Abstract
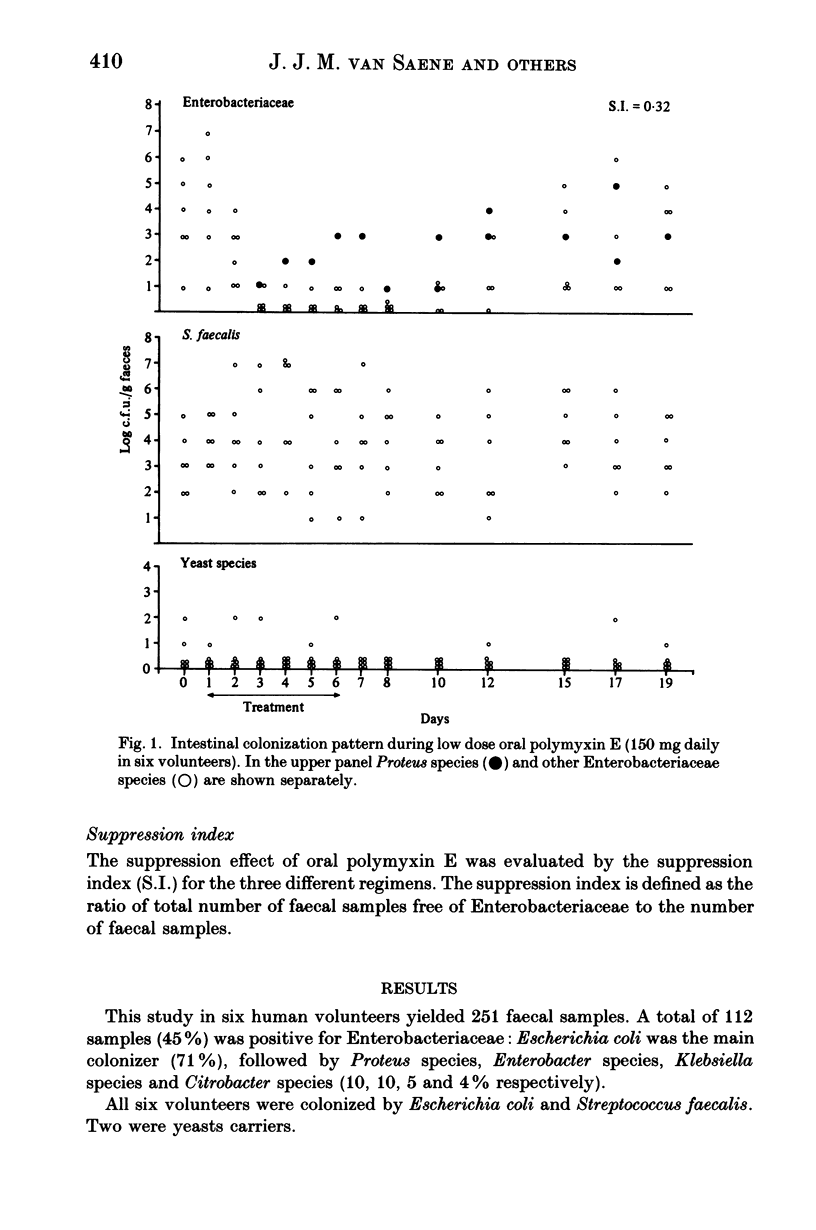
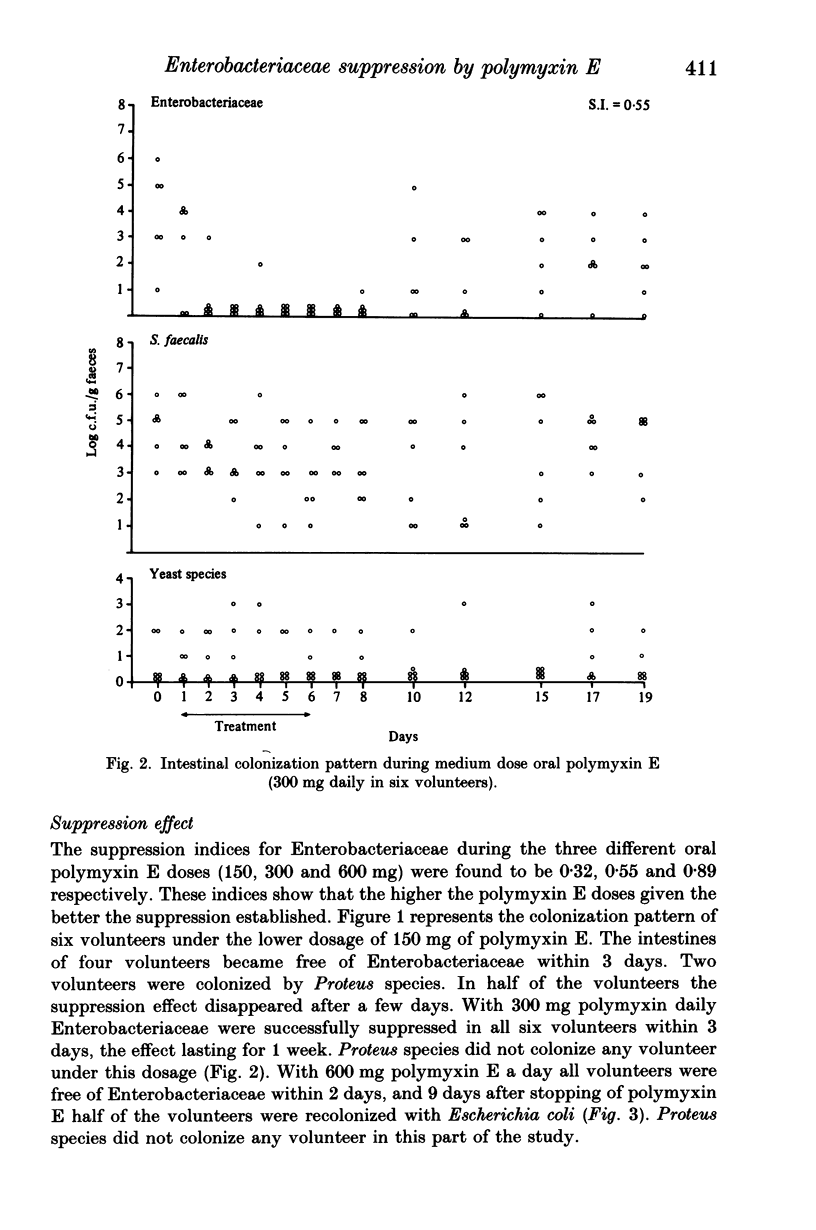
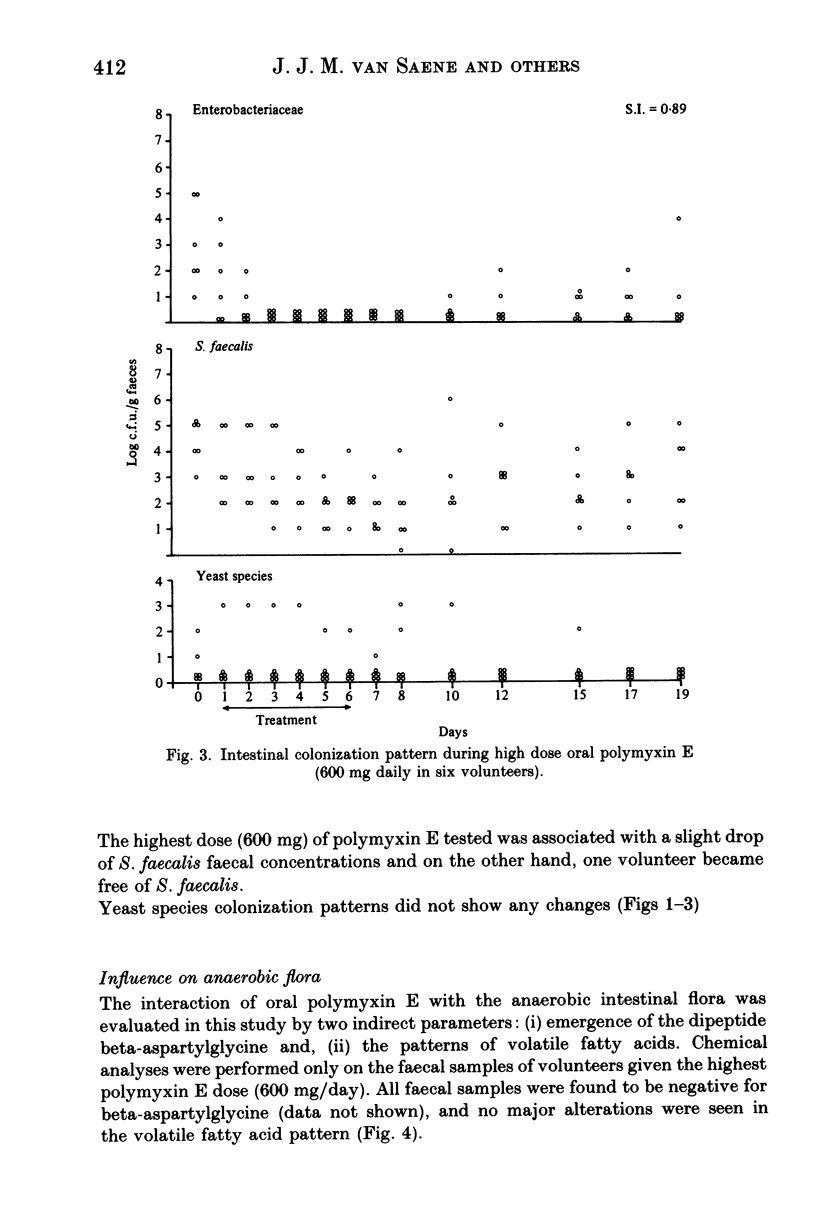
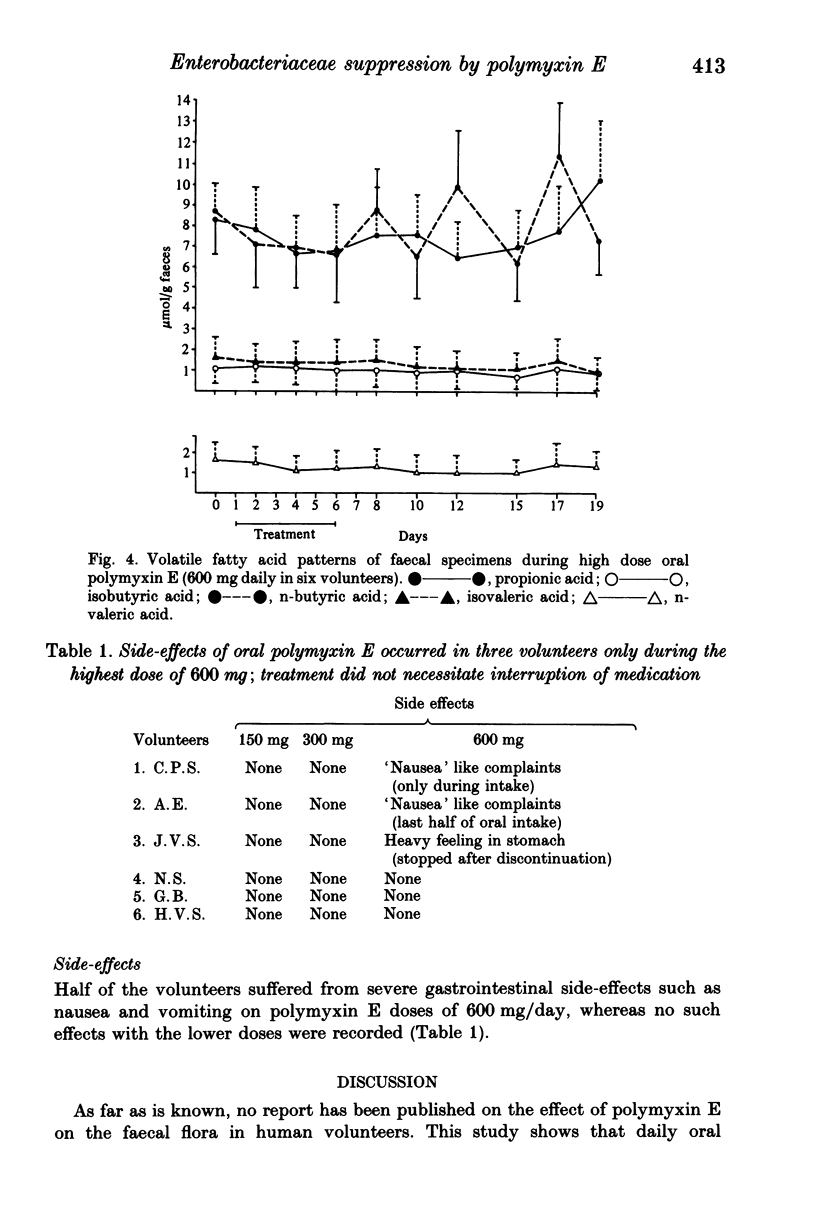
Polymyxin E is frequently used as an oral drug for flora suppression of the gastrointestinal canal. The suppression effect is dose dependent because polymyxin E is moderately inactivated by faecal and food compounds. Three oral polymyxin E doses (150, 300, 600 mg daily) were given to six volunteers for 6 days. The Enterobacteriaceae suppression effect was compared by means of the suppression index i.e. ratio of total number of faecal samples free of Enterobacteriaceae to the total number of faecal samples. The impact on the indigenous (mostly anaerobic) flora was measured in four ways: (i) beta-aspartylglycine content; (ii) volatile fatty acid pattern; (iii) yeast overgrowth and (iv) Streptococcus faecalis decrease. Enterobacteriaceae suppression was most successful during 600 mg oral polymyxin E (suppression indices during 150, 300 and 600 mg were 0.32, 0.55 and 0.89 respectively). None of the four markers of indigenous flora alterations were positive. However, using this dosage half of the volunteers suffered rather severe