Abstract
Epidermis is renewed by a population of stem cells that have been defined in vivo by slow turnover, label retention, position in the epidermis, and enrichment in β1 integrin, and in vitro by clonogenic growth, prolonged serial passage, and rapid adherence to extracellular matrix. The goal of this study is to determine whether clonogenic cells with long-term growth potential in vitro persist in vivo and give rise to a fully differentiated epidermis. Human keratinocytes were genetically labeled in culture by transduction with a retrovirus encoding the lacZ gene and grafted to athymic mice. Analysis of the cultures before grafting showed that 21.1–27.8% of clonogenic cells with the capacity for >30 generations were successfully transduced. In vivo, β-galactosidase (β-gal) positive cells participated in the formation of a fully differentiated epithelium and were detected throughout the 40-week postgraft period, initially as loosely scattered clusters and later as distinct vertical columns. Viable cells recovered from excised grafts were seeded at clonal densities and 23.3–33.3% of the colonies thus formed were β-gal positive. In addition, no evidence of transgene inactivation was obtained: all keratinocyte colonies recovered from grafted tissue that were β-gal negative also lacked the lacZ transgene. These results show that cells with long-term growth properties in vitro do indeed persist in vivo and form a fully differentiated epidermis, thereby exhibiting the properties of stem cells.
Keratinocytes within the epidermis are renewed by two categories of replicating cells: (i) stem cells that have the capacity for extended or unlimited growth and (ii) transient amplifying cells that arise from stem cells and replicate a limited number of cycles before undergoing terminal differentiation (1, 2). Stem cells are thought to cycle slowly and be labeled infrequently with nucleotide analogues, but once labeled retain that label for prolonged periods, producing what have been termed “label retaining cells” (3). Stem cells and transient amplifying cells are located within the basal compartment of epidermis with terminally differentiated cells forming the stratified, suprabasal layers. A stem cell and its descendant amplifying and terminally differentiated cells are clustered in a distinct spatial array termed the “epidermal proliferation unit” (EPU) (1).
With the advent of culture systems for clonal and serial growth of epidermal keratinocytes (4), it became possible to examine putative stem cells and other replicating cells in vitro. In submerged culture, keratinocytes are heterogeneous with respect to their cycling times (5, 6), rates of cell cycle withdrawal (7), and clonal growth properties (8). Holoclones have properties suggestive of stem cells in that they have a high colony forming efficiency (CFE) and give rise to meroclones and paraclones, which have progressively less colony forming capacity (8). When isolated from human hair follicles, holoclones undergo ≈130 doublings in culture before senescence (9). By using rates of attachment to various extracellular matrices, it was shown that cultured cells with high levels of β1 integrin also have high CFE and the capacity for substantial expansion in culture (10).
However, the relationship between putative stem cells in vitro and persistence in vivo has never been examined in detail. Specifically, it has never been shown that cells with a high capacity for growth in vitro persist when placed in an in vivo environment. Primary mouse keratinocytes that have been genetically marked in vitro and transplanted to in vivo sites persist for the 12-week observation period (11, 12); however, limited growth capacity of mouse keratinocytes prevents characterization of marked cells before grafting. Grafts of autologous epidermal keratinocytes applied in the treatment of human burn victims have been successfully maintained for over a decade (13). However, in the absence of reports on tracking of these cells, it is not certain that grafted cells have persisted or have been slowly replaced in the intervening years. Keratinocytes from skin and from culture that have high levels of β1 integrin have a high CFE, suggesting that putative stem cells in vivo and in vitro share this surface property (10, 14). The present study was initiated to explore the relationship between clonal and sustained growth in culture with persistence in vivo. We believe this is the first direct evidence linking putative stem cells in vitro with stem cells in vivo.
MATERIALS AND METHODS
Submerged Culture and Transduction of Human Keratinocytes.
Keratinocytes from newborn foreskin were grown in submerged culture with irradiated 3T3 cells (4) by using a serum-containing medium (15). These cells exhibited a CFE of 25.5%. Transduction was achieved by incubating 5 × 105 keratinocytes for 4 hr with MFGlacZ retrovirus (3 ml virus for an moi of 6) and 8 μg/ml polybrene (16). The titer of MFGlacZ, as determined by transducing cultures of freshly seeded 3T3 cells and measuring the number of subsequent colonies that contained a β-galactosidase (β-gal) positive cell(s), was 106 colony forming units (cfu)/ml.
