specific PCR primers: sense RT1, CGCGGGCCCAGAGCGACGCTCCGGGACG, and antisense RT2, GGGGTACCGGGCCCACTTAACAGAACCAAATC, to cover a full length of PEX1 open reading frame. Mutation polymorphism was analyzed by using a set of RT-PCR primers: F5, ATGCATGCCGTAGTCAGG, and R6, GAGATTGCTGAGACTGAC, to amplify the sequence between nucleotide residues 1276 and 2152. The nucleotide sequence of the PCR products cloned in pBluescript was determined as above. Patient PEX1 cDNA was inserted into the pCMVSPORT I vector by replacing a BglII–XhoI fragment (residues 1327–3192) of PEX1 from a control with that of the patient PEX1 in pBluescript. Transfection was done on fibroblasts by electroporation and on ZP107 by lipofection.
Other Methods.
Western blot analysis was done with rabbit antibodies and a second antibody, donkey antibody to rabbit IgG conjugated to horseradish peroxidase (Amersham). Catalase latency assay using digitonin was done as described (9). For Northern blotting, the blot of poly(A)+ RNA from fibroblasts of a control and patient PBDE-04 was hybridized with a PstI–SalI fragment (nucleotide residues 2305–2990) of HsPEX1 labeled with [α-32P]dCTP (Amersham). P12/UV and P9OH/UV resistance was determined under conditions of 2 μM/1 min and 6 μM/2 min (3), respectively.
RESULTS
Cloning of a Human PEX1 cDNA.
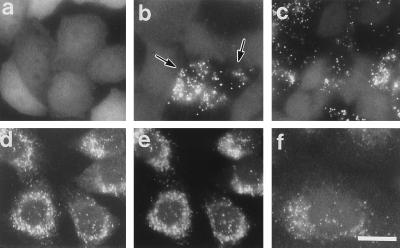
We used a transient expression assay as a cDNA cloning strategy (15, 16), using a human liver cDNA library and ZP107EG1 cells, a transformant of ZP107 stably expressing EGFP-PTS1. EGFP-PTS1 diffused in cytosol and nucleus in ZP107EG1 (Fig. 1a), whereas it was properly localized in peroxisomes in the wild-type CHO-K1 cells (data not shown), implying that EGFP-PTS1 is a useful marker protein for peroxisomal protein import. Peroxisome-restoring positive cDNA clones were searched for by directly observing peroxisomal localization of EGFP-PTS1 in unfixed ZP107EG1 cells. Twenty-seven pools (see Materials and Methods), which contained about 1.1 × 105 independent cDNA clones, were screened, and one pool (F6-17) yielded several peroxisome-restored cells of ZP107EG1 in a single dish (Fig. 1b, arrows). After a third round of screening, we isolated one positive clone, F6-17-6-34, containing a 4378-bp cDNA with an open reading frame encoding a 1283 amino acid protein of 142,865 Da (Fig. 2). A homology search suggested that this open reading frame was likely to be related to PEX1 from the yeasts Saccharomyces cerevisiae (28) and Pichia pastoris (29). We termed this cDNA HsPEX1. HsPEX1 complemented peroxisomal import of EGFP-PTS1 in ZP107EG1 (Fig. 1c). The human PEX1 protein, Pex1p, was 126 and 240 amino acids longer than the proteins of P. pastoris and S. cerevisiae, respectively (Fig. 2). The average amino acid identity to the Pex1p of P. pastoris and S. cerevisiae was 30% and 28%, respectively, in which the C-terminal half was more conserved (≈40%) than the N-terminal half (≈22%). Moreover, 60% identity was found in the region related to the AAA protein motifs of an ATPase family participating in a broad range of cellular processes (30–32). Moreover, the ATP/GTP-binding motifs, Walker motifs A and B, and AAA-protein family signature showed 90% homology, suggesting this region to be responsible for function of Pex1p. It is noteworthy that higher homology, ≈40% to 100%, was evident in these motifs between the human Pex1p and Pex6p, another AAA-family protein (20, 21), although the overall homology was only 23% (data not shown). Hydropathy analysis suggested that Pex1p is a hydrophilic protein with no apparent membrane-spanning region (data not shown).
Figure 1.
