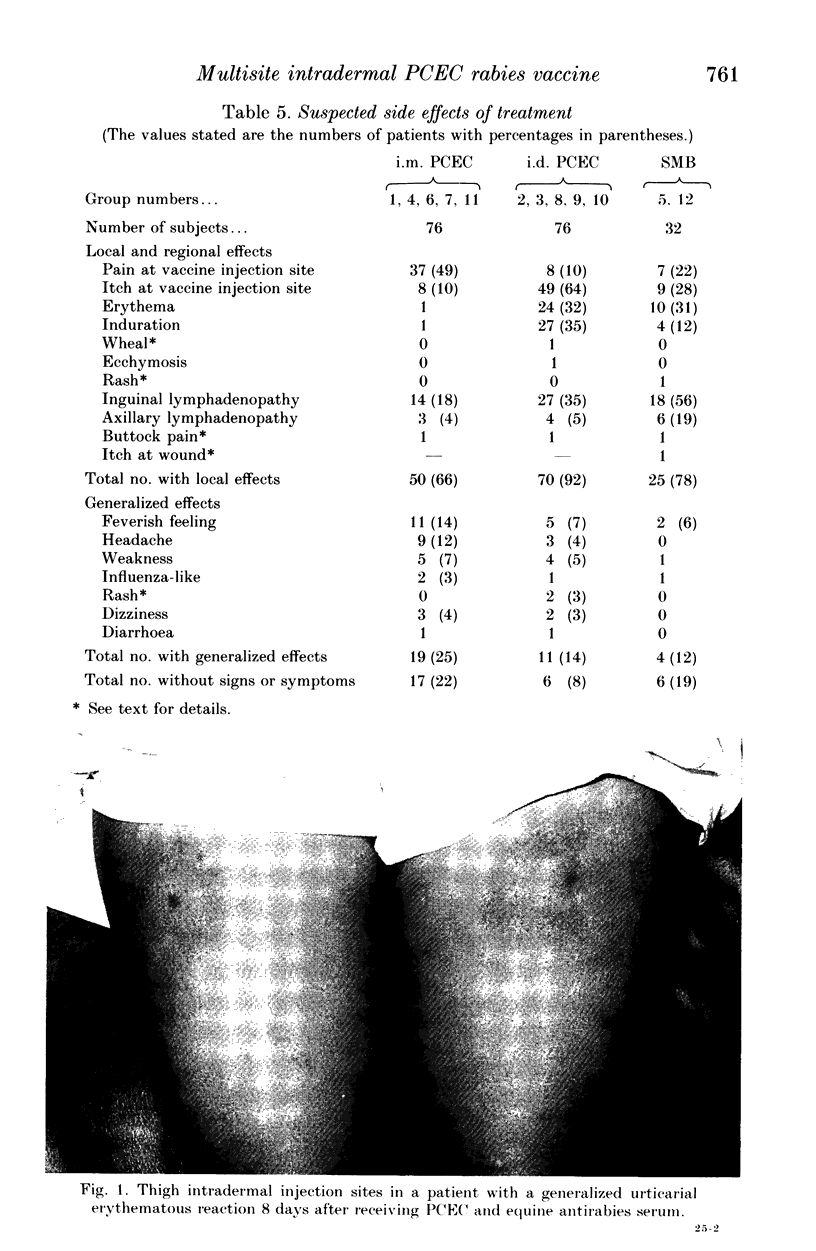
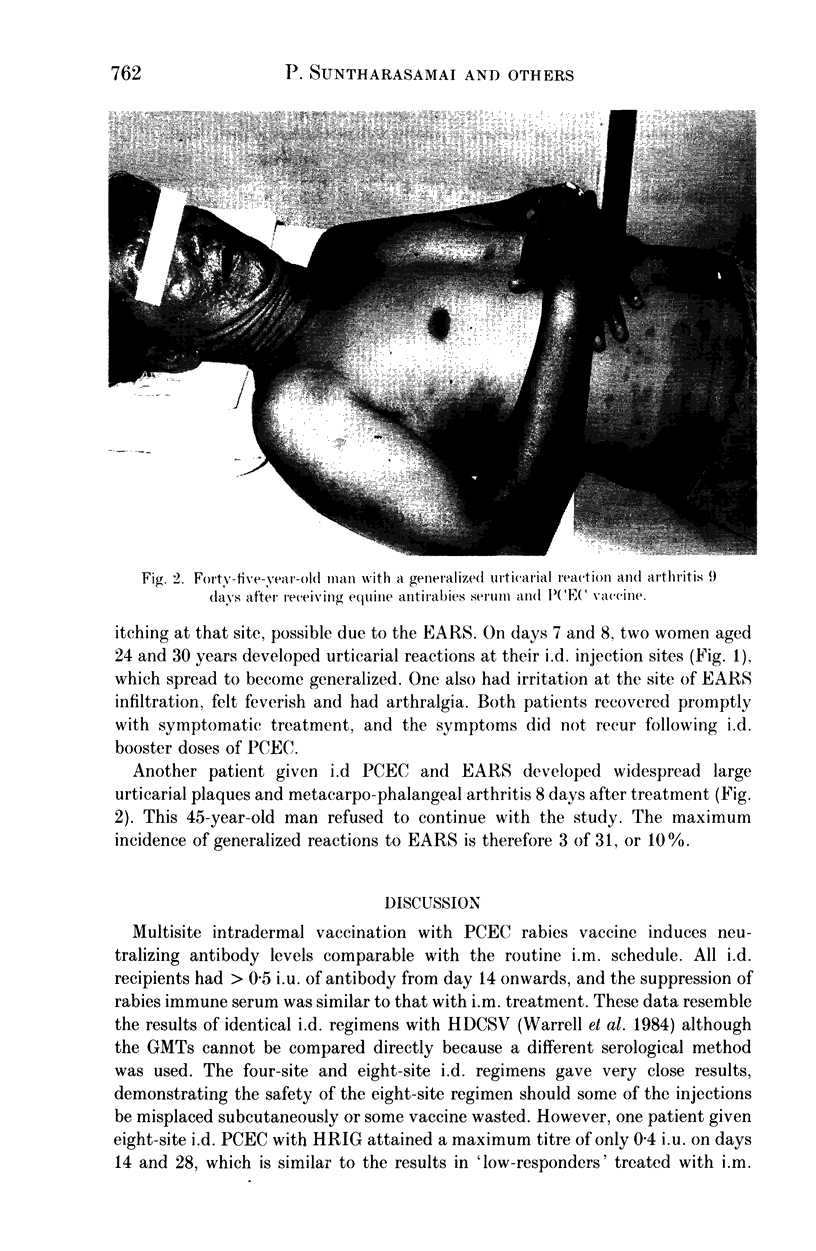
Abstract
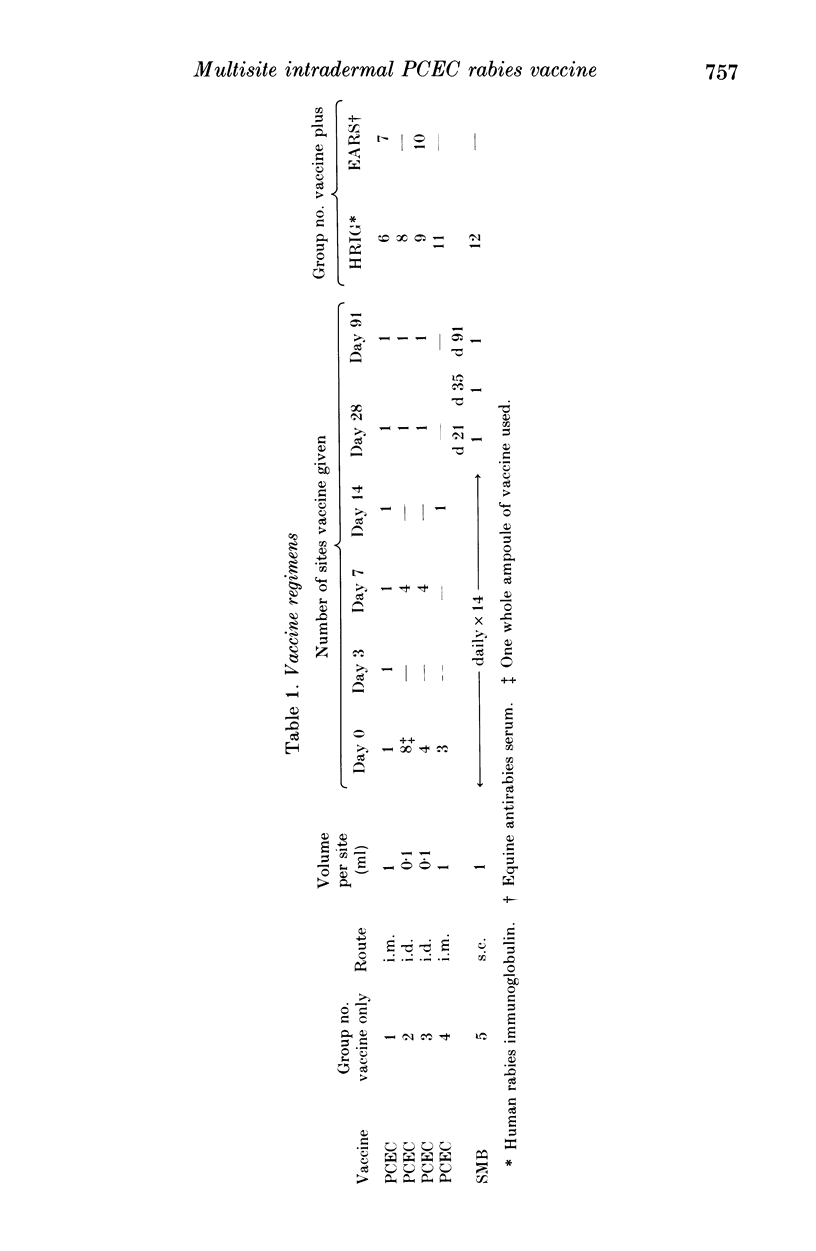
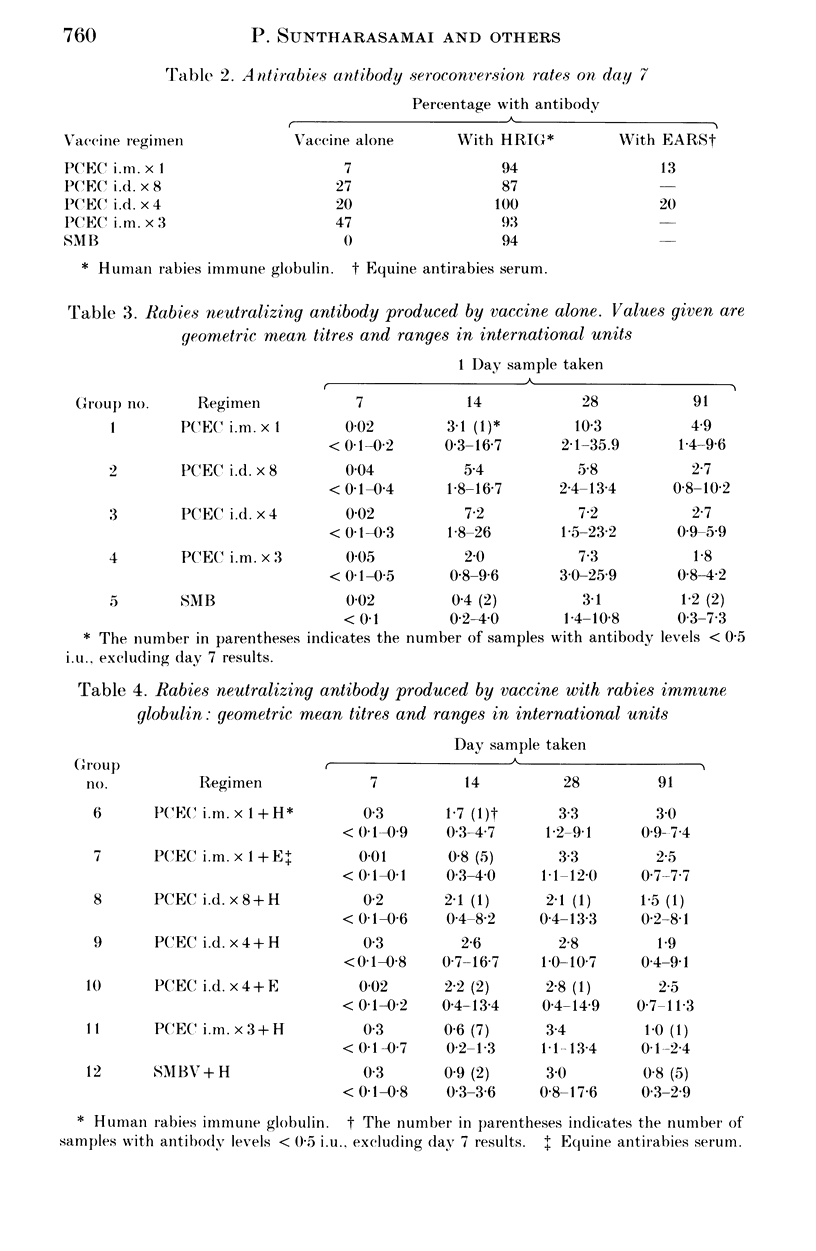
The standard six-dose intramuscular (i.m.) rabies post-exposure vaccine regimen using a new purified chick embryo cell (PCEC) vaccine was compared with two economical multisite intradermal (i.d.) PCEC regimens, a multisite i.m. PCEC schedule and a subcutaneous regimen using a suckling mouse brain (SMB) rabies vaccine manufactured in Thailand. The neutralizing antibody results for the four-site and eight-site i.d. and the standard i.m. PCEC regimens were similar over 3 months. A three-site i.m. PCEC regimen had no advantage. The SMB vaccine gave the lowest antibody levels. Human rabies immune globulin therapy significantly increased the GMT of all groups on day 7, unlike equine antirabies serum (EARS). Both antisera suppressed antibody responses to PCEC on days 14 and 28. Three generalized reactions probably related to EARS were the only serious side effects. An eight-site