Abstract
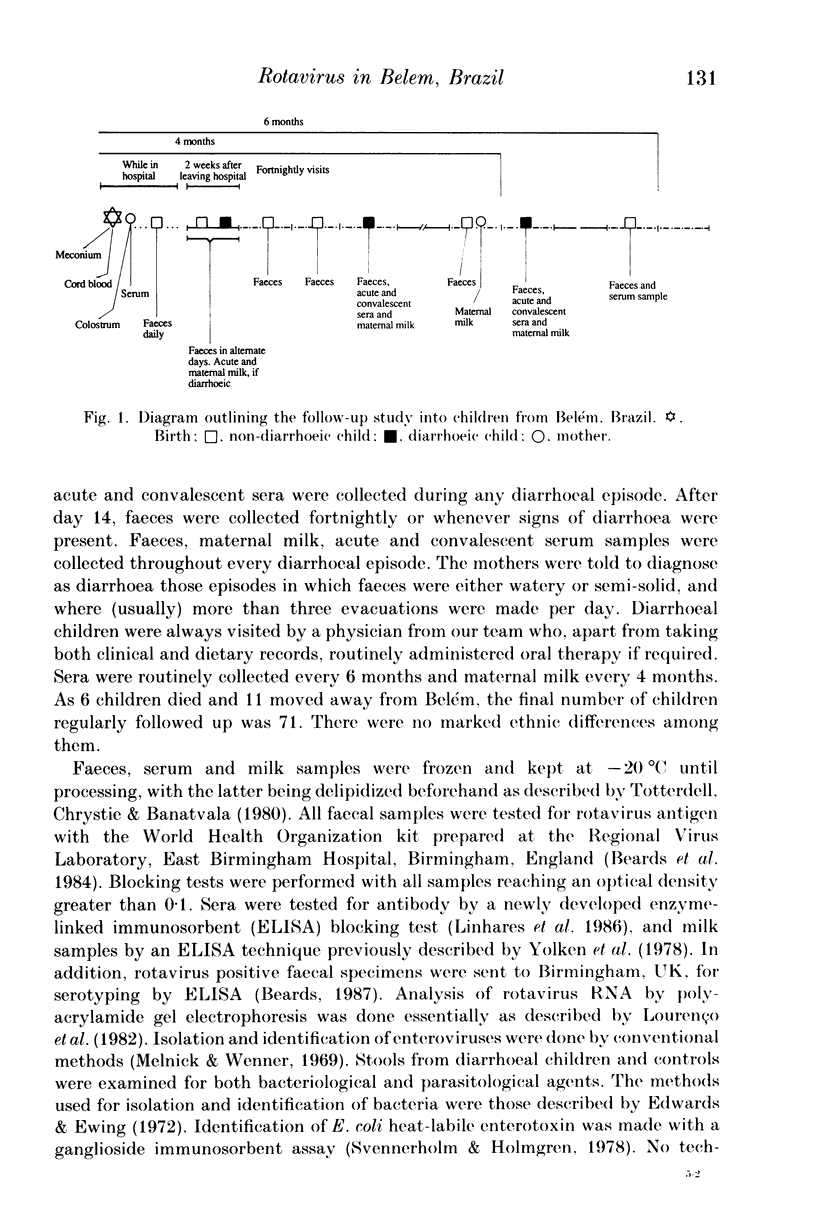
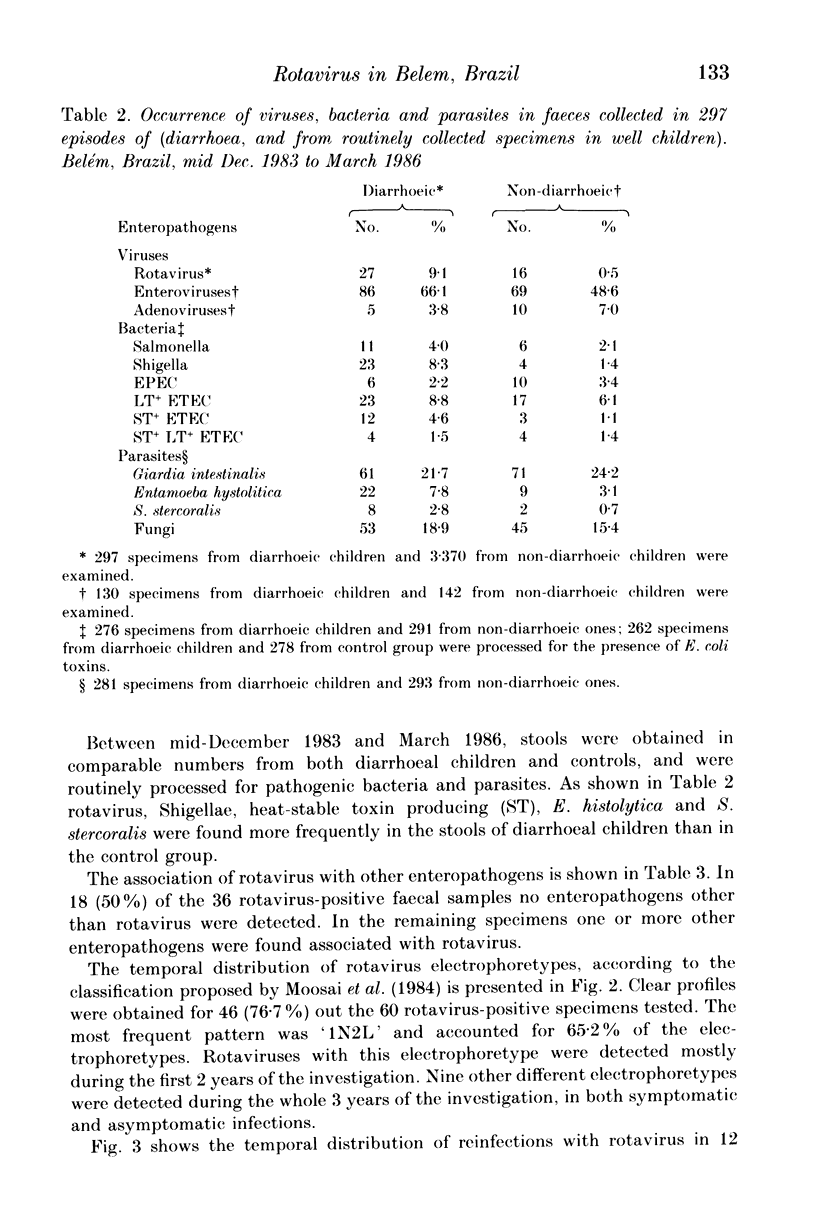
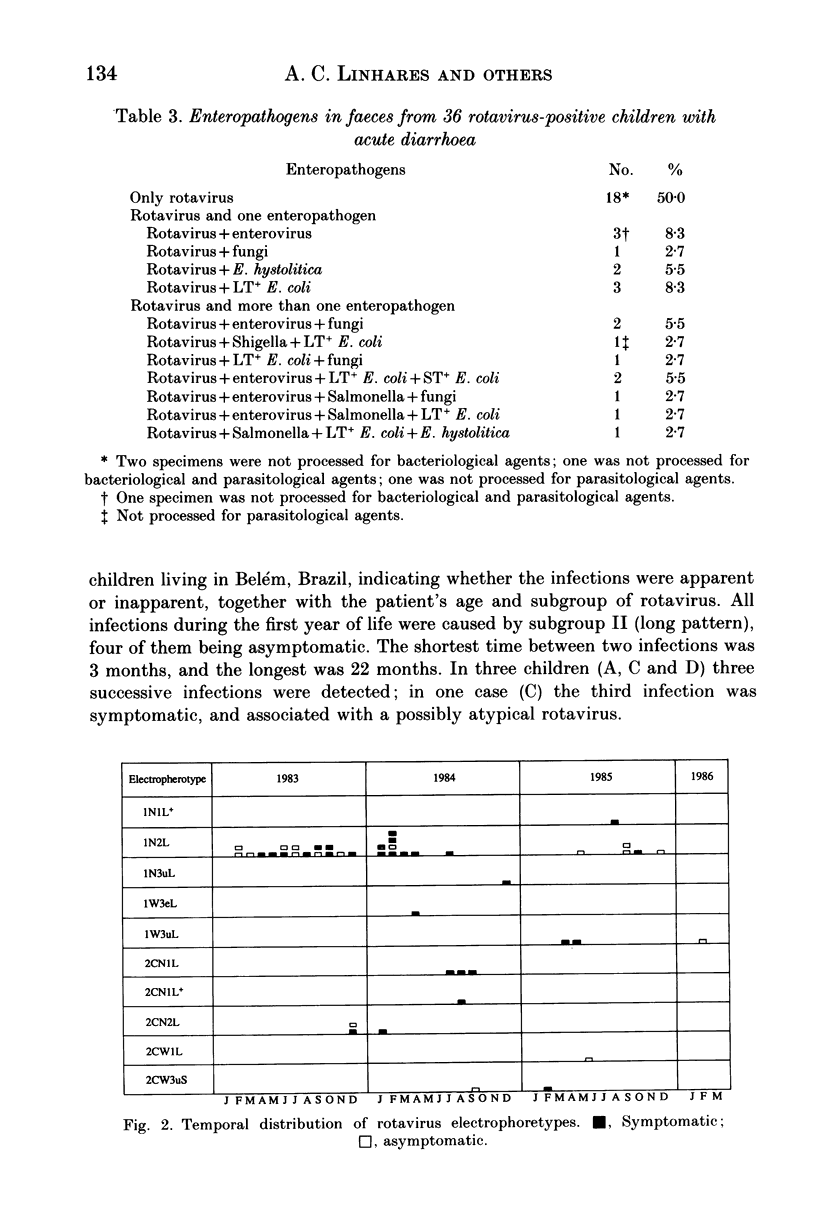
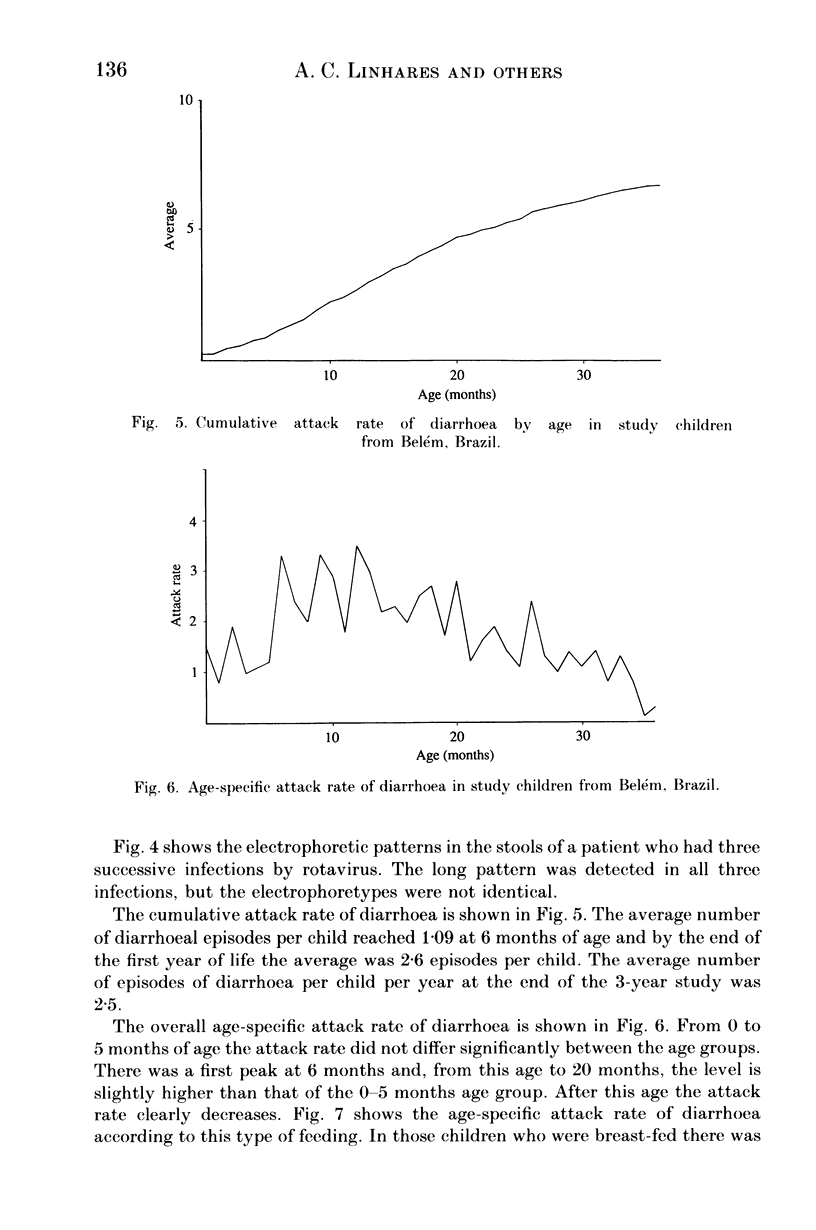
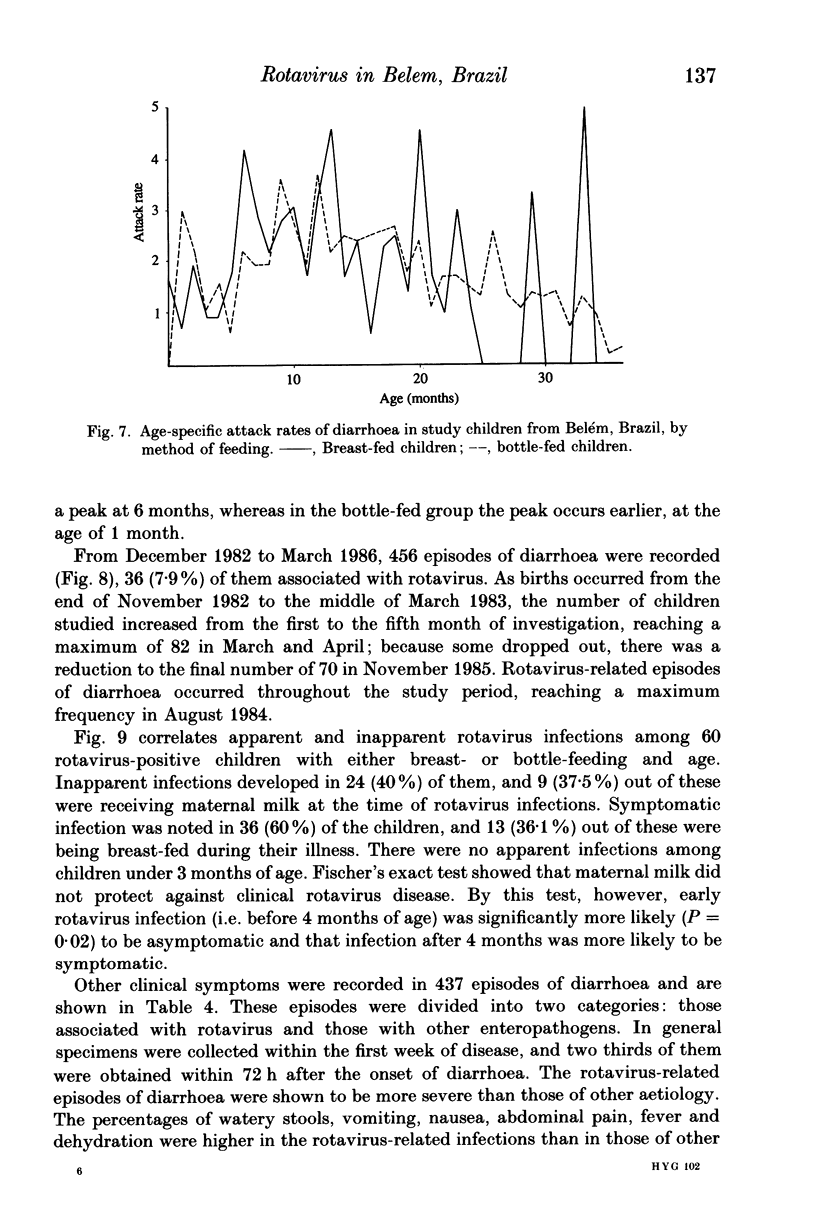
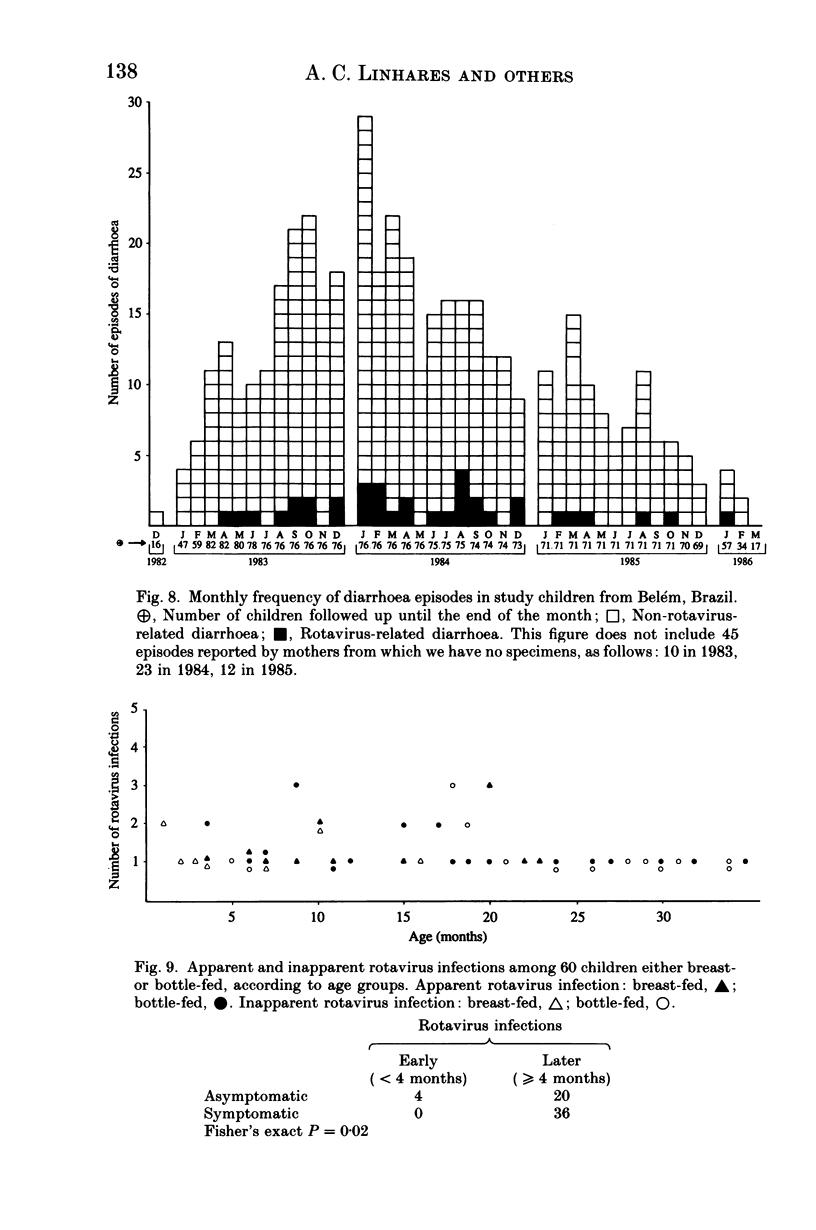
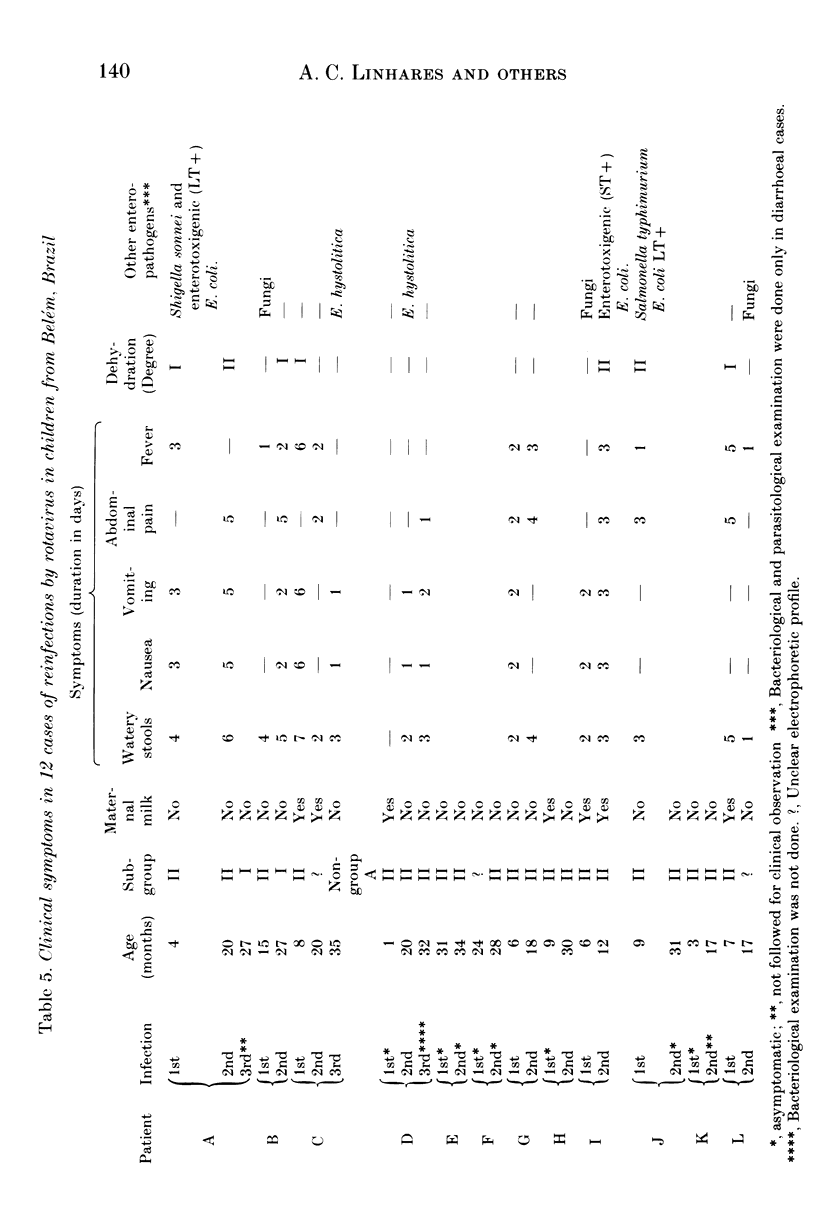
From December 1982 to March 1986 a group of 80 children between 0 and 3 years old who lived in the peripheral area of Belém, Brazil, were followed up for episodes of diarrhoea. A total of 441 diarrhoeal episodes were recorded and 36 (8.2%) were associated with rotavirus. This agent was the only pathogen in 50% of rotavirus-related episodes of acute diarrhoea, and strains were characterized by analysis of RNA in polyacrylamide gels. Forty-one belonged to subgroup II (long pattern) and five to subgroup I. Reinfections by rotavirus were noted in 12 children involving either the same or different subgroups. Ten distinct electrophoretypes were detected in the study period and the predominant one had the '1N2L' profile. The cumulative age-specific attack rate for diarrhoea reached 2.8 by the end of the first year of life; a frequency of 2.3 episodes of diarrhoea per child