Abstract
Dendritic cells (DC) are critical for stimulation of naive T cells. Little is known about the effect of herpes simplex virus type 2 (HSV-2) infection on DC structure or function or if the observed effects of HSV-1 on human DC are reproduced in murine DC. Here, we demonstrate that by 12 h postinfection, wild-type (wt) HSV-2 (186) abortively infected murine bone marrow-derived DC and induced early cell death compared to UV-inactivated HSV-2 or mock-infected DC. HSV-2-induced loss of DC viability was more rapid than that induced by HSV-1 and was due, in part, to apoptosis, as shown by TEM, caspase-3 activation, and terminal deoxynucleotidyl transferase-mediated dCTP biotin nick end labeling. HSV induced type-specific changes in the murine DC immunophenotype. At 12 h postinfection, wt HSV-2 upregulated DC major histocompatibility complex (MHC) class II expression, and in contrast to UV-inactivated HSV-2, downregulated expression of MHC class I, but it had no effect on surface CD40, CD80, or CD86. Wt HSV-1 (MC-1) induced only CD40 upregulation. More-profound effects on the DC immunophenotype were observed in HSV-2-infected neonatal DC. Wt HSV of either serotype impaired murine DC-induced T-cell alloproliferation and lipopolysaccharide-induced DC interleukin-12 secretion. Thus, there are marked differences in the levels of HSV-induced cytolysis in DC according to the HSV serotype, although HSV-2 displays immunomodulatory effects on the DC immunophenotype and function similar to those of HSV-1.
Dendritic cells (DC) play a key role in the induction of the primary cellular immune response to intracellular pathogens like herpes simplex virus (HSV), as they are the main cell type that stimulates naive T cells in the draining lymph nodes (23). The strength and character of the antigen-specific T-cell response are determined by factors such as the level of costimulatory molecule (B-7) expression and the density of antigen expressed on the surfaces of DC (1, 33). DC are also thought to be the major source of interleukin-12 (IL-12) (31), which has been demonstrated to play a pivotal role in the differentiation of naive CD4+ T cells into type 1 T helper (Th1) cells (18), although recent studies suggest that IL-12 produced by DC is required for optimal T-cell gamma interferon production rather than for CD4+-T-cell polarization (26).
HSV type 1 (HSV-1) infection of adult human DC derived from peripheral blood monocytes (MoDC) has been shown by a number of groups (15, 19, 27) to impair costimulatory molecule upregulation of immature DC. However, the timing and degree of this impairment and the presence or absence of associated effects on major histocompatibility complex (MHC) class I (MHC-I) and MHC-II molecule expression were different, possibly due to differences in the types of HSV-1 strain used. Infection of immature MoDC with a disabled infectious single-cycle mutant (DISC-HSV-1-GFP) inhibited DC maturation (as shown by downregulation of costimulatory molecules) and induced marked downregulation of MHC-I expression, attributed to ICP47 inhibition of the transporter associated with antigen presentation (TAP) (27). HSV-1 infection of mature MoDC with either the disabled single-cycle mutant or a laboratory-adapted HSV-1 strain (Ang) was also nonproductive and induced downregulation of CD83 and many defects in DC function, including reductions in DC-induced T-cell alloproliferation, DC maturation and migration, and DC cytokine secretion (15, 27). In contrast, infection of immature MoDC with a clinical HSV-1 isolate was productive of small amounts of infectious virus and induced asynchronous downregulation of costimulatory molecules with no effect on either MHC-I or MHC-II expression (19).
HSV infection of murine DC has not been well characterized. DC have been shown to be a major source of cytokine secretion in HSV-infected murine skin (30) and to constitute an effective vaccination strategy when primed with HSV epitopes against HSV challenge in murine models (28). Similarly, little is known about HSV-2 infection in any DC system. Therefore, the aim of our study was to test the effect of HSV-2 infection on murine bone marrow-derived DC (BMDC) in vitro compared to that of HSV-1 and to compare results with MoDC from humans to those observed in BMDC from mice. Our ultimate goal was to test whether murine DC would serve as a suitable in vivo model of human DC function with which to compare neonatal and adult murine DC function in later studies of the immunopathogenesis of neonatal HSV disease.
(This work was presented in part at the 27th International Herpesvirus Workshop, July 2002.)
MATERIALS AND METHODS
Mice.
Five- to 6-week old BALB/c (H-2d) and C57BL/6 (H-2b) mice were purchased from the Animal Resource Centre (Perth, Australia) and acclimatized for at least 1 week before use. Bone marrow was harvested from female adult mice at 6 to 12 weeks of age and from neonatal mice at 1 week of age. All experiments were conducted with the approval of The Children's Hospital at Westmead and Westmead Hospital Animal Ethics committees.
Generation of murine BMDC.
DC were generated from the long bones of BALB/c mice by culture in sterile bacterial petri dishes in the presence of granuloctye-macrophage colony-stimulating factor (GM-CSF) (20 ng/ml) (BD PharMingen, San Diego, Calif.) as described elsewhere (17). Nonadherent cells were collected on day 10 and positively selected for surface CD11c expression using MidiMACS with LS separation columns (BD Biosciences, San Jose, Calif.) to give a >97% pure population of CD11c+ MHCII+ cells, which were defined as BMDC. The few remaining contaminants were mostly granulocytes and not B cells, T cells, or macrophages, as shown by the absence of staining for B220, F4/80, CD3, and CD4 on fluorescence-activated cell sorter (FACS) analysis (not shown) after incubation with the relevant specific monoclonal antibodies (MAb) outlined below. The generated population predominantly consisted of immature BMDC (MHCIIlo CD11c+) and 20 to 30% mature BMDC (MHCIIhi CD11c+).
Virus preparation.
