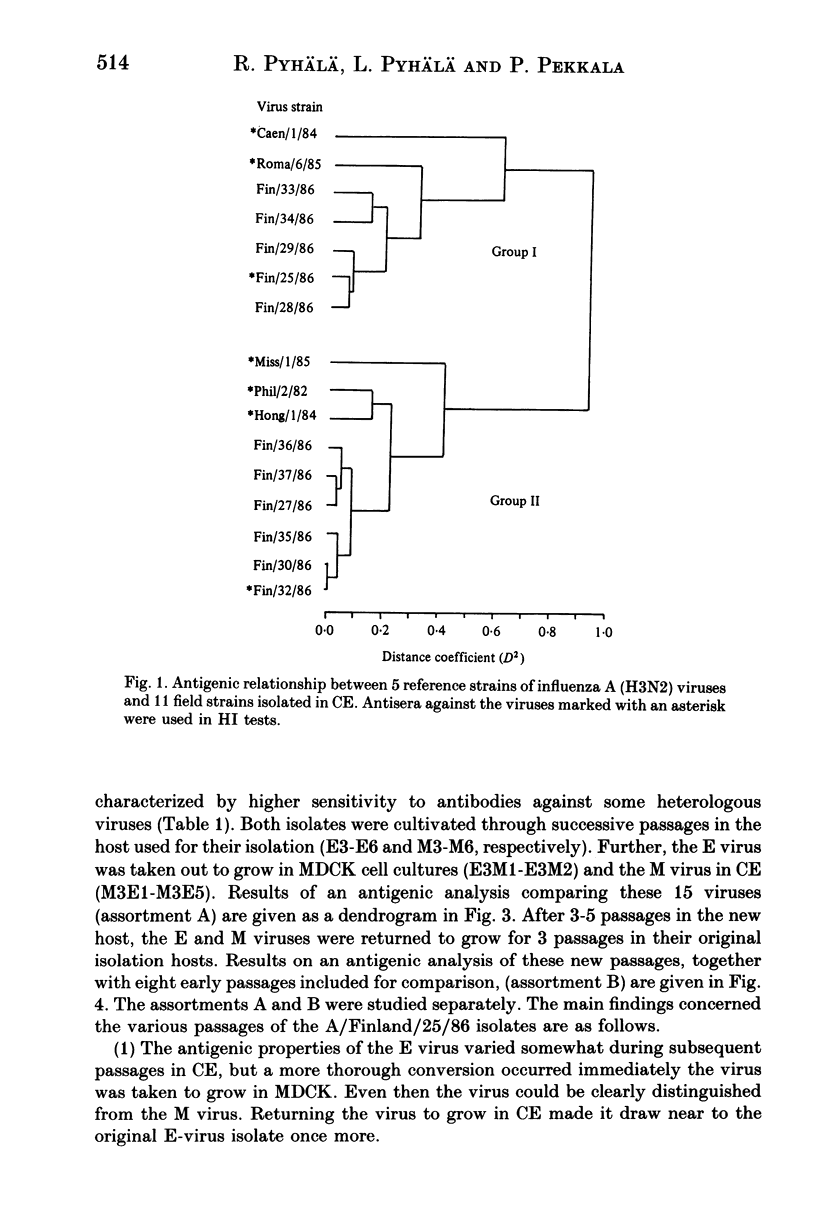
Abstract
During the outbreak of influenza due to A (H3N3) viruses in Finland in 1985/6 virus pairs were isolated from the same clinical specimens in embryonated hens' eggs (CE) and in canine kidney cell cultures (MDCK). Some of these isolates, the E and M pairs, were distinguished by their reactions in haemagglutination inhibition (HI) tests carried out using polyclonal antisera, and by receptor-binding properties, as evidenced by differences in their elution activity from erythrocytes. Passage of the E- and M-virus isolates in the foreign host affected their serological characteristics, but the E virus did not convert to an M-like virus and the M virus did not convert to an E-like virus. Returning the viruses to grow in the host used for their isolation changed the serological reactions so that they were once more close to the reactions of the original isolates. This contrasts with the changes in receptor-binding properties. Rapid elution from hen erythrocytes, which has been described as a property of viruses binding to the SA alpha 2,3Gal sequence in preference to SA alpha 2,6Gal, characterized the virus passages grown solely in MDCK cell cultures. Cultivation of the M virus in CE, at any stage of its passage history, made the virus irreversibly incapable of elution. The M virus was more sensitive than the E virus to HI antibodies against heterologous viruses of the H3N2 subtype, and, when used as an antigen in HI serology, it more frequently (90% vs. 69%; P less than 0.01) detected diagnostic antibody responses in patients infected with viruses of this subtype in 1985/6. Use of antigens with a different passage history in HI serology provided evidence that this superiority, which may be due to the ability of the virus to pick out anamnestic antibody responses, is unrelated to the receptor-binding peculiarity of the M virus under consideration. The results support the concept that the host cell can select a diversity of virus variant subpopulations from a single clinical specimen during isolation and subsequent cultivation procedures. Moreover, the MDCK-grown influenza viruses may correspond better than the egg-grown isolates to the natural epidemic viruses.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer W. E., Masurel N. Antigenic heterogeneity among influenza A(H3N2) field isolates during an outbreak in 1982/83, estimated by methods of numerical taxonomy. J Hyg (Lond) 1985 Feb;94(1):97–109. doi: 10.1017/s0022172400061179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. M., Higa H. H., Paulson J. C. Different cell-surface receptor determinants of antigenically similar influenza virus hemagglutinins. J Biol Chem. 1981 Aug 25;256(16):8357–8363. [PubMed] [Google Scholar]
- Daniels P. S., Jeffries S., Yates P., Schild G. C., Rogers G. N., Paulson J. C., Wharton S. A., Douglas A. R., Skehel J. J., Wiley D. C. The receptor-binding and membrane-fusion properties of influenza virus variants selected using anti-haemagglutinin monoclonal antibodies. EMBO J. 1987 May;6(5):1459–1465. doi: 10.1002/j.1460-2075.1987.tb02387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deom C. M., Caton A. J., Schulze I. T. Host cell-mediated selection of a mutant influenza A virus that has lost a complex oligosaccharide from the tip of the hemagglutinin. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3771–3775. doi: 10.1073/pnas.83.11.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J. M., Naeve C. W., Webster R. G. Host cell-mediated variation in H3N2 influenza viruses. Virology. 1987 Feb;156(2):386–395. doi: 10.1016/0042-6822(87)90418-1. [DOI] [PubMed] [Google Scholar]