per year was observed throughout the complete investigation. In comparing the age-specific attack rates for diarrhoea between breast-fed and bottle-fed children, a peak at 6 months of age was noted in the former, and at 1 month in the latter. A comparison by Fischer's exact test (P = 0.21) provided no evidence for protection against clinical rotavirus disease by maternal milk. By the same test, however (P = 0.021), we found significant evidence that early rotavirus infections were more likely to be asymptomatic and that infections after 4 months were more likely to be symptomatic. The clinical picture in children with rotavirus-related diarrhoea was more severe than in those suffering from acute diarrhoea due to another agent.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldacci E. R., Candeias J. A., Breviglieri J. C., Grisi S. J. Etiologia viral e bacteriana de casos de gastroenterite infantil: uma caracterizaço clínica. Rev Saude Publica. 1979 Mar;13(1):47–53. doi: 10.1590/s0034-89101979000100007. [DOI] [PubMed] [Google Scholar]
- Beards G. M., Campbell A. D., Cottrell N. R., Peiris J. S., Rees N., Sanders R. C., Shirley J. A., Wood H. C., Flewett T. H. Enzyme-linked immunosorbent assays based on polyclonal and monoclonal antibodies for rotavirus detection. J Clin Microbiol. 1984 Feb;19(2):248–254. doi: 10.1128/jcm.19.2.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beards G. M. Serotyping of rotavirus by NADP-enhanced enzyme-immunoassay. J Virol Methods. 1987 Nov;18(2-3):77–85. doi: 10.1016/0166-0934(87)90113-3. [DOI] [PubMed] [Google Scholar]
- Beasley R. P., Hwang L. Y. Postnatal infectivity of hepatitis B surface antigen-carrier mothers. J Infect Dis. 1983 Feb;147(2):185–190. doi: 10.1093/infdis/147.2.185. [DOI] [PubMed] [Google Scholar]
- Bishop R. F., Barnes G. L., Cipriani E., Lund J. S. Clinical immunity after neonatal rotavirus infection. A prospective longitudinal study in young children. N Engl J Med. 1983 Jul 14;309(2):72–76. doi: 10.1056/NEJM198307143090203. [DOI] [PubMed] [Google Scholar]
- Clemens J. D., Ahmed M., Butler T., Greenough W. B., 3rd, Sack D. A., Stanton B. F. Rotavirus diarrhoea: an expanding clinical spectrum. J Trop Med Hyg. 1983 Jun;86(3):117–122. [PubMed] [Google Scholar]