Stocks of the wild-type (wt) HSV-2 strain 186syn+-1 (29) and the wt clinical HSV-1 strain MC-1 (19) were propagated and assayed as described elsewhere (4). UV-inactivated 186 syn+-1 virus (UV-HSV-2) and UV-inactivated HSV-1 (UV-HSV-1) were prepared as previously reported so that the postirradiation titer was <100 PFU/ml (4). All stocks were kept at −80°C and thawed immediately before use.
HSV infection of BMDC.
Unless stated otherwise, 5 × 105 BMDC were infected with wt HSV-2, wt HSV-1, UV-186 (preirradiation titer), or UV-HSV-1 (preirradiation titer) at multiplicities of infection (MOI) from 0.1 to 10 or mock infected and incubated for 1 h at 37°C and 5% CO2. Infected BMDC were washed three times with phosphate-buffered saline (PBS) to remove any cell surface-bound virus and resuspended at 2 × 105/ml in RPMI 1640 (Invitrogen, Groningen, The Netherlands) supplemented with a penicillin-streptomycin mixture (Invitrogen), β-mercaptoethanol (Sigma Aldrich, Castle Hill, Australia), 10% heat-inactivated fetal calf serum (FCS), 2 mM glutamine, and 20 ng of GM-CSF (BD PharMingen)/ml and then cultured in a bacterial culture dish at 37°C and 5% CO2 for 12 h in GM-CSF-supplemented medium unless otherwise indicated. The cells were then assessed for viability by the trypan blue exclusion method, and viable DC were used to set up experiments as indicated.
Detection of HSV-infected BMDC.
BMDC were infected with wt HSV-2, UV-HSV-2, wt HSV-1, or UV-HSV-1 at an MOI of 4 and, together with uninfected controls, were incubated for 12 h and then washed as described above. Cytospin preparations were mounted on slides with 105 cells/slide. The cells were fixed in acetone for 5 min, blocked with 20% donkey serum (Jackson ImmunoResearch Laboratories, West Grove, Pa.) for 30 min at room temperature, permeabilized with PBS- 0.1% Triton X-100, and then stained with a polycolonal antibody against HSV-2 (1:1,000; Cortex Biochemical, San Leandro, Calif.), followed by donkey anti-sheep immunoglobulin G-fluorescein isothiocyanate (FITC) (1:1,000; Jackson ImmunoResearch), for HSV-2 strains or MAb against HSV-1 gC directly conjugated to FITC for HSV-1 strains. DC were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) (Molecular Probes, Eugene, Oreg.) and mounted on diazabicyclo(2.2.2.)octane (Sigma Aldrich). The slides were examined using an Olympus BX50 immunofluorescence microscope. Five photographs were taken of each slide with the fluorescence microscope, and the percentage of infected DC was calculated by two independent observers.
To analyze the pattern of HSV gene expression after infection of BMDC with wt HSV-2, DC were infected at an MOI of 4 and collected at 0, 2, 4, 6, 8, 12, 14, 16, and 18 h postinfection (p.i.). Cytospin slides were prepared and fixed as described above, blocked with 20% goat serum (Jackson ImmunoResearch), and then stained with antibodies against HSV ICP4 (M612446; BioClone), ICP8 (383; a kind gift from David Knipe, Harvard Medical School, Boston, Mass.), or HSV-2 glycoprotein D (gD) (910; Biodesign International, Saco, Maine), followed by FITC-conjugated goat anti-mouse MAb (Jackson ImmunoResearch). Washes and mounting were performed as described above.
To determine if infection of BMDC with wt HSV-2 or wt HSV-1 was productive of infectious progeny, DC were infected for 1 h as described above at an MOI of 0.4 or 4 or left untreated. Viable cells were plated in triplicate for each serotype and dose (4 × 105 DC/2 ml of RPMI medium supplemented with GM-CSF) in 24-well plates. The supernatant was collected at 0, 3, 6, 9, 12, 14, 16, 18, and 22 or 24 h p.i. and stored at −80°C until it was assayed. The titer of infectious virus was determined by standard plaque assay as described elsewhere (4).
Detection of apoptosis.
To determine if HSV infection of BMDC induced apoptosis, three methods were used: caspase-3 activation, analysis of ultrastructural morphology by transmission electron microscopy (TEM), and terminal deoxynucleotide transferase-mediated (TdT) dCTP biotin nick end labeling (TUNEL). Unless otherwise stated, BMDC were infected as described above at an MOI of 4 with wt HSV-2 (strain 186), wt HSV-1 (MC-1), UV-HSV-2, or UV-HSV-1 or left untreated and incubated at 37°C and 5% CO2 for the appropriate times.
For caspase-3 activation, 106 DC were infected with each HSV strain (wt HSV-2, UV-HSV-2, wt HSV-1, and UV-HSV-1) and, together with uninfected DC controls, were pelleted in triplicate; the supernatant was removed, and the pellets were stored at −80°C until the time of assay. The level of caspase-3 activity in each DC pellet was determined by a colorimetric assay for caspase-3 in a 96-well plate (R&D Systems, Minneapolis, Minn.). In brief, the cell pellets were lysed and mixed with reaction buffer, dithiothreonal, and colorimetric substrate and then incubated for 1 h in accordance with the manufacturer's instructions. The levels of caspase-3 activity in infected DC cell lysates were then determined spectrophotometrically by comparing the optical density reading at a wavelength of 405 nm using a microplate reader (Bio-Tek Bio-Kinetics) with the activities of uninfected DC controls and background controls (no cell lysate).