gastrointestinal side-effects. Oral polymyxin E in a dosage of minimum 600 mg daily seems to possess the ideal properties of a flora suppression agent, if the gastrointestinal side-effects could be mitigated.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brodie J., Macqueen I. A., Livingstone D. Effect of trimethoprim-sulphamethoxazole on typhoid and salmonella carriers. Br Med J. 1970 Aug 8;3(5718):318–319. doi: 10.1136/bmj.3.5718.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTOFF S. P., LEPPER M. H., FIEDLER M. A. TREATMENT OF SALMONELLA CARRIERS WITH COLISTIN SULFATE. Am J Med Sci. 1965 Apr;249:399–403. doi: 10.1097/00000441-196504000-00005. [DOI] [PubMed] [Google Scholar]
- Grylack L., Neugebauer D., Scanlon J. W. Effects of oral antibiotics on stool flora and overall sensitivity patterns in an intensive care nursery. Pediatr Res. 1982 Jul;16(7):509–511. doi: 10.1203/00006450-198207000-00001. [DOI] [PubMed] [Google Scholar]
- Hazenberg M. P., Pennock-Schröder A. M., Van den Boom M., Van de Merwe J. P. Binding to and antibacterial effect of ampicillin, neomycin and polymyxin B on human faeces. J Hyg (Lond) 1984 Aug;93(1):27–34. doi: 10.1017/s0022172400060897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIENITZ M. DARMFLORAVERAENDERUNGEN WAEHREND DER BEHANDLUNG AKUTER DURCHFALLSERKRANKUNGEN JUNGER SAEUGLINGE MIT COLISTIN. Zentralbl Bakteriol Orig. 1963 Oct;190:219–224. [PubMed] [Google Scholar]
- King K. Prophylactic non-absorbable antibiotics in leukaemic patients. J Hyg (Lond) 1980 Aug;85(1):141–151. doi: 10.1017/s0022172400027157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert-Zechovsky N., Bingen E., Beaufils F., Bourrillon A., Mathieu H. Etude de l'écosystème intestinal de l'enfant. Influence de la colistine. Pathol Biol (Paris) 1981 May;29(5):293–297. [PubMed] [Google Scholar]
- LeFrock J. L., Ellis C. A., Weinstein L. The impact of hospitalization on the aerobic fecal microflora. Am J Med Sci. 1979 May-Jun;277(3):269–274. doi: 10.1097/00000441-197905000-00004. [DOI] [PubMed] [Google Scholar]
- Löffler A., Grafvon Westphalen H. Successful treatment of chronic Salmonella excretor with ofloxacin. Lancet. 1986 May 24;1(8491):1206–1206. doi: 10.1016/s0140-6736(86)91179-7. [DOI] [PubMed] [Google Scholar]
- Poth E. J. Historical development of intestinal antisepsis. World J Surg. 1982 Mar;6(2):153–159. doi: 10.1007/BF01654682. [DOI] [PubMed] [Google Scholar]