Complementation of peroxisomes in CG-I CHO mutant cells. (a–c) Intracellular location of EGFP in ZP107EG1 cells, ZP107 stably expressing EGFP-PTS1, was monitored on unfixed cells grown on a coverslip, under a fluorescence microscope. (d–f) Immunofluorescent staining of peroxisomes in 107P1 cells, stable HsPEX1-transformants of ZP107. (a) Peroxisome-deficient mutant ZP107EG1 cells. (b) Peroxisome-restored ZP107EG1, after lipofection with a subpool (F6-17) of human cDNA library. Arrows indicate the complemented cells. Cytosolic appearance of EGFP-PTS1 was apparent in the other cells. (c) ZP107EG1 transfected with pUcD2Hyg⋅HsPEX1 plasmid. (d and e) 107P1 cells were stained with goat antibody to rat catalase plus donkey antibody to goat IgG conjugated to rhodamine (Chemicon) and antibody to PMP70, respectively. Note that punctate structures (peroxisomes) stained in d and e are superimposable. (f) 107P1 cells stained with antibody to 3-ketoacyl-CoA thiolase. (×630; bar = 20 μm.)
Figure 2.
Deduced amino acid sequence alignment of human (Hs) PEX1 protein and Pex1p from the yeasts Pichia pastoris (Pp) and Saccharomyces cerevisiae (Sc). A hyphen indicates a space. Amino acids identical between human and other species are shaded. Boxed are ATP/GTP-binding Walker motifs A (A-1 and A-2: A/GX4GKS/T; X, any residue), motifs B (B-1 and B-2: R/K/HXn>5ΦXΦ2D/E; Φ, hydrophobic residue), and AAA-protein family signature (C: A/G/M/ΦXTXR/H/ΦXD/E/N/SXL/I/V/MDXAL/I/V/MXRXGRL/IV/M/Φ/YD/E), respectively. Open and solid arrowheads indicate the position of mutation in each allele from a CG-I patient, PBDE-04 (see Fig. 5A). The database accession number for the human PEX1 cDNA is AB008112.
PEX1 Restored Peroxisome Biogenesis in ZP107.
In a stable HsPEX1-transformant of ZP107, named 107P1, numerous particles, presumably peroxisomes, were detected by immunofluorescent staining of catalase, and the particles were superimposable on those stained with anti-PMP70 antibody (Fig. 1 d and e). Peroxisomal particles positive for thiolase, a PTS2 protein, were also as numerous as those seen by immunostaining of catalase and PMP70 (Fig. 1f). These results demonstrated that 107P1 cells had morphologically normal peroxisomes, as seen in the wild-type CHO-K1 cells.
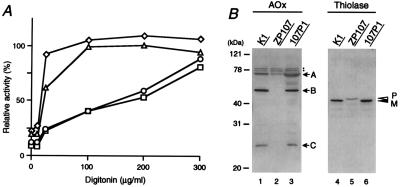
In peroxisome-deficient cells, peroxisomal proteins are mislocalized to the cytosol, rapidly degraded, or not converted to mature forms, despite normal synthesis (3, 8, 9, 11–13). In the digitonin titration assay, nearly 60% of catalase activity was latent at the digitonin concentration of 100 μg/ml in the wild-type, findings consistent with our earlier observation (9, 11–13) (Fig. 3A; Table 1). In ZP107 cells, full activity of catalase was detected at 100 μg/ml of digitonin, as was activity of a cytosolic enzyme, lactate dehydrogenase, indicating that catalase was present in the cytosol (Fig. 3A), compatible with morphological observation (11). In 107P1 cells, catalase showed the same latency as in the wild-type CHO-K1 cells. Moreover, 107P1 was resistant to P12/UV [12-(1′-pyrene)dodecanoic acid/long wavelength ultraviolet light] treatment (3, 33) and sensitive to P9OH/UV [9-(1′-pyrene)nonanol/UV] (3, 34), as was the case for CHO-K1 cells (Table 1). In contrast, mutant ZP107 was resistant to P9OH/UV-treatment and sensitive to P12/UV. These properties are characteristics for wild-type and peroxisome-defective mutants (3, 11–13). Acyl-CoA oxidase is synthesized as a 75-kDa polypeptide (A component) and is proteolytically converted into 53-kDa and 22-kDa polypeptides (B and C, respectively) in peroxisomes (35). All three polypeptide components, A, B, and C, were evident in CHO-K1 as well as 107P1 (Fig. 3B, lanes 1 and 3), but only the A component was seen in ZP107, and it was in a much smaller amount (lane 2). Peroxisomal 3-ketoacyl-CoA thiolase is synthesized as a larger precursor with an N-terminal, cleavable PTS2 (36, 37). In wild-type CHO-K1 and 107P1 cells, only the mature thiolase was detected (Fig. 3B, lanes 4 and 6), thereby reflecting rapid processing of the precursor form. In ZP107 cells, only the larger precursor was found (lane 5), implying a defect of import and processing activity.