- Coelho C. A., Moreira F. L., Maffei H. V., Coelho K. I. Incidência de partículas virais em crianças com diarréia aguda ou protraída, atendidas no Hospital das Clínicas da Faculdade de Medicina de Botucatu, no período de fevereiro de 1980 a fevereiro de 1981. Rev Inst Med Trop Sao Paulo. 1983 May-Jun;25(3):113–119. [PubMed] [Google Scholar]
- Coiro J. R., De Almeida Neto A. J., Heuser M. C., Bendati M. M., Vasconcellos V. L. Acute enteritis associated with rotavirus presence in Brazilian children: evaluations on prevalence, therapy and age group. J Diarrhoeal Dis Res. 1985 Jun;3(2):78–83. [PubMed] [Google Scholar]
- Davidson G. P., Bishop R. F., Townley R. R., Holmes I. H. Importance of a new virus in acute sporadic enteritis in children. Lancet. 1975 Feb 1;1(7901):242–246. doi: 10.1016/s0140-6736(75)91140-x. [DOI] [PubMed] [Google Scholar]
- Guerrant R. L., Kirchhoff L. V., Shields D. S., Nations M. K., Leslie J., de Sousa M. A., Araujo J. G., Correia L. L., Sauer K. T., McClelland K. E. Prospective study of diarrheal illnesses in northeastern Brazil: patterns of disease, nutritional impact, etiologies, and risk factors. J Infect Dis. 1983 Dec;148(6):986–997. doi: 10.1093/infdis/148.6.986. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Kim H. W., Wyatt R. G., Cline W. L., Arrobio J. O., Brandt C. D., Rodriguez W. J., Sack D. A., Chanock R. M., Parrott R. H. Human reovirus-like agent as the major pathogen associated with "winter" gastroenteritis in hospitalized infants and young children. N Engl J Med. 1976 Apr 29;294(18):965–972. doi: 10.1056/NEJM197604292941801. [DOI] [PubMed] [Google Scholar]
- Konno T., Suzuki H., Katsushima N., Imai A., Tazawa F., Kutsuzawa T., Kitaoka S., Sakamoto M., Yazaki N., Ishida N. Influence of temperature and relative humidity on human rotavirus infection in Japan. J Infect Dis. 1983 Jan;147(1):125–128. doi: 10.1093/infdis/147.1.125. [DOI] [PubMed] [Google Scholar]
- Linhares A. C., Monço H. C., Gabbay Y. B., de Araújo V. L., Serruya A. C., Loureiro E. C. Acute diarrhoea associated with rotavirus among children living in Belém, Brazil. Trans R Soc Trop Med Hyg. 1983;77(3):384–390. doi: 10.1016/0035-9203(83)90170-0. [DOI] [PubMed] [Google Scholar]
- Linhares A. C., Pinheiro F. P., Freitas R. B., Gabbay Y. B., Shirley J. A., Beards G. M. An outbreak of rotavirus diarrhea among a nonimmune, isolated South American Indian community. Am J Epidemiol. 1981 Jun;113(6):703–710. doi: 10.1093/oxfordjournals.aje.a113151. [DOI] [PubMed] [Google Scholar]