- Pulaski E. J., Baker H. J., Rosenberg M. L., Connell J. F. LABORATORY AND CLINICAL STUDIES OF POLYMYXIN B AND E. J Clin Invest. 1949 Sep;28(5 Pt 1):1028–1031. doi: 10.1172/JCI102134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E. L. Infectious diseases in the elderly. Ann Intern Med. 1983 Mar;98(3):395–400. doi: 10.7326/0003-4819-98-3-395. [DOI] [PubMed] [Google Scholar]
- Stoutenbeek C. P., van Saene H. K., Miranda D. R., Zandstra D. F. The effect of selective decontamination of the digestive tract on colonisation and infection rate in multiple trauma patients. Intensive Care Med. 1984;10(4):185–192. doi: 10.1007/BF00259435. [DOI] [PubMed] [Google Scholar]
- URBAN N. [Treatment of "ward dyspepsia" in infants with colistin]. Dtsch Med Wochenschr. 1960 Dec 16;85:2242–2245. doi: 10.1055/s-0028-1112724. [DOI] [PubMed] [Google Scholar]
- Veringa E. M., van der Waaij D. Biological inactivation by faeces of antimicrobial drugs applicable in selective decontamination of the digestive tract. J Antimicrob Chemother. 1984 Dec;14(6):605–612. doi: 10.1093/jac/14.6.605. [DOI] [PubMed] [Google Scholar]