Figure 3.
Restoration of peroxisome biogenesis. (A) Latency of catalase activity in CHO-K1, ZP107, and 107P1 cells. Catalase: ○, CHO-K1; ▵, ZP107; □, 107P1. Lactate dehydrogenase in ZP107 is shown by ⋄. Relative free enzyme activity is expressed as a percentage of the total activity measured in the presence of 1% Triton X-100 (9). The results represent a mean of duplicate assays. (B) Biogenesis of peroxisomal proteins. Cell lysates (≈1.8 × 105 cells) were subjected to SDS/PAGE and transferred to polyvinylidene difluoride (PVDF) membrane. Cell types are indicated at the top. Immunoblot analysis used rabbit antibodies to rat acyl-CoA oxidase (AOx) and 3-ketoacyl-CoA thiolase (Thiolase). Arrows show the positions of AOx polypeptide components A, B, and C; open and solid arrowheads indicate a larger precursor (P) and mature protein (M) of 3-ketoacyl-CoA thiolase, respectively. Dots indicate nonspecific bands.
Table 1.
Properties of wild-type CHO-K1, ZP107, and HsPEX1-transfected ZP107 (107P1) cells
| Cell | Peroxisome | Catalase latency, % | P12/UV, % | P9OH/UV, % |
|---|---|---|---|---|
| CHO-K1 | + | 60 | 85 | <0.001 |
| ZP107 | − | 0.8 | <0.1 | 92 |
| 107P1 | + | 59 | 93 | <0.1 |
Catalase latency represents peroxisomal catalase, calculated as described (9). For determination of resistance to 12-(1′-pyrene)dodecanoic acid (P12)/UV or 9-(1′-pyrene)nonanol (P9OH)/UV, 200 or 1 × 105 cells were inoculated into 60-mm dishes and selected (3). The numbers of colonies were counted in duplicate experiments and expressed as percentages of the number in the unselected control.
Taken together, these results indicate that HsPEX1 can fully complement the ZP107 mutation. HsPEX1 expression also complemented peroxisome assembly in Z24 cells, the same CG as ZP107, but did not restore peroxisomes in six other CGs of peroxisome-deficient CHO cell mutants thus far isolated (3, 9, 11, 13)—i.e., Z65, ZP92, ZP105, ZP109, ZP110, and ZP114, confirming that Pex1p is a peroxisome biogenesis factor for ZP107/Z24 (Table 2).
PEX1 Specifically Complements Fibroblasts from CG-I Patients.
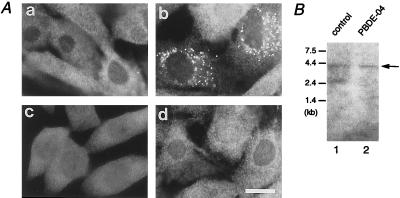
Z24 and ZP107 were found to belong to the same CG as CG-I ZS, by cell fusion analysis (3, 11). HsPEX1 was introduced into fibroblasts of ten CGs of peroxisomal diseases, i.e., CGs, A, B, C, D, E, F, and G of Gifu University, Japan, and groups I (the same as E), II, III, and VI of the Kennedy–Krieger Institute, Baltimore, MD. As expected, the fibroblasts derived from a CG-I patient (PBDE-04) were morphologically restored for peroxisome assembly (Fig. 4A, a and b), but none of the fibroblasts from the other nine CGs were complemented (Table 2). Fibroblasts from two unrelated NALD and ZS patients (PBDE-13 and PBDE-14) of CG-I were also complemented by HsPEX1 (Table 2). These results strongly suggested that HsPEX1 is the causal gene of CG-I peroxisome-deficiency disorders.
Figure 4.