- Linhares A. C., Pinheiro F. P., Schmetz C., Müller G., Peters D., Freitas R. B. Rotavirus em Belém do Pará, Brasil (estudo piloto). Rev Inst Med Trop Sao Paulo. 1982 Sep-Oct;24(5):292–297. [PubMed] [Google Scholar]
- Linhares A. C., Salbé E. V., Gabbay Y. B., Rees N. Prevalence of rotavirus antibody among isolated South American Indian communities. Am J Epidemiol. 1986 Apr;123(4):699–709. doi: 10.1093/oxfordjournals.aje.a114290. [DOI] [PubMed] [Google Scholar]
- Mata L., Simhon A., Padilla R., del Mar Gamboa M., Vargas G., Hernández F., Mohs E., Lizano C. Diarrhea associated with rotaviruses, enterotoxigenic Escherichia coli, Campylobacter, and other agents in Costa Rican children, 1976-1981. Am J Trop Med Hyg. 1983 Jan;32(1):146–153. doi: 10.4269/ajtmh.1983.32.146. [DOI] [PubMed] [Google Scholar]
- Mata L., Simhon A., Urrutia J. J., Kronmal R. A., Fernández R., García B. Epidemiology of rotaviruses in a cohort of 45 Guatamalan Mayan Indian children observed from birth to the age of three years. J Infect Dis. 1983 Sep;148(3):452–461. doi: 10.1093/infdis/148.3.452. [DOI] [PubMed] [Google Scholar]