For TEM, infected or uninfected DC pellets (106 cells for each strain and uninfected controls) were washed twice in RPMI medium containing no FCS, fixed in modified Karnovsky's fixative for 1 h at room temperature, and then prepared as described elsewhere (20). In brief, the cells were washed twice with 0.1 M morpholinopropanesulfonic acid (MOPS) buffer; postfixed in 2% buffered osmium tetroxide and then in 2% uranyl acetate, both for 1 h; dehydrated through graded ethanols; and embedded in Spurr epoxy resin. The blocks were polymerized for 10 h at 70°C. Ultrathin sections (70 nm) were collected from each DC pellet onto 400-mesh thin-bar copper grids, stained (2% uranyl acetate in 50% ethanol, followed by Reynold's lead citrate), and then examined by TEM using a Philips CM120 BioTWIN operated at 80 kV. As a positive control for apoptosis, uninfected BMDC were irradiated with UV light (30-W bulb at 20 cm for 2 min), incubated for 3 h in RPMI medium containing no FCS, washed twice, and then fixed and processed for TEM as described above. For semiquantitative analysis of the number of apoptotic cells, semithin sections (0.5 μm) were cut from the same DC pellets using an ultramicrotome (Leica Ultracut UCT), stained with toluidine blue, and then counted by light microscopy (100 cells in two high-power fields per slide, two slides per DC pellet, by two independent observers).
For the TUNEL assay, BMDC were infected at an MOI of 4 with wt HSV-2 or left untreated and collected at time points up to 12 h p.i. Cytospin slides were prepared with 104 cells/slide, fixed in 4% paraformaldehyde, permeabilized with PBS-0.1% Triton X-100 for 15 min at room temperature, washed in PBS, and then labeled with biotin-14-dCTP to detect DNA breaks by nick translation. Briefly, slides were treated with the labeling mix containing TdT reaction buffer (0.5 M potassium cacodylate, pH 7, 10 mM CoCl2, 1 mM dithiothreitol), 50 μM biotin-14-CTP, and 0.2 U of TdT/μl (all from Invitrogen) and then placed in a humidified chamber at 37°C for 30 min, together with positive controls (mock-infected slides pretreated with 50 μl of 3-U/50 μl of DNase I in 0.1 M sodium acetate-5 mM MgCl2) and negative controls (no TdT in labeling mix). Texas Red-avidin D (Vector Laboratories, Burlingame, Calif.) diluted 1:1,000 in PBS was used to colorimetrically detect biotin-dCTP end-labeled (apoptotic) cells. To test if the apoptotic cells were HSV-2 infected, the slides were blocked with 20% donkey serum (Jackson ImmunoResearch) for 30 min at room temperature and then stained with an antibody either against HSV ICP8 (383; David Knipe, Harvard Medical School) or against HSV-2 gD (910; Biodesign International), followed by FITC-conjugated goat anti-mouse MAb (Jackson ImmunoResearch Laboratories) and then washed and mounted on DABCO (Sigma Aldrich) and examined using Spot Advance software on an Olympus BX50 immunofluorescence microscope. The percentages of apoptotic cells (intense red nuclear staining), HSV-infected cells (intense green nuclear or cytoplasmic staining), and double-labeled, apoptotic HSV-infected cells (yellow-stained nucleus or green cytoplasm with red nucleus) were determined in 100 cells from 15 randomly chosen microscopic fields per sample.
Flow cytometry.
The immunophenotype of infected CD11c+ DC or uninfected controls was analyzed 12 h p.i. by fluorescent labeling of cell surface markers followed by FACs analysis (FACScan and Cell Quest software; BD BioSciences). The monoclonal antibodies against CD11c (clone HL-3), CD40 (clone HM-40-3), CD80 (clone 16-10A1), CD86 (clone GLI), and MHC class I H-2Kd (clone SF1-1.1) were all directly conjugated with FITC, and anti-MHC class II I-Ad (G155-178) was directly conjugated with phycoerythrin. The corresponding directly conjugated isotype MAb were used as negative controls; all were obtained from BD PharMingen. Dead cells were excluded by costaining them with propidium iodide (PI).
The purity of T-cell populations for alloproliferation assays were determined by labeling them with MAb against CD3 (A19-3) and then FITC-conjugated goat anti-hamster MAb (G70-204) or with MAb against CD45R (RA3-6B2) or CD11b (M1/70) and then polycloncal FITC-conjugated goat anti-rat MAb (all from BD PharMingen).
Measurement of T-cell proliferation.
T-cell responders were prepared from the spleens of C57BL/6 mice. Single-cell splenocyte suspensions were incubated with hypotonic lysis buffer (0.15 M NH4Cl,1 g of NaHCO3/liter, 0.1 mM EDTA); depleted of B cells and macrophages using CD45R (B220) and CD11b (MAC-1) microbeads, respectively; and then passaged through MidiMACs separation columns to give a population of 90 to 95% CD3 cells by FACS analysis. T cells were resuspended at 3 × 106/ml in RPMI 1640.
DC were infected at an MOI of 4 using wt HSV-2, wt HSV-1, or UV-HSV-2 or mock-infected with PBS; incubated for 12 h at 37°C in RMPI as described above; irradiated (3,000 rads); and washed, and the viable-cell count was determined by trypan blue exclusion. DC were added to responder T cells (105 T cells/well) at ratios of 0.01:1 up to 1:1 in triplicate (final volume, 100 μl) and then incubated for 3 days at 37°C and 5% CO2. The cells were then labeled with 1 μCi of [3H]thymidine (ICN) and harvested onto filter mats. The mats were dried and sealed in bags with scintillant, and samples were counted with a Wallac microbeta counter (Perkin-Elmer). Phorbol myristate acetate (10 ng/ml) (Sigma Aldrich)- ionomycin (500 ng/ml) stimulation of T-cell cultures was used as a positive control, and DC incubated with syngeneic BALB/c T cells were used as negative controls.
IL-12 secretion by DC.
