Abstract
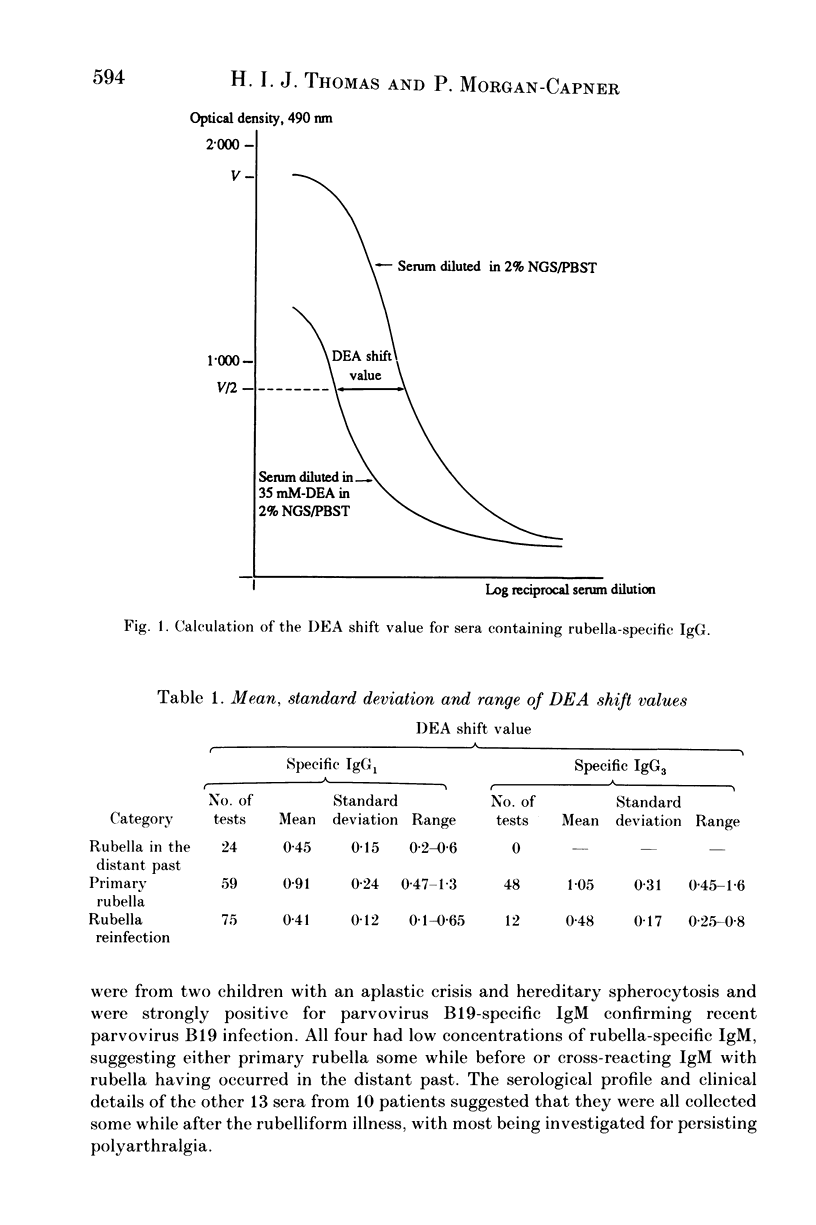
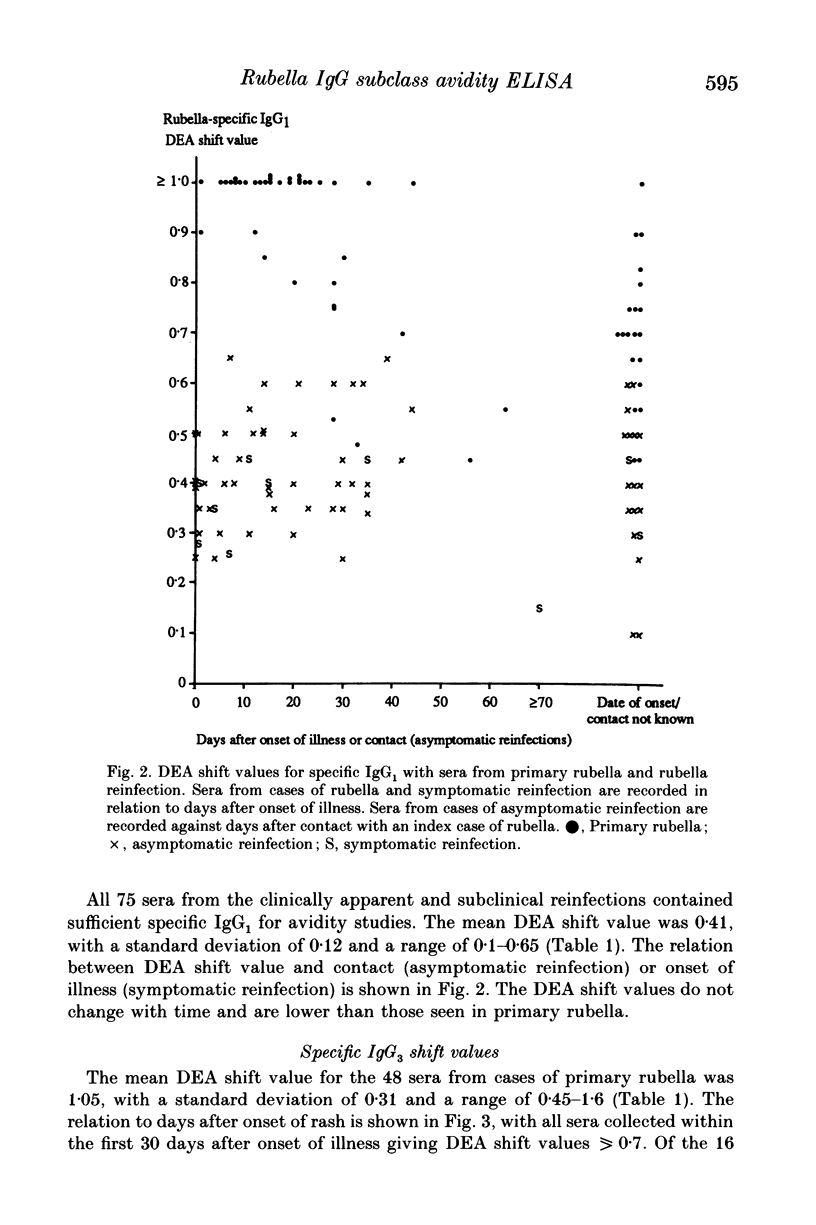
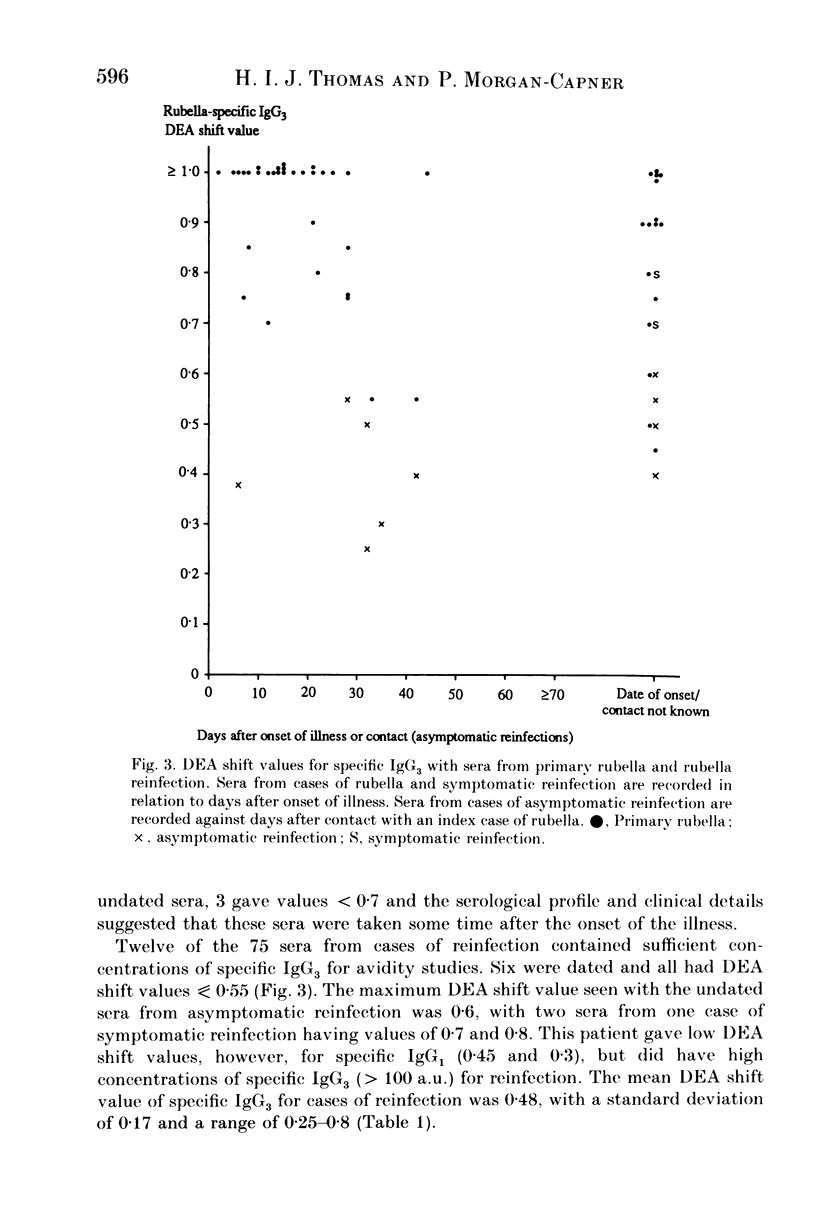
An antiglobulin enzyme-linked immunosorbent assay for rubella-specific IgG1 and IgG3 was adapted to measure antibody avidity by incorporating a mild protein denaturant, diethylamine (DEA), into the serum diluent. Sera were tested at varying dilutions, both with and without DEA, if they contained sufficient specific IgG1 or IgG3. The optical density (OD) was measured and curves were plotted. The highest OD (V) was noted and halved (V/2). The distance between the OD curves at V/2 was measured as the DEA shift value. Sera were examined from people whose sera contained rubella-specific antibodies as a consequence of infection or vaccination in the distant past (24 sera), recent primary rubella (66 sera), symptomatic reinfection (11 sera) or asymptomatic reinfection (64 sera). For specific IgG1 the DEA shift value was less than 0.6 for cases of rubella in the distant past, compared with greater than 0.8 for the first month after primary infection. The maximum DEA shift value for the sera from cases of reinfection was 0.65. No serum from cases of rubella in the distant past contained sufficient specific IgG3 to estimate avidity. The sera collected within 1 month of onset of primary rubella gave DEA shift values greater than 0.7 compared with sera from reinfections, which gave DEA shift values less than 0.6, except for two sera from a case of symptomatic reinfection. Thus the assessment of specific IgG subclass avidity is of value