Complementation of fibroblasts from a CG-I ZS patient. (A) Transfection of PEX1 from a normal control and a CG-I patient (PBDE-04) with ZS. (a) PBDE-04 fibroblasts. (b) PBDE-04 fibroblasts were transfected with pUcD2Hyg⋅HsPEX1. (c) ZP107 was transfected with PEX1 cDNA, PEX1L664P, derived from PBDE-04. (d) PBDE-04 fibroblasts were back-transfected with PBDE-04-derived PEX1 cDNA, PEX1e/s. Cells were stained with antisera to human catalase (a, b, and d) or PTS1 peptide (c). Note that peroxisomes were restored in b, but not in c and d. (×630; bar, 20 μm.) (B) Northern blot analysis of PEX1 mRNA. Poly(A)+ RNA (1 μg) from fibroblasts of a control (lane 1) and the patient PBDE-04 (lane 2) was separated, transferred to Zeta-Probe GT membrane (Bio-Rad), and hybridized with 32P-labeled cDNA probes for human PEX1. Washing was done twice with 0.15 M NaCl/10 mM sodium phosphate, pH 7.4/1 mM EDTA/0.5% SDS at 55°C. Arrow indicates PEX1 mRNA; the identity of the 2.5-kb band in the control is presently unknown. Exposure, 4 days.
CG-I Patient Analysis.
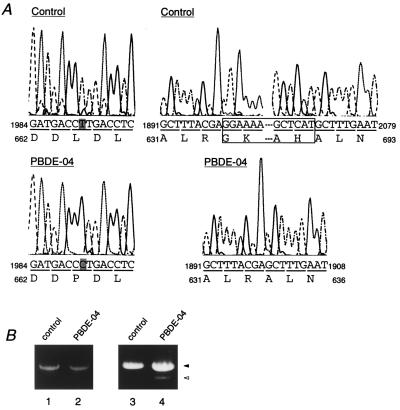
On Northern blotting, PEX1 mRNA was detected as a single band of about 4.3 kb in a control and in CG-I patient PBDE-04, suggesting that the transcription of PEX1 in this patient was not apparently affected (Fig. 4B). To determine dysfunction of PEX1 in this patient, we isolated PEX1 cDNA from the fibroblasts, by means of RT-PCR. A compound heterozygous mutation was detected by subsequent sequencing: in one allele, a missense point mutation, T-1991 to C in the codon for Leu-664, resulting in Pro-664, named PEX1L664P; in another allele, a 171-bp deletion of nucleotide residues 1900 to 2070, presumably because of defective splicing, named PEX1e/s (Fig. 5A). All of the six cDNA clones isolated from each allele showed the same site mutation, evidently implying that the patient was a compound heterozygote for the mutation. A single band with the expected size was obtained when mRNA from a normal control and patient PBDE-04 was amplified with primers RT1 and RT2 (Fig. 5B, lanes 1 and 2). When primers F5 and R6 were used, a single band with ≈0.9 kb was detected in a control, whereas two products with a normal size and a smaller were obtained with the patient (Fig. 5B, lanes 3 and 4). Direct sequencing of the smaller product revealed a 171-base deletion, exactly the same sequence as a part of PEX1e/s (data not shown). These two types of mutations apparently inactivated PEX1, as assessed by back-transfection of PEX1L664P and PEX1e/s, to ZP107 and PBDE-04 fibroblasts, respectively (Fig. 4A, c and d). PEX1L664P and PEX1e/s were likewise incompetent in restoring peroxisome biogenesis in PBDE-04 fibroblasts and ZP107, respectively (not shown). These findings imply the importance of structural configuration and a AAA-family motif, B-1, of Pex1p in its biological activity. Collectively, these data demonstrate that the dysfunction of PEX1 is responsible for peroxisome deficiency in CG-I disorders.
Figure 5.