DC were infected with wt HSV-1 or HSV-2 or with UV-HSV-2, all at an MOI of 4, or left untreated. Immediately p.i., the DC were resuspended at 107/ml, and 106 cells/well were added to 96-well tissue culture plates in triplicate for each sample or control. The cells were either left untreated or stimulated with lipopolysaccharide (LPS) (50 ng/ml; Sigma Aldrich) or anti-CD40 (0.01 μg/ml; BD PharMingen) in a final volume of 300 μl. After incubation for 12, 18, or 24 h at 37°C and 5% CO2, the DC were pelleted and the supernatant was collected and stored at −80°C until it was used. Levels of IL-12 were assayed by standard enzyme-linked immunosorbent assay (OptEIA kit; BD PharMingen) on immunopure Maxisorp microtiter plates (Nunc, Roskilde, Denmark), using 100 μl of cell supernatant in triplicate per sample.
Statistical analysis.
The differences in the observed values of loss of DC viability and DC-induced T-cell alloproliferation were determined by the nonparametric Wilcoxon rank sum test. The differences in the mean values of caspase-3 induction and DC IL-12 cytokine production compared to controls were determined by two-tailed Student's t test. A P value of <0.05 was considered a significant difference.
RESULTS
A population of BMDC remains refractory to infection with wt HSV even when exposed to large infectious doses of HSV.
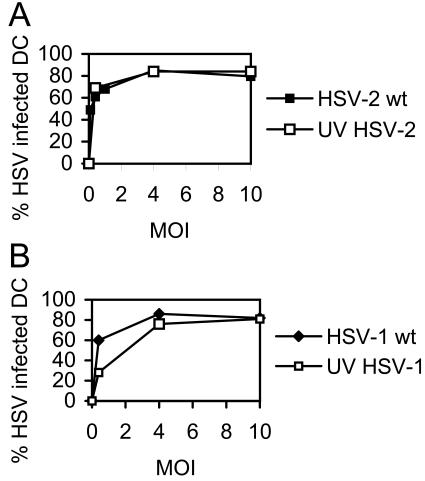
The percentage of immature human MoDC infected with HSV-1 has been shown to be proportional to the level of input virus until a threshold of 80% is reached at a dose of 5 PFU/cell (19). Increasing the amount of input HSV-1 beyond this level did not increase the percentage of infected DC, suggesting that a subpopulation was refractory to infection. To test the susceptibility of immature murine DC to HSV-2 or HSV-1 infection, BMDC from adult mice were infected with wt HSV-2 (strain 186), wt HSV-1 (MC-1), UV-HSV-2, or UV-HSV-1 at various doses, and the percentage of HSV antigen-positive cells was determined 12 h p.i. by immunofluorescence microscopy after staining the cells with antibody or isotype controls. As shown in Fig. 1, the proportion of HSV-infected DC peaked and plateaued at an MOI of 4 after infection with all HSV strains, at which ∼80% of the viable DC population displayed HSV antigens. Increasing the infectious dose to an MOI of 10 did not result in an increase in the number of HSV antigen-positive DC beyond this value. Thus, consistent with previous reports of HSV-1 infection of immature human DC, a population of murine BMDC remains refractory to either HSV-2 or HSV-1 infection even at a high MOI. Of note, the percentage of DC that had taken up either UV-inactivated strain was approximately the same as for the wt strains (except for UV-HSV-1 at an MOI of 1).
FIG. 1.
Infection of murine BMDC by HSV according to virus type and dose. CD11c+ BMDC were infected at a range of MOI as shown with either wt HSV-2 or UV-HSV-2 (A) or with wt HSV-1 or UV-HSV-1 (B). DC were mounted on slides, fixed, and permeabilized at 12 h p.i and then stained with HSV type-specific antibody and counterstained with DAPI. The proportion of DC expressing HSV proteins was determined by immunofluorescence microscopy using five slides per strain. Mock-infected cells did not stain for HSV proteins. Shown are average values ± standard errors of the mean (error bars are too small to be visualized) from one of three independent experiments with similar results.
HSV-2 undergoes de novo viral gene expression in immature murine BMDC but does not generate free infectious progeny.
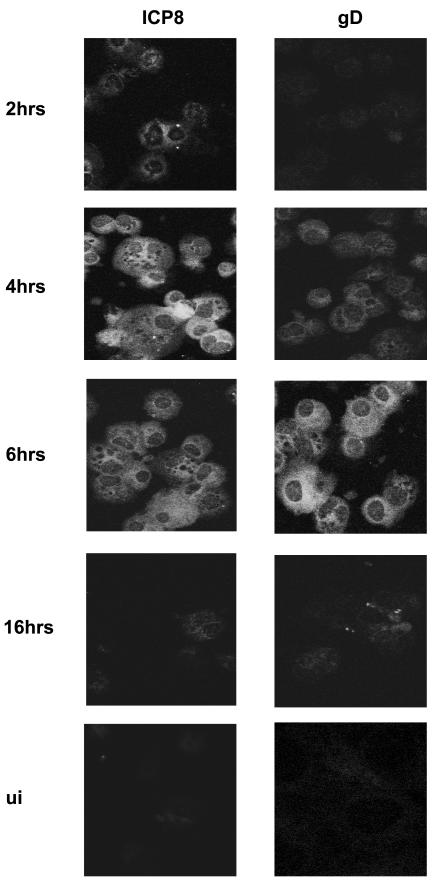
To test if wt HSV-2 undergoes de novo gene expression in murine DC after entry, the expression of either the HSV early protein, ICP8, or the late protein, gD, was examined in HSV-infected adult BMDC or mock-infected controls by immunofluorescence microscopy. The results are shown in Fig. 2. ICP8 was detected in ∼10% of the cells at 2 h p.i. in a predominantly perinuclear pattern and then in 80% of DC by 4 h p.i. in a fine, granular pattern spread evenly throughout the cytoplasm. The intensity of ICP8 staining fell after that time point, reaching undetectable levels by 18 h p.i. (not shown). At no time was the characteristic localization of ICP8 (13) to the nucleus observed, although faint nuclear staining could be observed in a small proportion of HSV-infected cells at 2 h p.i. Staining for the late protein, gD, was detected by 4 h, with peak signal intensity and percentage of infected cells seen at 6 h p.i., after which expression fell, becoming undetectable by 18 h p.i. (not shown). No ICP8 staining was detected in UV-HSV-2-infected DC up to 12 h p.i. (not shown) or in mock-infected controls. Thus, there is de novo expression of HSV-2 gene products in immature murine DC in the early stages of infection but not at later time points (i.e., 18 h p.i.), by which time up to 90% of DC are nonviable.