i.d. PCEC vaccine regimen proved as immunogenic as the routine i.m. schedule and, if implemented as post-exposure prophylaxis, would be the cheapest widely available tissue culture vaccine regimen. The protective efficiency should now be tested in patients bitten by rabid animals.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth R., Gruschkau H., Bijok U., Hilfenhaus J., Hinz J., Milcke L., Moser H., Jaeger O., Ronneberger H., Weinmann E. A new inactivated tissue culture rabies vaccine for use in man. Evaluation of PCEC-vaccine by laboratory tests. J Biol Stand. 1984 Jan;12(1):29–46. doi: 10.1016/s0092-1157(84)80019-0. [DOI] [PubMed] [Google Scholar]
- Bijok U., Vodopija I., Smerdel S., Thongcharoen P., Nicholson K., Dietrich M., Gonzalez de Cosio A. Purified chick embryo cell (PCEC) rabies vaccine for human use: clinical trials. Behring Inst Mitt. 1984 Nov;(76):155–164. [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) Rabies prevention--United States, 1984. MMWR Morb Mortal Wkly Rep. 1984 Jul 20;33(28):393-402, 407-8. [PubMed] [Google Scholar]
- Chanthavanich P., Suntharasamai P., Warrell M. J., Viravan C., Looareesuwan S., Supanaranond W., Karbwang J., Warrell D. A., Phillips R. E., Sinhaseni A. Antibody response to suckling mouse brain rabies vaccines for post exposure treatment. Trans R Soc Trop Med Hyg. 1987;81(2):260–263. doi: 10.1016/0035-9203(87)90234-3. [DOI] [PubMed] [Google Scholar]
- HOSTY T. S., HUNTER F. R. Incidence of reactions to antirabies horse serum. Public Health Rep. 1953 Aug;68(8):789–791. [PMC free article] [PubMed] [Google Scholar]
- Harverson G. Post-exposure intradermal antirabies vaccine: a cheaper alternative for developing countries. Trop Doct. 1984 Apr;14(2):67–70. doi: 10.1177/004947558401400206. [DOI] [PubMed] [Google Scholar]
- Hattwick M. A., Corey L., Creech W. B. Clinical use of human globulin immune to rabies virus. J Infect Dis. 1976 Jun;133 (Suppl):A266–A272. doi: 10.1093/infdis/133.supplement_2.a266. [DOI] [PubMed] [Google Scholar]