Organotypic Raft Culture and Grafting Method.
Organotypic cultures were constructed by established methods (17) and maintained at the air/liquid interface for 3 days until grafting. A full thickness, circular 12 mm wound was created in the interscapular region on the dorsum of 4–6-week-old NIH male Swiss nu/nu mice (Taconic Farms). Organotypic cultures were trimmed to match the 12 mm circular wound, placed onto the fascia, and held in place with a bandage according to procedures published elsewhere (18). The dressing was changed at 1 week and removed after 2 weeks.
Histology of Grafted Tissue.
Mice were euthanized by CO2 asphyxiation and grafts were excised along with about 2 mm of surrounding mouse skin. Part of the graft was immediately fixed in 10% buffered formalin for paraffin embedding, and the remainder was incubated in 2 M sucrose at 4°C overnight and frozen in Tissue-Tek O.C.T. embedding medium (Sakura Finetek, Torrance, CA). For detection of β-gal activity, 7 μm frozen sections were fixed briefly in paraformaldehyde, reacted for 2 hr with 4-chromo-5-bromo-3-indolyl-β-d-galactoside (X-Gal) and counterstained with hematoxylin and eosin. For fluorescent immunohistochemistry, 4–6 μm frozen sections were stained with antibodies against involucrin and filaggrin (Biomedical Technologies, Stoughton, MA), keratin 1 (Enzo Biolabs, Farmingdale, NY), and β-gal (Cortex Biochem, San Leandro, CA). Antibody to keratin K16 was kindly provided Pierre A. Coulombe (The Johns Hopkins University) and to collagen VII by Robert E. Burgeson (Harvard University).
Clonal Analysis of Cells from Submerged Cultures, Organotypic Cultures, and Graft Tissue.
Submerged cultures were rinsed in phosphate buffered saline (PBS) containing 0.5 mM EDTA (pH 7.4) and incubated at 37°C in trypsin (2 mg/ml) until a single cell suspension was obtained. Portions of organotypic cultures and freshly removed graft tissue were washed in PBS, cut into fragments (≈1 mm2), and incubated with trypsin with agitation every 15 min and fresh exchanges at hourly intervals for a total of 2 hr (organotypic culture) or 4 hr (graft tissue). Trypsin was neutralized by addition of an equal volume of culture medium, and recovered cells (0.1–5 × 105 cells) were seeded into 10-cm culture dishes. When colonies were ≈5 mm in diameter, some cultures were fixed briefly with gluteraldehyde, stained with X-Gal to detect β-gal positive clones, and counterstained with rhodamine to detect β-gal negative clones. In other cultures, colonies that were ≥5 mm were isolated with cloning rings, trypsinized, and reseeded into 24-well and 6-well culture dishes. After ≈1 week in culture, cells in the 24-well dish were stained with X-Gal and cells in the 6-well plate were expanded by serial passage.
DNA Isolation, PCR Analysis, and Southern Blot Analysis.
DNA was extracted from intact cells by 16 hr digestion with proteinase K (25 μg/ml) and SDS (0.5%) at 37°C, according to procedures described elsewhere (19). For PCR analysis, 50–100 ng of genomic DNA was used as a template. The specific primers used to detect lacZ sequences were 5′-GATCGACAGATTTGATCCAGCGATA-3′ and 5′-TGCGTGACTACCTACGGGTAACAGT-3′ and to detect filaggrin sequences 5′-CCAGATGACCCAGATATGGTTGATG-3′ and 5′-GATCTTGGATCTTCCCTTATTCCC-3′. For Southern blots, 5 μg of genomic DNA was digested with HindIII or NcoI, electrophoresed on a 0.5% agarose gel, and transferred to a nylon membrane. A 689-bp digoxigenin-labeled probe specific for lacZ DNA was generated by PCR using the DIG/Genius Labeling Kit (Boehringer Mannheim) and the above primers. Hybridization and detection were performed according to manufacturer’s recommendations.