Mutation analysis of PEX1 from a CG-I patient. (A) Nucleotide sequence analysis of PBDE-04 PEX1. Partial sequence and deduced amino acid sequence of PEX1 cDNA isolated from patient PBDE-04 and a normal control are shown. (Left) One-base point mutation (shaded), T to C in the codon for Leu-664, resulting in a codon for Pro-664 in one allele (see the open arrowhead in Fig. 2). (Right) A 171-bp deletion of nucleotide residues 1900–2070 (boxed), in another allele (solid arrowheads in Fig. 2). (B) RT-PCR analysis of PEX1 transcript from a control and PBDE-04. RT-PCR of poly(A)+ RNA was done with two sets of primers, RT1 and RT2 to amplify full-length PEX1 (lanes 1 and 2), and F5 and R6 to amplify a partial PEX1 fragment, nucleotide residues from 1276 to 2152 (lanes 3 and 4). Lanes: 1 and 3, a control; 2 and 4, PBDE-04. Note that two products with a normal size, ≈0.9 kb (solid arrowhead), and a smaller, ≈0.7 kb (open arrowhead), were obtained in PBDE-04 (lane 4).
DISCUSSION
In the present work, we isolated a human Pex1p cDNA by functional complementation of a peroxisome-deficient CHO cell mutant, ZP107. ZP107 cells were restored for peroxisome assembly, by both morphological and biochemical criteria. Complementation of peroxisome deficiency only in fibroblasts from CG-I patients strongly suggested that the PEX1 is the causal gene of this group. We delineated the PEX1 in a CG-I patient: a missense point mutation resulting in Leu-664 → Pro and a deletion of the sequence from Gly-634 to His-690, presumably caused by splice site mutation. The findings by back-transfection of these mutated forms of PEX1 into the patient’s fibroblasts and CHO mutant ZP107 confirmed that impaired PEX1 is the genetic cause of CG-I disorders. Thereby, PEX1 is the fifth gene thus far identified responsible for the peroxisome-deficiency diseases (Table 2). CG-I has the largest incidence of ten PBD genotypes thus far reported (7). Such higher incidence may simply be inferred from the rather larger size of PEX1 gene presumed from the mass of Pex1p, 143 kDa, resulting in higher risk of mutation. It is also possible that site(s) with a high frequency of mutation, so-called hot spot(s), are present in the PEX1 gene.
Pex1p and Pex6p are members of a growing family of proteins known as the AAA-type ATPases (30–32), but they are in two distinct complementation groups—i.e., CG-I and CG-IV (ref. 15 and this study). Deletion analysis of PEX1 and PEX6 and mutual domain shuffling experiments may provide pertinent clues to understanding their structural and functional relationship. Peroxisomal remnants, “ghosts,” were present in CG-I CHO cell mutants (3) and patients’ fibroblasts (38–40), implying that membrane protein transport was not affected, consistent with the notion in other CGs (5). Pex1p may function as a factor in matrix protein-translocation process, possibly by interacting with other PEX proteins (5, 41, 42), including not only another AAA ATPase, Pex6p (15, 20, 21), but also Pex2p (14, 17, 43), Pex5p (PTS1 receptor) (12, 18, 19, 44), Pex7p (PTS2 receptor) (22–24), and Pex12p (16, 25). It is equally possible that Pex1p is involved in membrane recognition and fusion events in peroxisome assembly, in concert with Pex6p, probably requiring the activity of these AAA ATPases. Such AAA factors have recently been identified, including those in vesicular transport (31, 32). It would be intriguing to investigate at which step(s) Pex1p and Pex6p function in peroxisome biogenesis.
Two point mutations (a donor splice site mutation and a missense mutation) in the gene for PMP70, one of each mutation in one allele of two ZS patients, were noted in 21 CG-I patients (45). However, peroxisome assembly was not complemented by a stable transfection of a human PMP70 cDNA into fibroblasts from seven patients of CG-E (the same group as CG-I) (46). The functional significance of PMP70 in peroxisome biogenesis remains ambiguous.
We have shown that a cloning strategy using a mammalian expression vector is highly efficient in delineating peroxisome biogenesis factors in mammals (refs. 14–17 and 20, and the present work). We introduced EGFP to further accelerate the screening process for the cDNA clone that restores peroxisome assembly in mutant cells. We detected such cells without treatment with fixatives such as formaldehyde, as was the successfully introduced GFP in PEX12 cDNA cloning (ref. 16 and K.O. et al., unpublished work). A modified, efficient genetic function-complementation assay described in this report can now make it readily possible to isolate cDNA clones of other mammalian peroxisome assembly factors. Generally, isolation of mutants and gene cloning by genetic complementation are much more readily facilitated by using yeast than by using mammalian cells. While human homologues of yeast genes have recently been cloned by EST (expressed sequence tag) search, direct cDNA cloning by functional complementation is an independent, parallel approach that often complements the cloning of human cDNAs of interest by using EST search.
Acknowledgments
This work was done with the technical assistance of T. Sakaguchi and N. Matsumoto. We thank the members of the Fujiki laboratory for comments. This work was supported in part by CREST grant (to Y.F.) from the Science and Technology Corporation of Japan; Grants-in-Aid for Scientific Research (07408016, 08249232 and 08557011 to Y.F.) from The Ministry of Education, Science, Sports and Culture.
ABBREVIATIONS
- CG
complementation group
- CHO
Chinese hamster ovary
- CMV
cytomegalovirus
- EGFP
enhanced green fluorescent protein
- NALD
neonatal adrenoleukodystrophy
- P9OH/UV
9-(1′-pyrene)nonanol/ultraviolet
- P12
12-(1′-pyrene)dodecanoic acid
- PBD
peroxisome biogenesis disorders
- PEX1
cDNA encoding the peroxin Pex1p
- PMP70
70-kDa peroxisomal integral membrane protein
- PTS1 and PTS2
peroxisome targeting signal types 1 and 2
- RT
reverse transcription
- ZS
Zellweger syndrome
Note
While this manuscript was in review, two papers (47, 48) reported mutations in CG-I patients of PEX1 isolated by homology search on a human EST database, using yeast PEX1.
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AB008112).
References
- 1.van den Bosch H, Schutgens R B H, Wanders R J A, Tager J M. Annu Rev Biochem. 1992;61:157–197. doi: 10.1146/annurev.bi.61.070192.001105. [DOI] [PubMed] [Google Scholar]
- 2.Lazarow P B, Fujiki Y. Annu Rev Cell Biol. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- 3.Shimozawa N, Tsukamoto T, Suzuki Y, Orii T, Fujiki Y. J Clin Invest. 1992;90:1864–1870. doi: 10.1172/JCI116063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazarow P B, Moser H W. In: The Metabolic Basis of Inherited Disease. 7th Ed. Scriver C R, Beaudet A I, Sly W S, Valle D, editors. New York: McGraw-Hill; 1995. pp. 2287–2324. [Google Scholar]
- 5.Fujiki Y. Biochim Biophys Acta. 1997;1361:235–250. doi: 10.1016/s0925-4439(97)00051-3. [DOI] [PubMed] [Google Scholar]
- 6.Poulos A, Christodoulou J, Chow C W, Goldblatt J, Paton B C, Orii T, Suzuki Y, Shimozawa N. J Pediatr. 1995;127:596–599. doi: 10.1016/s0022-3476(95)70121-4. [DOI] [PubMed] [Google Scholar]
- 7.Moser A B, Rasmussen M, Naidu S, Watkins P A, McGuiness M, Hajra A K, Chen G, Raymond G, Liu A, Gordon D, Garnaas K, Walton D S, Skjeldal O H, Guggenheim M A, Jackson L G, Elias E R, Moser H W. J Pediatr. 1995;127:13–22. doi: 10.1016/s0022-3476(95)70250-4. [DOI] [PubMed] [Google Scholar]
- 8.Zoeller R A, Allen L-A H, Santos M J, Lazarow P B, Hashimoto T, Tartakoff A M, Raetz C R H. J Biol Chem. 1989;264:21872–21878. [PubMed] [Google Scholar]
- 9.Tsukamoto T, Yokota S, Fujiki Y. J Cell Biol. 1990;110:651–660. doi: 10.1083/jcb.110.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsukamoto T, Bogaki A, Okumoto K, Tateishi K, Fujiki Y, Shimozawa N, Suzuki Y, Kondo N, Osumi T. Biochem Biophys Res Commun. 1997;230:402–406. doi: 10.1006/bbrc.1996.5971. [DOI] [PubMed] [Google Scholar]
- 11.Okumoto K, Bogaki A, Tateishi K, Tsukamoto T, Osumi T, Shimozawa N, Suzuki Y, Orii T, Fujiki Y. Exp Cell Res. 1997;233:11–20. doi: 10.1006/excr.1997.3552. [DOI] [PubMed] [Google Scholar]