- Helmick C. G., Johnstone C., Sumner J., Winkler W. G., Fager S. A clinical study of Merieux human rabies immune globulin. J Biol Stand. 1982 Oct;10(4):357–367. doi: 10.1016/s0092-1157(82)80013-9. [DOI] [PubMed] [Google Scholar]
- Monson M. H. Practical management of rabies and the 1982 outbreak in Zorzor District, Liberia. Trop Doct. 1985 Apr;15(2):50–54. doi: 10.1177/004947558501500202. [DOI] [PubMed] [Google Scholar]
- Nicholson K. G., Cole P. J., Turner G. S., Harrison P. Immune responses of humans to a human diploid cell strain of rabies virus vaccine: lymphocyte transformation, production of virus-neutralizing antibody, and induction of interferon. J Infect Dis. 1979 Aug;140(2):176–182. doi: 10.1093/infdis/140.2.176. [DOI] [PubMed] [Google Scholar]
- Nicholson K. G., Turner G. S. Studies with human diploid cell strain rabies vaccine and human antirabies immunoglobulin in man. Dev Biol Stand. 1978;40:115–120. [PubMed] [Google Scholar]
- Suntharasamai P., Chanthavanich P., Warrell M. J., Looareesuwan S., Karbwang J., Supanaranond W., Phillips R. E., Jansawan W., Xueref C., Pouradier-Duteil X. Purified Vero cell rabies vaccine and human diploid cell