RESULTS
Normal human foreskin keratinocytes were transduced in submerged culture with a single exposure to the recombinant retrovirus MFGlacZ at a titer of 106 cfu/ml and moi estimated at 6. Three days after transduction, 79% of the cells in culture stained positive for β-gal. The transduced cells were expanded for two additional passages at which point they were subjected to (i) in vitro clonal analysis and (ii) in vivo analysis. For the in vitro clonal analysis, cells were seeded at low cell densities and 2 weeks later, 26.9% of the colonies present were observed to be β-gal positive (Table 1). Colonies >5 mm in diameter from nonstained cultures were isolated and passaged three times until >107 cells were obtained. At a CFE of 25.5%, this expansion is equivalent to >30 cumulative cell doublings. Of the 24 colonies selected, 18 could be serially passaged and did not appear to undergo senescence. Among these expanded colonies, 27.8% were β-gal positive.
Table 1.
β-gal expression in keratinocyte colonies
| Samples | Initial colonies
|
Selected for expansion
|
Expanded colonies
|
||
|---|---|---|---|---|---|
| Total | % β-gal + | Total | Total | % β-gal + | |
| Pregraft | |||||
| Submerged culture | 256 | 26.9 | 24 | 18 | 27.8 |
| Organotypic raft culture | 131 | 23.6 | 24 | 19 | 21.1 |
| Graft, weeks | |||||
| 2 | 408 | 33.3 | ND | ||
| 5 | 203 | 30.0 | ND | ||
| 10 | 980 | 23.3 | ND | ||
| 20 | 353 | 26.1 | ND | ||
| 40 | Insufficient material | ||||
Keratinocytes were collected from submerged culture, organotypic culture, and excised grafts and seeded at clonal densities. Two weeks later, the cultures were stained with X-Gal. The percentage of β-gal positive (β-gal +) colonies is noted. A number of colonies from submerged and organotypic cultures were selected and expanded to >107 cells. The percentage of these expanded colonies that were β-gal positive is noted. Colonies from graft tissue were not expanded to >107 cells and this is indicated by ND.
For the in vivo analysis, the polyclonal mixture of transduced cells was assembled into organotypic cultures and grafted to full thickness wounds in nude mice. Clonal analysis of cells recovered from organotypic cultures before grafting showed no change in the percentage of β-gal positive colonies (Table 1). In the first 2 weeks after grafting, contraction of the graft was noted, but after that, further shrinkage occurred at a minimal rate. At various times up to 40 weeks after grafting, grafted tissue was excised and a portion examined histologically and a portion used for recovery of viable cells. There was insufficient material from the 40-week grafts to allow recovery of viable cells. Histological examination showed a fully differentiated epithelium with expression of human collagen VII in the basement membrane zone and suprabasal expression of human involucrin, filaggrin, K1, and K16 (data not shown). At 1 week after grafting, β-gal positive cells were present in scattered locations throughout the epidermis (Fig. 1B). By 10–20 weeks after grafting, β-gal positive cells appeared less dispersed and more clustered (Fig. 1 C and D) and by 40 weeks, distinct columns of β-gal positive cells were evident, reminiscent of the EPU (Fig. 1E). By using dual antibody staining, β-gal positive cells were shown to coexpress markers of keratinocyte differentiation including involucrin, filaggrin, K1, and K16 (data not shown). The basement membrane beneath columns of β-gal cells was also shown to contain human collagen VII (data not shown). Coexpression in the case of β-gal and filaggrin is illustrated in Fig. 1F. Viable keratinocytes recovered from 1–20-week graft tissue were seeded at clonal densities and the percentage of colonies that were β-gal positive ranged from 23.3–33.3 (Table 1).