- 12.Otera H, Tateishi K, Okumoto K, Ikoma Y, Matsuda E, Nishimura M, Tsukamoto T, Osumi T, Ohashi K, Higuchi O, Fujiki Y. Mol Cell Biol. 1998;18:388–399. doi: 10.1128/mcb.18.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tateishi K, Okumoto K, Shimozawa N, Tsukamoto T, Osumi T, Suzuki Y, Kondo N, Okano I, Fujiki Y. Eur J Cell Biol. 1997;73:352–359. [PubMed] [Google Scholar]
- 14.Tsukamoto T, Miura S, Fujiki Y. Nature (London) 1991;350:77–81. doi: 10.1038/350077a0. [DOI] [PubMed] [Google Scholar]
- 15.Tsukamoto T, Miura S, Nakai T, Yokota S, Shimozawa N, Suzuki Y, Orii T, Fujiki Y, Sakai F, Bogaki A, Yasumo H, Osumi T. Nat Genet. 1995;11:395–401. doi: 10.1038/ng1295-395. [DOI] [PubMed] [Google Scholar]
- 16.Okumoto K, Fujiki Y. Nat Genet. 1997;17:265–266. doi: 10.1038/ng1197-265. [DOI] [PubMed] [Google Scholar]
- 17.Shimozawa N, Tsukamoto T, Suzuki Y, Orii T, Shirayoshi Y, Mori T, Fujiki Y. Science. 1992;255:1132–1134. doi: 10.1126/science.1546315. [DOI] [PubMed] [Google Scholar]
- 18.Dodt G, Braverman N, Wong C S, Moser A, Moser H W, Watkins P, Valle D, Gould S J. Nat Genet. 1995;9:115–125. doi: 10.1038/ng0295-115. [DOI] [PubMed] [Google Scholar]
- 19.Wiemer E A, Nuttley W M, Bertolaet B L, Li X, Francke U, Wheelock M J, Anne U K, Johnson K R, Subramani S. J Cell Biol. 1995;130:51–65. doi: 10.1083/jcb.130.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuda S, Shimozawa N, Suzuki Y, Tomatsu S, Tsukamoto T, Hashiguchi N, Osumi T, Masuno M, Imaizumi K, Kuroki Y, Fujiki Y, Orii T, Kondo N. Am J Hum Genet. 1996;59:1210–1220. [PMC free article] [PubMed] [Google Scholar]
- 21.Yahraus T, Braverman N, Dodt G, Kalish J E, Morrell J C, Moser H W, Valle D, Gould S J. EMBO J. 1996;15:2914–2923. [PMC free article] [PubMed] [Google Scholar]
- 22.Braverman N, Steel G, Obie C, Moser A, Moser H, Gould S J, Valle D. Nat Genet. 1997;15:369–376. doi: 10.1038/ng0497-369. [DOI] [PubMed] [Google Scholar]
- 23.Motley A M, Hettema E H, Hogenhout E M, Brites P, ten Asbroek A L M A, Wijburg F A, Baas F, Heijmans H S, Tabak H F, Wanders R J A, Distel B. Nat Genet. 1997;15:377–380. doi: 10.1038/ng0497-377. [DOI] [PubMed] [Google Scholar]
- 24.Purdue P E, Zhang J W, Skoneczny M, Lazarow P B. Nat Genet. 1997;15:381–384. doi: 10.1038/ng0497-381. [DOI] [PubMed] [Google Scholar]
- 25.Chang C-C, Lee W-H, Moser H, Valle D, Gould S J. Nat Genet. 1997;15:385–388. doi: 10.1038/ng0497-385. [DOI] [PubMed] [Google Scholar]
- 26.Cormack B P, Valdivia R H, Falkow S. Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 27.Fujiki Y, Fowler S, Shio H, Hubbard A L, Lazarow P B. J Cell Biol. 1982;93:103–110. doi: 10.1083/jcb.93.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erdmann R, Wiebel F F, Flessau A, Rytka J, Beyer A, Frohlich K U, Kunau W H. Cell. 1991;64:499–510. doi: 10.1016/0092-8674(91)90234-p. [DOI] [PubMed] [Google Scholar]
- 29.Heyman J A, Mononsov E, Subramani S. J Cell Biol. 1994;127:1259–1273. doi: 10.1083/jcb.127.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunau W-H, Beyer A, Franken T, Goette K, Marzioch M, Saidowsky J, Skaletz-Rorowski A, Wiebel F F. Biochimie. 1993;75:209–224. doi: 10.1016/0300-9084(93)90079-8. [DOI] [PubMed] [Google Scholar]