strain vaccine: comparison of neutralizing antibody responses to post-exposure regimens. J Hyg (Lond) 1986 Jun;96(3):483–489. doi: 10.1017/s0022172400066286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrell M. J., Suntharasamai P., Nicholson K. G., Warrell D. A., Chanthavanich P., Viravan C., Sinhaseni A., Phanfung R., Xueref C., Vincent-Falquet J. C. Multi-site intradermal and multi-site subcutaneous rabies vaccination: improved economical regimens. Lancet. 1984 Apr 21;1(8382):874–876. doi: 10.1016/s0140-6736(84)91340-0. [DOI] [PubMed] [Google Scholar]
- Wasi C., Chaiprasithikul P., Chavanich L., Puthavathana P., Thongcharoen P., Trishanananda M. Purified chick embryo cell rabies vaccine. Lancet. 1986 Jan 4;1(8471):40–40. doi: 10.1016/s0140-6736(86)91918-5. [DOI] [PubMed] [Google Scholar]
- Wasi C., Chaiprasithikul P., Thongcharoen P., Rungpitarungsi V., Tharachan C., Likanontsakul S. Protective antibodies after vaccination with human diploid cell rabies vaccine. Asian Pac J Allergy Immunol. 1983 Dec;1(2):125–130. [PubMed] [Google Scholar]