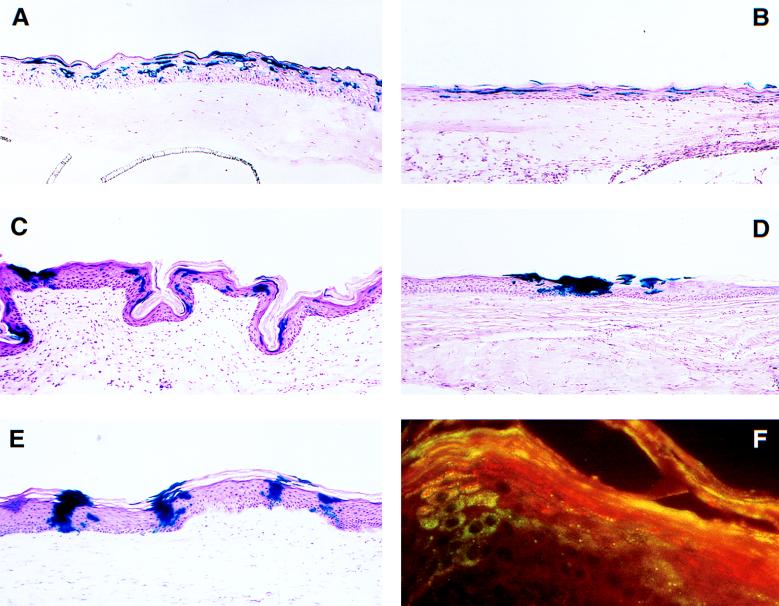
Figure 1.
Histochemical staining of grafted tissue for transgene expression. Organotypic raft cultures constructed with MFGlacZ-transduced keratinocytes were grafted to athymic mice. At the times noted, the tissue was excised and examined for β-gal expression by staining with X-Gal. β-gal positive cells are present in all grafts. (A) Organotypic raft culture before grafting. (B) One week after grafting. (C) Ten weeks after grafting. (D) Twenty weeks after grafting. (E) Forty weeks after grafting. (F) A 10-week graft stained with an antibody for β-gal (green) and an antibody for filaggrin (red). Note dual expression of β-gal and filaggrin (orange) in the column of cells on the left. (A–E, ×240.)
A number of studies with retrovirus-transduced keratinocytes grafted to immune-compromised mice have observed loss of transgene expression (20–25). These data have been interpreted as silencing of the transgene, possibly at the transcriptional level. To determine whether silencing had occurred during grafting, clones of viable keratinocytes were recovered from grafted material and expanded until sufficient material was available for PCR analysis. Silencing would be evident as cells that have lacZ sequences but fail to express the β-gal phenotype. Of the 63 β-gal negative colonies (21 from pregraft material and 44 from grafted tissue), none possessed the lacZ gene (Table 2). No evidence of transgene silencing could be obtained.
Table 2.
PCR analysis for lacZ sequences in keratinocyte colonies
| Sample | β-gal-negative colonies
|
β-gal-positive colonies
|
||
|---|---|---|---|---|
| Total examined | PCR negative | Total examined | PCR positive | |
| Submerged culture | 12 | 12 | 5 | 5 |
| Organotypic culture | 9 | 9 | 4 | 4 |
| 2 weeks after grafting | 13 | 13 | 6 | 5 |
| 5 weeks after grafting | 19 | 19 | 9 | 9 |
| 10 weeks after grafting | 12 | 12 | 12 | 12 |
DNA from a number of keratinocyte colonies was analyzed by PCR for lacZ sequencies. Expression of β-gal correlated with the presence of lacZ sequences, except in one β-gal positive colony that did not contain detectable lacZ sequences. All DNA samples were tested by PCR for the endogenous filaggrin gene and found to be positive.