FIG. 2.
HSV-2 undergoes de novo viral gene expression in infected murine BMDC. CD11c+ BMDC were infected with wt HSV-1 or -2 at an MOI of 4 or left untreated and then incubated for up to 18 h. DC were collected from separate wells of a 24-h plate for each time point, mounted on slides (n = 5 slides/group/time point), fixed, permeabilized, and then stained with antibody specific for either the HSV early protein, ICP8 (383), or the late protein, gD (910). Shown are immunofluorescence microscope images of HSV-2-infected DC stained for ICP8 or gD at 2, 4, 6, or 16 h p.i. (magnification, ×100). No signal was detected in mock-infected cells after staining them for ICP8 or gD (UI) nor for ICP8 in UV-HSV-2-infected DC to 12 h p.i. (not shown). The results shown are from one of three independent experiments with similar results.
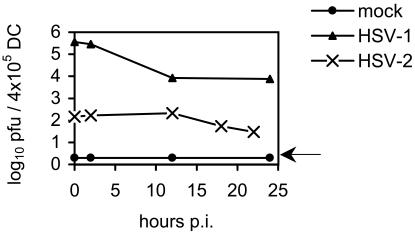
To test if wt HSV productively infects adult murine DC, BMDC were cultured with wt HSV-2 or wt HSV-1 or mock infected, acid washed to remove unbound and surface-bound virions, cultured for various times in medium supplemented with GM-CSF, and then analyzed for infectious virus by standard plaque assay. As shown in Fig. 3, we were unable to detect a significant increase in the infectious titer of HSV-2 or HSV-1 over the residual surface-bound virus left after acid stripping. Time points were not recorded beyond 24 h post-HSV-2 infection, as all DC were nonviable by that time. Thus, infection of murine BMDC with HSV of either serotype was nonproductive.
FIG. 3.
Infection of murine BMDC with HSV-1 or -2 is not productive of infectious progeny. CD11c+ DC were infected with wt HSV-2 or wt HSV-1, both at an MOI of 4, or left untreated; washed in acid three times to remove any excess bound virus; and then plated at a concentration of 4 × 105 DC/well in 24-well plates in 2 ml of GM-CSF-supplemented medium/well. At the times shown, the medium was aspirated (n = 3 wells of DC/group/time point) and frozen at −80°C until the assay. Medium was not harvested from HSV-2-infected DC beyond 24 h p.i., as they were all nonviable after that time. The titer of infectious virus in the supernatant was determined by standard plaque assay. Shown are the mean values ± standard errors of the mean. The results shown are from one of two independent experiments with similar results. The level of detection is indicated by the arrow.
HSV-2 causes rapid cytolysis of murine DC.
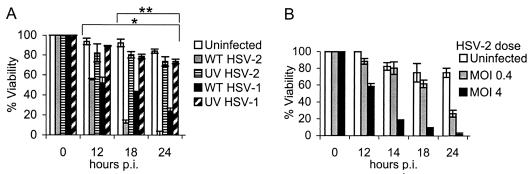
HSV-2 has a more rapid replication cycle than HSV-1. To compare the effect of HSV-2 on the viability of BMDC from adult mice, DC were infected with wt HSV-2, wt HSV-1, or the relevant UV-inactivated HSV strains (at the same preinactivation MOI) or left untreated. DC viability was determined by trypan blue exclusion after incubation for 0 to 24 h. Strikingly, wt HSV of either serotype induced rapid cell death in infected DC, with ∼40% cell death seen by 12 h and almost no viable DC observed 24 h after wt HSV-2 infection at an MOI of 4 (Fig. 4A). In contrast, the viability of DC infected with the respective UV-inactivated HSV strains was equivalent to the viability of uninfected DC cultured for the same period at all times p.i. (P < 0.01; wt HSV-1 or wt HSV-2 versus UV-HSV-1 or -2, respectively, at 12, 18, and 24 h p.i.; Wilcoxon's rank sum test) (Fig. 4A). At later time points, the level of cell death induced by HSV-1 was significantly less than the level of wt HSV-2-induced cell death (P < 0.01; wt HSV-1 versus wt HSV-2 at 18 and 24 h p.i.; Wilcoxon's rank sum test). Similar results were obtained when we used a clinical HSV-2 isolate (9836-4) or laboratory-adapted HSV-1 strains (KOS and HSV-1 mP) (not shown). Thus, HSV induces cytotoxicity in murine BMDC at a serotype-dependent rate.