- Lathey J. L., Van Voris L. P., Belshe R. B. Superiority of tissue-culture-grown antigens over egg-grown antigens for serologic diagnosis of influenza B virus infections. J Med Virol. 1986 Jun;19(2):155–159. doi: 10.1002/jmv.1890190208. [DOI] [PubMed] [Google Scholar]
- Oxford J. S., Corcoran T., Knott R., Bates J., Bartolomei O., Major D., Newman R. W., Yates P., Robertson J., Webster R. G. Serological studies with influenza A(H1N1) viruses cultivated in eggs or in a canine kidney cell line (MDCK). Bull World Health Organ. 1987;65(2):181–187. [PMC free article] [PubMed] [Google Scholar]
- Oxford J. S. What is the true nature of epidemic influenza virus and how do new epidemic viruses spread? Epidemiol Infect. 1987 Aug;99(1):1–3. doi: 10.1017/s095026880006684x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson S., Oxford J. S. Analysis of antigenic determinants on internal and external proteins of influenza virus and identification of antigenic subpopulations of virions in recent field isolates using monoclonal antibodies and immunogold labelling. Arch Virol. 1986;88(3-4):189–202. doi: 10.1007/BF01310874. [DOI] [PubMed] [Google Scholar]
- Pyhälä R., Pyhälä L. Antigenic analysis of intraepidemic variants of influenza A (H3N2) viruses by hyperimmune rat antisera. J Virol Methods. 1987 Mar;15(4):259–265. doi: 10.1016/0166-0934(87)90147-9. [DOI] [PubMed] [Google Scholar]
- Pyhälä R., Pyhälä L., Visakorpi R. Intraepidemic heterogeneity of influenza A (H3N2) viruses in 1985: antigenic analysis and sensitivity to non-specific inhibitors. Med Biol. 1986;64(5):277–284. [PubMed] [Google Scholar]
- Robertson J. S., Bootman J. S., Newman R., Oxford J. S., Daniels R. S., Webster R. G., Schild G. C. Structural changes in the haemagglutinin which accompany egg adaptation of an influenza A(H1N1) virus. Virology. 1987 Sep;160(1):31–37. doi: 10.1016/0042-6822(87)90040-7. [DOI] [PubMed] [Google Scholar]
- Robertson J. S., Naeve C. W., Webster R. G., Bootman J. S., Newman R., Schild G. C. Alterations in the hemagglutinin associated with adaptation of influenza B virus to growth in eggs. Virology. 1985 May;143(1):166–174. doi: 10.1016/0042-6822(85)90105-9. [DOI] [PubMed] [Google Scholar]
- Rogers G. N., Daniels R. S., Skehel J. J., Wiley D. C., Wang X. F., Higa H. H., Paulson J. C. Host-mediated selection of influenza virus receptor variants. Sialic acid-alpha 2,6Gal-specific clones of A/duck/Ukraine/1/63 revert to sialic acid-alpha 2,3Gal-specific wild type in ovo. J Biol Chem. 1985 Jun 25;260(12):7362–7367. [PubMed] [Google Scholar]
- Rott R., Orlich M., Klenk H. D., Wang M. L., Skehel J. J., Wiley D. C. Studies on the adaptation of influenza viruses to MDCK cells. EMBO J. 1984 Dec 20;3(13):3329–3332. doi: 10.1002/j.1460-2075.1984.tb02299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild G. C., Oxford J. S., de Jong J. C., Webster R. G. Evidence for host-cell selection of influenza virus antigenic variants. Nature. 1983 Jun 23;303(5919):706–709. doi: 10.1038/303706a0. [DOI] [PubMed] [Google Scholar]
- Underwood P. A., Skehel J. J., Wiley D. C. Receptor-binding characteristics of monoclonal antibody-selected antigenic variants of influenza virus. J Virol. 1987 Jan;61(1):206–208. doi: 10.1128/jvi.61.1.206-208.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