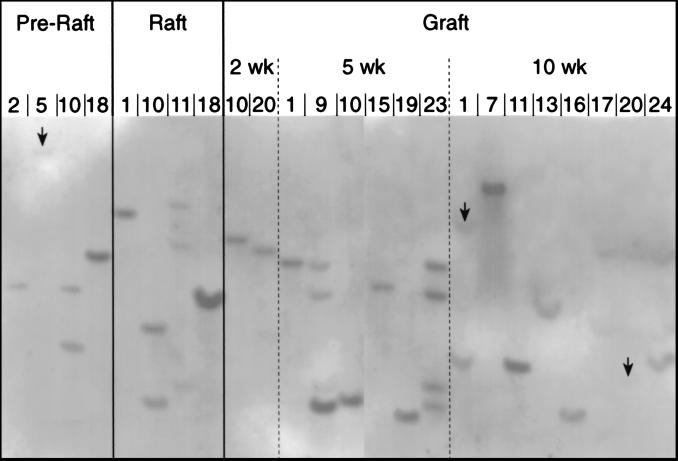
Mathor et al. (26) have shown that when a polyclonal mixture of transduced keratinocytes is serially passed in culture, there is a loss of polyclonality and emergence of a small number of clones, suggesting selection for stem cells with extended growth capacity. To determine whether the β-gal positive clones examined in this study represented a small subpopulation of transduced cells, or perhaps a single transformed cell, clones of keratinocytes recovered from grafted tissue were expanded until sufficient material was available for analysis. DNA from β-gal positive colonies was digested with either HindIII or NcoI and analyzed by Southern blot hybridization by using a labeled probe for lacZ sequences. HindIII does not cut within MFGlacZ and NcoI cuts once outside the lacZ sequence. Differences in sizes of labeled lacZ fragments indicate different integration sites and therefore different clonal origin. Analysis of DNA from 16 clones from the 2-, 5-, and 10-week grafts, as well as DNA from 8 pregraft clones indicated that none of the clones shared a common origin. Data from HindIII-restricted DNA is shown in Fig. 2. Some clones in this blot appeared to generate similar size fragments (pregraft-2 and 5 week-15, pregraft-18 and 2 week-20, raft-18 and 10 week-13, 5 week-19 and 10 week-16). When DNA from these clones was analyzed by using NcoI restriction enzyme, clear differences in fragment sizes were apparent (data not shown). These results show that the transduced cells examined in this study were not derived from a single clone or a small subset of clones, but did represent a true polyclonal mixture.
Figure 2.
Sites of integration of retrovirus transgene DNA isolated from various β-gal positive colonies was digested with HindIII enzyme and analyzed by Southern blotting using lacZ DNA as a probe. HindIII does not cut within the retrovirus genome and different sizes of restricted fragments indicate different sites of provirus integration. Arrows indicate the position of labeled fragments that did not appear in the photo reproduction, but were evident in the autoradiograph. The background, but not the bands, has been digitally enhanced to improve appearance.
DISCUSSION
We have used retrovirus marking to determine whether clonogenic cells with long-term growth potential in vitro persist in vivo. Cultures of epidermal keratinocytes were transduced by a single exposure to a retrovirus vector encoding the lacZ gene and 21.1–27.8% of clonogenic cells capable of >30 population doublings were β-gal positive. When a polyclonal culture of transduced cells was grafted to athymic mice, β-gal positive cells were evident throughout the 40-week observation period, formed distinct columns extending from the basal layer through the stratum corneum, and expressed markers indicative of complete keratinocyte differentiation. When viable cells were recovered from grafted tissue, 23.3–33.3% of the clonogenic isolates were β-gal positive, a figure quite similar to pregraft levels. These results mark the first direct evidence linking clonogenic cells with long-term growth potential in vitro with persistence in vivo.
Although there is no single definition for an epidermal keratinocyte stem cell, there is general agreement that such a cell would exhibit clonogenicity and long-term growth potential in vitro, as well as persistence and the capacity to generate a fully differentiated epithelium in vivo. Uncertainty rests primarily in defining a minimal number of generations in vitro that mark a cell as a stem cell. For example, although nonclonal cultures of keratinocytes were originally shown to be capable of 150 cell doublings (27), clones containing as few as 32 cells (5 replication cycles) have been interpreted as of stem cell origin (14). To further confuse the issue, paraclones, which presumably represent transient amplifying cells, have been shown to undergo as many as 15 doublings (8). In this study, a population of lacZ-marked keratinocytes was defined in vitro by clonogenicity and the capacity for >30 cycles of replication, and in vivo by persistence for at least 40 weeks and the capacity to generate progeny that undergo full differentiation. The link that connects the genetically marked cells in vivo with their in vitro counterparts is the fact that the percentage of lacZ positive clones was the same in each. Although we have not defined a minimal number of cell doublings for a stem cell, we have established a connection between in vitro clonogenicity and long-term growth potential with in vivo persistence and full differentiation.