in differentiating serologically primary rubella from reinfection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cradock-Watson J. E., Ridehalgh M. K., Anderson M. J., Pattison J. R. Outcome of asymptomatic infection with rubella virus during pregnancy. J Hyg (Lond) 1981 Oct;87(2):147–154. doi: 10.1017/s0022172400069345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devey M. E., Bleasdale K., Lee S., Rath S. Determination of the functional affinity of IgG1 and IgG4 antibodies to tetanus toxoid by isotype-specific solid-phase assays. J Immunol Methods. 1988 Jan 21;106(1):119–125. doi: 10.1016/0022-1759(88)90279-7. [DOI] [PubMed] [Google Scholar]
- EISEN H. N., SISKIND G. W. VARIATIONS IN AFFINITIES OF ANTIBODIES DURING THE IMMUNE RESPONSE. Biochemistry. 1964 Jul;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- Inouye S., Hasegawa A., Matsuno S., Katow S. Changes in antibody avidity after virus infections: detection by an immunosorbent assay in which a mild protein-denaturing agent is employed. J Clin Microbiol. 1984 Sep;20(3):525–529. doi: 10.1128/jcm.20.3.525-529.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz J. B., Mortimer P. P., Mortimer P. R., Morgan-Capner P., Shafi M. S., White G. B. Rubella antibody measured by radial haemolysis. Characteristics and performance of a simple screening method for use in diagnostic laboratories. J Hyg (Lond) 1980 Apr;84(2):213–222. doi: 10.1017/s0022172400026711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen O. P., Meurman O. H. An ELISA for the estimation of high-avidity and total specific IgG and IgM antibodies to rubella virus. J Virol Methods. 1982 Sep;5(1):1–10. doi: 10.1016/0166-0934(82)90091-x. [DOI] [PubMed] [Google Scholar]