- Moosai R. B., Alcock R., Madeley C. R. A cryptogram for recording rotavirus strains: the Rotacode. J Hyg (Lond) 1984 Oct;93(2):237–249. doi: 10.1017/s0022172400064767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul M. O., Erinle E. A. Rotavirus infection in Nigerian infants and young children with gastroenteritis. Am J Trop Med Hyg. 1982 Mar;31(2):374–375. doi: 10.4269/ajtmh.1982.31.374. [DOI] [PubMed] [Google Scholar]
- Pereira H. G., Azeredo R. S., Leite J. P., Candeias J. A., Rácz M. L., Linhares A. C., Gabbay Y. B., Trabulsi J. R. Electrophoretic study of the genome of human rotaviruses from Rio de Janeiro, São Paulo and Pará, Brazil. J Hyg (Lond) 1983 Feb;90(1):117–125. doi: 10.1017/s0022172400063919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez W. J., Kim H. W., Brandt C. D., Yolken R. H., Arrobio J. O., Kapikian A. Z., Chanock R. M., Parrott R. H. Sequential enteric illnesses associated with different rotavirus serotypes. Lancet. 1978 Jul 1;2(8079):37–37. doi: 10.1016/s0140-6736(78)91339-9. [DOI] [PubMed] [Google Scholar]