Epidermal renewal takes place by sequential replication of stem cells, which replicate infrequently but have the capacity for unlimited growth, and transient amplifying cells, which replicate frequently but cycle only a limited number of times before undergoing terminal differentiation (1). In culture, a similar heterogeneity in proliferative capacity is seen and has been attributed to the presence of both stem cells and amplifying cells (5, 6, 10, 28). At any single moment, the predominant replicating cell in tissue (1) as well as in culture (7, 8) is the transient amplifying cell. As retroviruses require cells to be in or close to mitosis for successful provirus integration (29), infection of a culture of keratinocytes is likely to result in preferential transduction of the amplifying population. Preferential transduction of the amplifying population helps to explain a key observation made in this study: whereas 79% of the cells were positive for β-gal 3 days following transduction, only 21.1–27.8% of clonogenic cells with the capacity for >30 doublings were β-gal positive. The 79% β-gal positive cells seen 3 days after infection represented a mixture of transduced stem cells, transduced amplifying cells, and derived terminally differentiated cells. However, the β-gal positive clones with a capacity for >30 doublings would likely have come from stem cells, not amplifying cells. Values of 21.1–27.8% are not a measure of the fraction of cells that are stem cells, but rather the fraction of stem cells that were transduced. The fact that the percentage of β-gal colonies recovered from grafted tissue closely matched pregraft levels suggests that there was no preferential loss or enrichment of transduced cells in vivo.
A persistent problem for keratinocyte gene therapy with retrovirus vectors has been the transient nature of transgene expression in vivo (20–23, 25, 30, 31). Although some prolongation of expression is seen when an internal promoter is present in the vector genome (32), the usual course of events is loss of expression when keratinocytes transduced ex vivo are grafted to immunocompromised mice. The most favored explanation for this phenomenon is silencing of the transgene, possibly through methylation of promoter sequences (33, 34). However, preferential transduction of transient amplifying cells offers an alternative explanation. As shown in Fig. 3, transduction of amplifying cells and stem cells have different consequences. Transduction of amplifying cells results in an initial increase in labeled cells, but with repeated cycles of replication and desquamation from the surface overall transgene levels fall. Transduction of stem cells, however, results in sustained levels of expression. With time, the resultant level of transgene expression is determined by the fraction of stem cells that are transduced, not the fraction of cells that initially are transduced. In the studies that have documented loss of expression in vivo (20–23, 25, 30, 31), transgene expression was assessed initially in submerged cultures that contained mixtures of stem, amplifying, and differentiated cells. Even when these cultures are reported to contain >99% transduced cells (20), the possibility exists that slowly cycling stem cells are underrepresented in the transduced population. A useful in vitro test to predict long-term expression in vivo would be to measure the fraction of clonogenic cells that express the transgene and have the capacity for expanded growth. The importance of transducing stem cells for gene therapy has been emphasized by De Luca and Pellegrini (35) who suggest that successful epidermal gene therapy will require careful attention to genetic modification, specifically of the stem cell population.
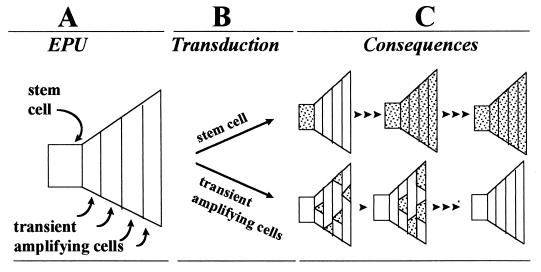
Figure 3.
Consequences of transduction of stem cells and transient amplifying cells. (A) EPU is illustrated by a polygonal figure. The stem cell is a square located at the left side of the polygon and sequential cycles of transient amplifying cells are depicted by areas that expand to the right. Terminal differentiation is not shown in the figure, but would occur after the last round of amplification division. (B) When an EPU is infected with a retrovirus, the stem cell or transient amplifying cells are transduced depending on the position of each in the cell cycle. (C) Transduced cells and their progeny are noted by shaded areas. Cell replication is indicated by an arrowhead. When the stem cell is transduced, all cells in the EPU become and remain labeled with repeated cycles of cell replication (Upper). When transient amplifying cells are transduced, there is an initial rise in the number of transgene-positive cells; however, with further replication, the EPU becomes depleted of transduced cells (Lower).
In epidermis, a stem cell, its descendant transient amplifying cells, and the subsequent terminally differentiated cells are thought to be organized in a spatially distinct unit, the EPU (1). In slowly cycling epithelium, the EPU has a distinct organization with a single label retaining cell in the basal layer and vertical columns of cornified cells in the stratum corneum (36). When transduced mouse keratinocytes are grafted onto inbred histocompatible mice, vertical columns of β-gal-stained cornified cells are not seen until about 6 weeks after grafting (11). We have noted a lack of distinctive arrangement in the early postgrafting period with the appearance of β-gal positive clusters at ≈10 weeks and distinct columns at 40 weeks. We also noted that cells within these clusters expressed markers of differentiation characteristic of normal keratinocytes.