- Morgan-Capner P., Hodgson J., Hambling M. H., Dulake C., Coleman T. J., Boswell P. A., Watkins R. P., Booth J., Stern H., Best J. M. Detection of rubella-specific IgM in subclinical rubella reinfection in pregnancy. Lancet. 1985 Feb 2;1(8423):244–246. doi: 10.1016/s0140-6736(85)91027-x. [DOI] [PubMed] [Google Scholar]
- Mortimer P. P., Tedder R. S., Hamblig M. H., Shafi M. S., Burkhardt F., Schilt U. Antibody capture radioimmunoassay for anti-rubella IgM. J Hyg (Lond) 1981 Apr;86(2):139–153. doi: 10.1017/s0022172400068856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodkey L. S., Freeman M. J. Variations in the properties of rabbit antibodies during prolonged immunization by various routes with serum albumin in Freund's complete adjuvant. Immunology. 1970 Aug;19(2):219–224. [PMC free article] [PubMed] [Google Scholar]
- Rousseau S., Hedman K. Rubella infection and reinfection distinguished by avidity of IgG. Lancet. 1988 May 14;1(8594):1108–1109. doi: 10.1016/s0140-6736(88)91926-5. [DOI] [PubMed] [Google Scholar]
- Siskind G. W., Benacerraf B. Cell selection by antigen in the immune response. Adv Immunol. 1969;10:1–50. doi: 10.1016/s0065-2776(08)60414-9. [DOI] [PubMed] [Google Scholar]
- Thomas H. I., Morgan-Capner P. Specific IgG subclass antibody in rubella virus infections. Epidemiol Infect. 1988 Jun;100(3):443–454. doi: 10.1017/s0950268800067182. [DOI] [PMC free article] [PubMed] [Google Scholar]