FIG. 4.
wt HSV induces rapid cytolysis of murine BMDC at a serotype-dependent rate. CD11c+ BMDC were infected with wt HSV-2, UV-HSV-2, wt HSV-1, or UV-HSV-1, all at an MOI of 4 (preirradiation titer) (A) or with wt HSV-2 at an MOI of 0.4 or 4 (B) or left untreated (Uninfected) (A and B) and then incubated in medium supplemented with GM-CSF. At the times shown, the percentage of viable DC was determined by trypan blue exclusion (n = 3 to 7 dishes of DC/group/time). Shown are the mean values ± standard errors of the mean (some error bars are too small to be visualized). The results shown are from concurrent experiments and are representative of one of three independent experiments with similar results. *, P < 0.01 (wt HSV-1 or -2 versus UV-HSV-1 or -2, respectively, at 12, 18, and 24 h p.i.; Wilcoxon's rank sum test; n = 7/group); **, P < 0.01 (wt HSV-1 versus wt HSV-2 at 18 and 24 h p.i.; Wilcoxon's rank sum test; n = 7/group).
The detrimental effects of wt HSV-2 (Fig. 4B) and wt HSV-1 (not shown) on BMDC viability was observed to be dose dependent. Thus, wt HSV induces rapid cytolysis of murine BMDC that is dependent upon viral gene expression.
HSV-2 induces a mixed picture of spontaneous apoptosis and necrosis in murine BMDC.
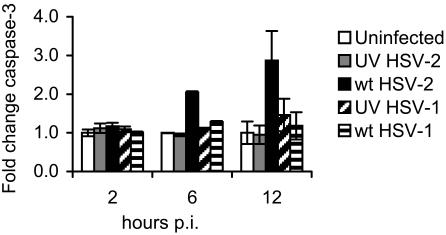
To test if the loss of viability observed after HSV infection of murine BMDC was due to apoptosis or to necrosis, DC were infected with the wt and UV-inactivated strains of either HSV-1 or HSV-2 at an MOI of 4. The level of caspase-3 activity in HSV-infected DC lysates compared to untreated controls was determined at the times listed above. We observed activation of caspase-3 in DC infected with wt HSV-2 by 6 h p.i., and it had increased to almost three times the level of activity in untreated controls by 12 h p.i. (Fig. 5) (P < 0.05; wt HSV-2-infected DC compared to uninfected controls at 12 h p.i.; Student's t test). In contrast, no increase in the activation of caspase-3 was observed in DC infected with wt HSV-1 or either UV-inactivated strain as late as 12 h p.i.
FIG. 5.
HSV-2 induces activation of caspase-3 in murine BMDC. BMDC were infected with wt HSV-2, wt HSV-1, UV-HSV-2, or UV-HSV-1 (all at an MOI of 4 [preirradiation titer for UV strains]) or left untreated (Uninfected) and incubated for 2, 6, or 12 h. The level of caspase-3 activity in the supernatant-free cell pellet of HSV-infected DC from each strain (106 cells/strain in triplicate) was determined using a colorimetric assay for caspase-3 in a 96-well plate (optical density reading at a wavelength of 405 nm using a microplate reader) and compared with the activities of uninfected DC controls and background controls (no cell lysate), which were normalized to a value of 1. Shown are average values ± standard errors of the mean from one of three independent experiments with similar results. P < 0.01 (Student's t test; wt HSV-2 versus uninfected DC; 12 h p.i.).
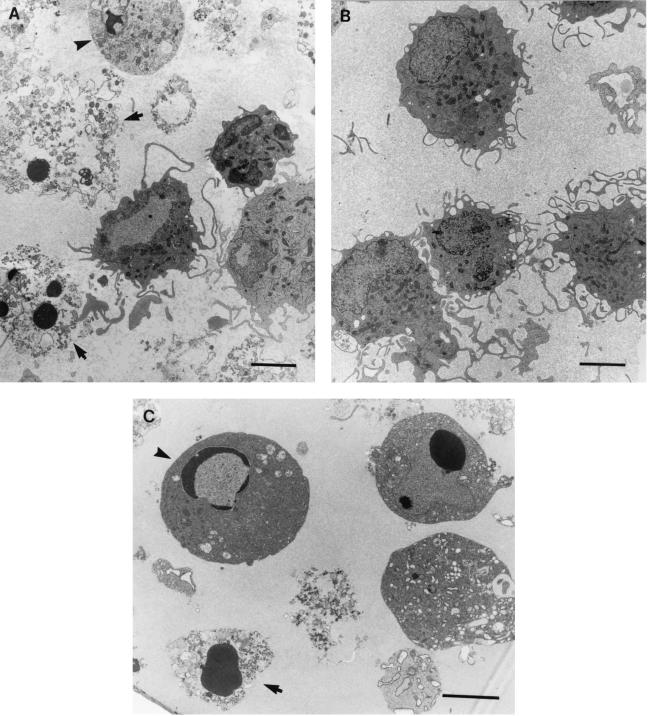
As it is possible that caspase-3 became activated in the HSV-infected DC but did not result in apoptosis, we sought further evidence of the presence of apoptosis in murine DC induced by wt HSV-2 infection. We examined DC by TEM at 2 and 6 h p.i. with wt HSV-2 or wt HSV-1, together with mock-infected controls. Although most DC remained viable at these times post-HSV infection, we observed characteristic ultrastructural evidence of various stages of apoptosis in many HSV-2-infected DC even at 6 h p.i. (Fig. 6A), ranging from early changes, in which the nuclear chromatin was condensed in apposition to an intact nuclear envelope, to condensed chromatin with loss of the nuclear envelope, to frank necrosis. Similar findings were observed after HSV-1 infection of BMDC, although there were notably fewer apoptotic DC at that time (not shown). In contrast, uninfected control DC showed no evidence of necrosis or apoptosis (Fig. 6B). The observed ultrastructural features of apoptosis post-HSV infection were similar to apoptosis induced by treatment of BMDC with UV light (Fig. 6C) and to published reports of ultrastructural features of apoptotic DC (14). To determine the proportion of apoptotic DC in the HSV-2-infected DC or uninfected controls, we performed quantitative analysis of adjacent toluidine blue-stained semithin sections of the same DC pellets by light microscopy. We observed that ∼15% of HSV-2-infected DC were apoptotic by 6 h p.i., equivalent to the percentage observed to be nonviable by trypan blue exclusion at that time (not shown). Thus, wt HSV-2 infection of murine BMDC induces a mixed picture of apoptosis and necrosis in murine BMDC shortly after infection.