Mathor et al. (26) have noted that when a culture of transduced keratinocytes is serially passaged, the number of clones of transduced cells is reduced so that after ≈14 passages (or ≈80 cell doublings), the cultures become dominated by a small number of clones, and as the culture approaches senescence, by a single clone. During the 40-week in vivo graft period in our study, there did appear to be a reduction in the number of β-gal positive foci histologically (Fig. 1), as though the number of β-gal positive clones were diminishing. However, an analysis of integration sites in a limited number of clones recovered from graft tissue failed to reveal any restriction in clonality. All colonies revealed a unique origin. One explanation for this lack of restriction may be that restriction occurs only in culture, not in vivo. It is also possible that the number of cell cycles in vivo were insufficient to generate this restriction.
Acknowledgments
We wish to thank Ashkan Javaherian, Michael Vaccariello, Brian Hill, and Anne B. Katz for assistance at various steps in this project. We also thank members of our respective labs, Dr. Marcia Simon (Living Skin Bank, State University of New York at Stony Brook) and Dr. Ian Mackenzie (University of Michigan) for helpful discussions. This research was funded by Grants R37 DEO4511 and RO1 DK49093 from the National Institutes of Health (to L.B.T.) and RO1 DE11250 (to J.A.G).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: CFE, colony forming efficiency; β-gal, β-galactosidase; EPU, epidermal proliferation unit.
References
- 1.Potten C S. Int Rev Cytol. 1981;69:271–318. doi: 10.1016/s0074-7696(08)62326-8. [DOI] [PubMed] [Google Scholar]
- 2.Hall P A, Watt F M. Development (Cambridge, UK) 1989;106:619–633. doi: 10.1242/dev.106.4.619. [DOI] [PubMed] [Google Scholar]
- 3.Bickenbach J R, Mackenzie I C. J Invest Dermatol. 1984;82:618–622. doi: 10.1111/1523-1747.ep12261460. [DOI] [PubMed] [Google Scholar]
- 4.Rheinwald J G, Green H. Cell. 1975;6:331–344. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 5.Albers K M, Setzer R W, Taichman L B. Differentiation. 1986;31:134–140. doi: 10.1111/j.1432-0436.1986.tb00394.x. [DOI] [PubMed] [Google Scholar]
- 6.Dover R, Potten C S. J Invest Dermatol. 1983;80:423–429. doi: 10.1111/1523-1747.ep12555494. [DOI] [PubMed] [Google Scholar]
- 7.Albers K M, Greif F, Setzer R W, Taichman L B. Differentiation. 1987;34:236–240. doi: 10.1111/j.1432-0436.1987.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 8.Barrandon Y, Green H. Proc Natl Acad Sci USA. 1987;84:2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rochat A, Kobayashi K, Barrandon Y. Cell. 1994;76:1063–1073. doi: 10.1016/0092-8674(94)90383-2. [DOI] [PubMed] [Google Scholar]
- 10.Jones P H, Watt F M. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 11.Mackenzie I C. J Invest Dermatol. 1997;109:377–383. doi: 10.1111/1523-1747.ep12336255. [DOI] [PubMed] [Google Scholar]
- 12.Kamimura J, Lee D, Baden H P, Brissette J, Dotto G P. J Invest Dermatol. 1997;109:534–540. doi: 10.1111/1523-1747.ep12336704. [DOI] [PubMed] [Google Scholar]
- 13.Gallico G G, III, O’Connor N E, Compton C C, Kehinde O, Green H. N Engl J Med. 1984;311:448–451. doi: 10.1056/NEJM198408163110706. [DOI] [PubMed] [Google Scholar]