- 31.Confalonieri F, Duguet M. BioEssays. 1995;17:639–650. doi: 10.1002/bies.950170710. [DOI] [PubMed] [Google Scholar]
- 32.Rowe T, Balch W E. Nature (London) 1997;388:20–21. doi: 10.1038/40269. [DOI] [PubMed] [Google Scholar]
- 33.Zoeller R A, Morand O H, Raetz C R H. J Biol Chem. 1988;263:11590–11596. [PubMed] [Google Scholar]
- 34.Morand O H, Allen L-A H, Zoeller R A, Raetz C R H. Biochim Biophys Acta. 1990;1034:132–141. doi: 10.1016/0304-4165(90)90066-6. [DOI] [PubMed] [Google Scholar]
- 35.Miyazawa S, Osumi T, Hashimoto T, Ohno K, Miura S, Fujiki Y. Mol Cell Biol. 1989;9:83–91. doi: 10.1128/mcb.9.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osumi T, Tsukamoto T, Hata S, Yokota S, Miura S, Fujiki Y, Hijikata M, Miyazawa S, Hashimoto T. Biochem Biophys Res Commun. 1991;181:947–954. doi: 10.1016/0006-291x(91)92028-i. [DOI] [PubMed] [Google Scholar]
- 37.Swinkels B W, Gould S J, Bodnar A G, Rachubinski R A, Subramani S. EMBO J. 1991;10:3255–3262. doi: 10.1002/j.1460-2075.1991.tb04889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiemer E A C, Brul S, Just W W, van Driel R, Brouwer-Kelder E, van den Berg M, Weijers P J, Schutgens R B H, van den Bosch H, Schram A, Wanders R J A, Tager J M. Eur J Cell Biol. 1989;50:407–417. [PubMed] [Google Scholar]
- 39.Santos M J, Hoefler S, Moser A B, Moser H W, Lazarow P B. J Cell Physiol. 1992;151:103–112. doi: 10.1002/jcp.1041510115. [DOI] [PubMed] [Google Scholar]
- 40.Wendland M, Subramani S. J Clin Invest. 1993;92:2462–2468. doi: 10.1172/JCI116854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Distel B, Erdmann R, Gould S J, Blobel G, Crane D I, Cregg J M, Dodt G, Fujiki Y, Goodman J M, Just W W, Kiel J A K W, Kunau W-H, Lazarow P B, Mannaerts G P, Moser H, Osumi T, Rachubinski R A, Roscher A, Subramani S, Tabak H F, Tsukamoto T, Valle D, van der Klei I, van Veldhoven P P, Veenhuis M. J Cell Biol. 1996;135:1–3. doi: 10.1083/jcb.135.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subramani S. Nat Genet. 1997;15:331–333. doi: 10.1038/ng0497-331. [DOI] [PubMed] [Google Scholar]
- 43.Tsukamoto T, Shimozawa N, Fujiki Y. Mol Cell Biol. 1994;14:5458–5465. doi: 10.1128/mcb.14.8.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fransen M, Brees C, Baumgart E, Vanhooren J C, Baes M, Mannaerts G P, van Veldhoven P P. J Biol Chem. 1995;270:7731–7736. doi: 10.1074/jbc.270.13.7731. [DOI] [PubMed] [Google Scholar]
- 45.Gaertner J, Moser H, Valle D. Nat Genet. 1992;1:16–23. doi: 10.1038/ng0492-16. [DOI] [PubMed] [Google Scholar]
- 46.Shimozawa N, Suzuki Y, Tomatsu S, Tsukamoto T, Osumi T, Fujiki Y, Kamijo K, Hashimoto T, Kondo N, Orii T. Pediatr Res. 1996;39:812–815. doi: 10.1203/00006450-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 47.Reuber B E, Germain-Lee E, Collins C S, Morrell J C, Ameritunga R, Moser H W, Valle D, Gould S J. Nat Genet. 1997;17:445–448. doi: 10.1038/ng1297-445. [DOI] [PubMed] [Google Scholar]
- 48.Portsteffen H, Beyer A, Becker E, Epplen C, Pawlak A, Kunau W-H, Dodt G. Nat Genet. 1997;17:449–452. doi: 10.1038/ng1297-449. [DOI] [PubMed] [Google Scholar]