FIG. 6.
HSV-2 induces ultrastructural changes of apoptosis in a subpopulation of BMDC by 6 h p.i. BMDC were infected with wt HSV-2 (186) or wt HSV-1 (MC-1), both at an MOI of 4, or left untreated and incubated for 2 or 6 h in GM-CSF-supplemented medium. The DC were pelleted (106 cells for each strain) and then fixed, embedded in resin, and cut as ultrathin sections on a microtome; the sections were collected on thin-bar copper grids, stained (1% uranyl acetate in 50% ethanol and Reynold's lead citrate), and examined by TEM. Shown are electron micrographs of BMDC infected with wt-HSV-2 and displaying typical nuclear features of apoptosis (condensation of chromatin) at different stages, ranging from early apoptosis (arrowhead) to apoptotic necrosis (arrows) (A), uninfected BMDC which are uniformly healthy (B), and early (arrowhead) and late (arrow) stages of apoptosis in uninfected BMDC induced by treatment with UV light for 2 min and incubation in serum starvation medium for 3 h (C). Bars = 3 μm.
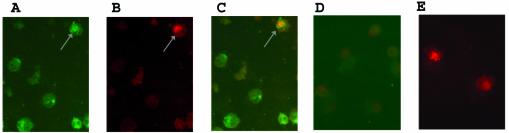
To test if the apoptotic DC in the BMDC cultures were themselves HSV infected or were uninfected bystander DC, cells were collected at times up to 12 h p.i. and then examined by double immunofluorescence (TUNEL assay) for DNA breaks and the HSV protein ICP8 or gD. Consistent with earlier experiments, we observed that by 2 h p.i., cell viability was 100% (trypan blue) and that ∼40% of the cells stained positive for HSV ICP8 in the cytoplasm and, on occasion, the nucleus (Fig. 7A). Approximately 15 to 20% of DC showed evidence of apoptosis by TUNEL staining at that time (Fig. 7B), all of which were found to be HSV infected (Fig. 7C). No staining or apoptosis was observed in uninfected BMDC at that time (Fig. 7D), but TUNEL-positive cells could be detected after treatment with DNase (Fig. 7E). The level of ICP8 staining was too low at 6 and 12 h p.i. to allow accurate determination of the percentage of HSV-infected, apoptotic DC. Therefore, we tested by double staining for gD and apoptosis (TUNEL). We observed that although 80% of DC expressed HSV gD by 12 h p.i. and the total viability of the DC (by trypan blue exclusion) had fallen to 60%, the proportion of apoptotic DC remained constant at 20%, and they were once again seen only in the HSV-infected (gD-positive) DC population (not shown).
FIG. 7.
Subpopulation of HSV-2-infected BMDC exhibit DNA breaks shortly after infection. BMDC were infected with wt HSV-2 at an MOI of 4 or left untreated, incubated for up to 12 h p.i., and then double stained for HSV ICP8 (anti-ICP8 MAb 383 plus FITC-conjugated goat anti-mouse MAb) and for apoptosis (biotin-labeled dCTP followed by avidin-Texas Red). Shown are immunofluorescence microscope images of the same optical section of a population of HSV-2-infected DC collected 2 h p.i. to demonstrate staining for ICP8 (green) (A), nick end-labeled DNA breaks (TUNEL) (red) (B), and a double-labeled, HSV-2-infected, apoptotic BMDC (yellow). Arrow in panels A, B, and C indicates positive staining of an apoptotic, HSV-2-infected BMDC. Included were double-stained uninfected BMDC as a negative control (D) and DNase-treated uninfected DC stained with biotin-dCTP-avidin-Texas red as a positive control (E) for the TUNEL assay. Magnification, ×40.
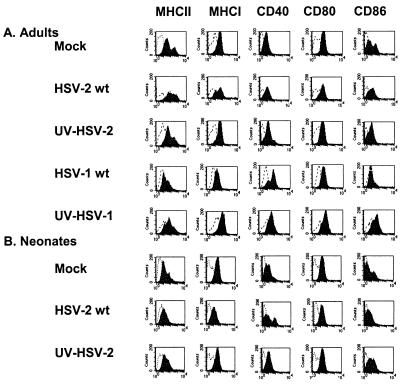
HSV infection of immature murine BMDC induces modest type-specific effects on the DC immunophenotype by 12 h p.i.
HSV-1 has been shown to alter the cell surface phenotypes of both immature (19, 27) and mature (15, 19) human MoDC. To test the effect of HSV on the surface immunophenotype of murine BMDC, DC were infected with either wt HSV strain or with the UV-inactivated strains at the same preinactivation dose or left untreated. FACS analysis for MHC-I and -II molecules and for the costimulatory markers CD80, CD86, and CD40 was performed 12 h p.i. Although we had previously shown that a proportion of HSV-infected DC were nonviable at 12 h p.i., this time was chosen to reflect the approximate time that DC signal naive T cells in draining lymph nodes in vivo after migration from the site of HSV infection (22). Nonviable cells were excluded by counterstaining them with PI, and the degrees of HSV infection (∼80% of viable DC) for all groups were confirmed to be equivalent by immunofluorescence microscopy for HSV antigens. The mock-infected BMDC population was shown to consist of ∼80% immature DC (MHC-IIlo CD86lo) and 20% mature DC (MHC-IIhi CD86hi) (Fig. 8A). By 12 h p.i., wt HSV-2 upregulated DC MHC-II expression in the MHC-IIlo population, resulting in loss of the biphasic pattern of expression observed in mock-infected controls, but did not significantly alter the level of surface CD40, CD80, or CD86 compared to that in mock-infected controls (Fig. 8A). MHC-I expression was downregulated in a subpopulation of DC by wt HSV-2 but not by UV-HSV-2, suggesting that de novo expression of an HSV gene product(s) was responsible for this phenomenon. In contrast to wt HSV-2, wt HSV-1 had little effect on surface MHC-II, MHC-I, or costimulatory molecule expression in murine DC, aside from upregulation of surface CD40 (Fig. 8A). This upregulation was also seen after infection with UV-inactivated HSV-1, which in addition upregulated expression of MHC-I, CD80, and CD86 but had no effect on MHC-II expression. Thus, HSV infection of murine BMDC has serotype-specific effects on the DC surface immunophenotype.