- 14.Jones P H, Harper S, Watt F M. Cell. 1995;80:83–93. doi: 10.1016/0092-8674(95)90453-0. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y-J, Parker L M, Binder N E, Beckett M A, Sinard J H, Griffiths C T, Rheinwald J G. Cell. 1982;31:693–703. doi: 10.1016/0092-8674(82)90324-5. [DOI] [PubMed] [Google Scholar]
- 16.Garlick J A, Katz A B, Fenjves E S, Taichman L B. J Invest Dermatol. 1991;97:824–829. doi: 10.1111/1523-1747.ep12489019. [DOI] [PubMed] [Google Scholar]
- 17.Garlick J A, Taichman L B. J Invest Dermatol. 1994;103:554–559. doi: 10.1111/1523-1747.ep12396847. [DOI] [PubMed] [Google Scholar]
- 18.Parenteau N L, Sabolinski M, Prosky S, Nolte C, Oleson M, Kriwet K, Bilbo P. Biotechnol Bioeng. 1996;52:3–14. doi: 10.1002/(SICI)1097-0290(19961005)52:1<3::AID-BIT1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 19.Miller S A, Dykes D D, Polesky H F. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choate K A, Khavari P A. Hum Gene Ther. 1997;8:895–901. doi: 10.1089/hum.1997.8.8-895. [DOI] [PubMed] [Google Scholar]
- 21.Choate K A, Kinsella T M, Williams M L, Nolan G P, Khavari P A. Hum Gene Ther. 1996;7:2247–2253. doi: 10.1089/hum.1996.7.18-2247. [DOI] [PubMed] [Google Scholar]
- 22.Choate K A, Medalie D A, Morgan J R, Khavari P A. Nat Med. 1996;2:1263–1267. doi: 10.1038/nm1196-1263. [DOI] [PubMed] [Google Scholar]
- 23.Gerrard A J, Hudson D L, Brownlee G G, Watt F M. Nat Genet. 1993;3:180–183. doi: 10.1038/ng0293-180. [DOI] [PubMed] [Google Scholar]
- 24.Page S M, Brownlee G G. J Invest Dermatol. 1997;109:139–145. doi: 10.1111/1523-1747.ep12319194. [DOI] [PubMed] [Google Scholar]
- 25.Morgan J R, Barrandon Y, Green H, Mulligan R C. Science. 1987;237:1476–1479. doi: 10.1126/science.3629250. [DOI] [PubMed] [Google Scholar]
- 26.Mathor M B, Ferrari G, Dellambra E, Cilli M, Mavilio F, Cancedda R, De Luca M. Proc Natl Acad Sci USA. 1996;93:10371–10376. doi: 10.1073/pnas.93.19.10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rheinwald J G, Green H. Nature (London) 1977;265:421–424. doi: 10.1038/265421a0. [DOI] [PubMed] [Google Scholar]
- 28.Milstone L M, LaVigne J F. J Invest Dermatol. 1985;84:504–507. doi: 10.1111/1523-1747.ep12273479. [DOI] [PubMed] [Google Scholar]
- 29.Miller D G, Adam M A, Miller A D. Mol Cell Biol. 1990;10:4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fenjves E S, Yao S-N, Kurachi K, Taichman L B. J Invest Dermatol. 1996;106:576–578. doi: 10.1111/1523-1747.ep12344976. [DOI] [PubMed] [Google Scholar]
- 31.Petersen M J, Kaplan J, Jorgensen C M, Schmidt L A, Li L, Morgan J R, Kwan M K, Krueger G G. J Invest Dermatol. 1995;104:171–176. doi: 10.1111/1523-1747.ep12612734. [DOI] [PubMed] [Google Scholar]
- 32.Deng H, Lin Q, Khavari P A. Nature Biotechnol. 1997;15:1388–1391. doi: 10.1038/nbt1297-1388. [DOI] [PubMed] [Google Scholar]
- 33.Challita P-M, Kohn D B. Proc Natl Acad Sci USA. 1994;91:2567–2571. doi: 10.1073/pnas.91.7.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoeben R C, Migchielsen A A, van der Jagt R C, van Ormondt H, van der Eb A J. J Virol. 1991;65:904–912. doi: 10.1128/jvi.65.2.904-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Luca M, Pellegrini G. Gene Ther. 1997;4:381–383. doi: 10.1038/sj.gt.3300434. [DOI] [PubMed] [Google Scholar]
- 36.Mackenzie I C, Bickenbach J R. Cell Tissue Res. 1985;242:551–556. doi: 10.1007/BF00225420. [DOI] [PubMed] [Google Scholar]