- Welling G. W. Comparison of methods for the determination of beta-aspartylglycine in fecal supernatants of leukemic patients treated with antimicrobial agents. J Chromatogr. 1982 Oct 8;232(1):55–62. doi: 10.1016/s0378-4347(00)86007-7. [DOI] [PubMed] [Google Scholar]
- van Saene H. K., Stoutenbeek C. P. Selective decontamination. J Antimicrob Chemother. 1987 Oct;20(4):462–465. doi: 10.1093/jac/20.4.462. [DOI] [PubMed] [Google Scholar]
- van Saene J. J., van Saene H. K., Stoutenbeek C. P., Lerk C. F. Influence of faeces on the activity of antimicrobial agents used for decontamination of the alimentary canal. Scand J Infect Dis. 1985;17(3):295–300. [PubMed] [Google Scholar]
- van den Bogaard A. E., Hazen M. J., Van Boven C. P. Quantitative gas chromatographic analysis of volatile fatty acids in spent culture media and body fluids. J Clin Microbiol. 1986 Mar;23(3):523–530. doi: 10.1128/jcm.23.3.523-530.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bogaard A. E., Weidema W. F., van Boven C. P., van der Waay D. Recolonization and colonization resistance of the large bowel after three methods of preoperative preparation of the gastrointestinal tract for elective colorectal surgery. J Hyg (Lond) 1986 Aug;97(1):49–59. doi: 10.1017/s0022172400064342. [DOI] [PMC free article] [PubMed] [Google Scholar]