- Totterdell B. M., Chrystie I. L., Banatvala J. E. Cord blood and breast-milk antibodies in neonatal rotavirus infection. Br Med J. 1980 Mar 22;280(6217):828–830. doi: 10.1136/bmj.280.6217.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totterdell B. M., Nicholson K. G., MacLeod J., Chrystie I. L., Banatvala J. E. Neonatal rotavirus infection: role of lacteal neutralising alpha1-anti-trypsin and nonimmunoglobulin antiviral activity in protection. J Med Virol. 1982;10(1):37–44. doi: 10.1002/jmv.1890100106. [DOI] [PubMed] [Google Scholar]
- Wyatt R. G., Yolken R. H., Urrutia J. J., Mata L., Greenberg H. B., Chanock R. M., Kapikian A. Z. Diarrhea associated with rotavirus in rural Guatemala: a longitudinal study of 24 infants and young children. Am J Trop Med Hyg. 1979 Mar;28(2):325–328. doi: 10.4269/ajtmh.1979.28.325. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Wyatt R. G., Kim H. W., Kapikian A. Z., Chanock R. M. Immunological response to infection with human reovirus-like agent: measurement of anti-human reovirus-like agent immunoglobulin G and M levels by the method of enzyme-linked immunosorbent assay. Infect Immun. 1978 Feb;19(2):540–546. doi: 10.1128/iai.19.2.540-546.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]