FIG. 8.
Effects of HSV on the immunophenotype of murine BMDC according to virus strain and age. CD11c+ BMDC derived from adult female mice (A) or from 1-week-old mice (B) were infected at an MOI of 4 with wt HSV-2 or UV-HSV-2 (A and B) or with wt HSV-1 or UV-HSV-1 (adult mice alone) (A) or left untreated (Mock) (A and B); incubated for 12 h; stained for surface MHC-I, MHC-II, CD40, CD80, and CD86 expression (solid curves) or with isotype controls (open curves); and then analyzed by FACS. Dead cells were excluded by costaining them with PI. The results shown are from one of three independent experiments.
HSV is highly virulent in newborn humans and mice. As part of ongoing studies to explore immunological mechanisms behind the age-related defective response to HSV, we tested the effect of HSV-2 infection on the surface immunophenotype of murine BMDC that had been derived from 1-week-old mice (Fig. 8B). The surface immunophenotype of uninfected neonatal BMDC generated after 10 days of culture in GM-CSF-supplemented medium was more immature than that of the BMDC from adult mice derived in the same manner in that there were lower levels of MHC-II and CD86 expression in both the MHC-IIlo and MHC-IIhi populations. Infection with wt HSV-2 had more profound effects on neonatal DC than it did on DC from adult counterparts. Wt HSV-2, but not UV-HSV-2, resulted in downregulation of surface MHC-I, MHC-II, and CD86 but had differential effects on the surface expression of CD40, with two populations becoming evident: CD40hi and CD40lo (Fig. 8B). Neonatal murine BMDC failed to upregulate MHC-II in response to wt HSV-2, as observed in adult mice. Thus, there are some age-related differences in the effects of HSV-2 infection on the surface immunophenotype of murine BMDC in vitro.
HSV-2 infection impairs murine BMDC alloproliferative response.
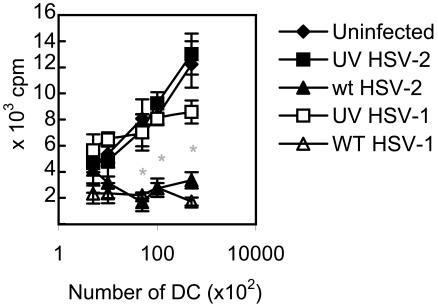
DC are potent stimulators of naive T cells. The ability of DC to induce an allogeneic T-cell proliferative response is therefore an important measure of their function. To test the effect of HSV-2 infection on the ability of DC to induce an alloproliferative T-cell response, BMDC from BALB/c mice (H-2b) were infected with either wt HSV or the UV-inactivated strains at an MOI of 4 or left untreated and then irradiated. After 12 h of incubation (to mimic the time p.i. of T-cell signaling in vivo) (22), serial dilutions of viable DC from each group were incubated with splenocytes from naive adult C57BL/6 mice (H-2d), cultured for 3 days, and labeled with [3H]thymidine, and the degree of DC-induced alloproliferation was determined by standard techniques. As expected, increasing numbers of uninfected DC resulted in a proportionate increase in the alloproliferative response (Fig. 9). Infection of DC with UV-inactivated HSV had no effect on the allostimulatory capacity of the DC. In marked contrast, infection of DC with either wt HSV strain reduced the T-cell proliferative response to background levels at all DC dilutions, reaching statistical significance for DC/T-cell ratios of 1:50 and above (Fig. 9) (P < 0.01; Wilcoxon's rank sum test; wt HSV-1 or -2 versus uninfected DC or versus UV-HSV-1- or UV-HSV-2-infected DC, respectively). The same phenomenon was observed when DC were incubated with mismatched splenocytes immediately after wt HSV-2 infection (not shown). Thus, wt HSV infection of murine BMDC inhibits the allostimulatory capacity of DC.
FIG. 9.
HSV-2 infection of murine BMDC impairs DC-induced T-cell alloproliferation. CD11c+ DC derived from BALB/c mice (H-2d) were infected with wt HSV-2, wt HSV-1, or the respective UV-inactivated strains (UV-HSV-2 or UV-HSV-1), all at an MOI of 4, or left untreated (Uninfected) and then incubated for 12 h. Viable DC were irradiated and then incubated with T cells derived from the spleens of C57BL/6 mice (H-2b) at ratios of 0.01:1 to 1:1 (105 T cells/well; n = 6 wells per DC/T cell ratio per strain) for 3 days. The cultures were then labeled with [3H]thymidine, and the levels of 3H incorporation were determined after 18 h. Shown are the mean values ± standard errors of the mean. The results shown are from one of two independent experiments with similar results. Proliferation of T cells in the absence of DC was negligible. *, P < 0.01 (Wilcoxon's rank sum test; wt HSV-2 or wt HSV-1 versus uninfected DC or versus the respective UV-inactivated strain; n = 6 observations/group).
HSV-2 infection impairs DC LPS-induced secretion of IL-12.
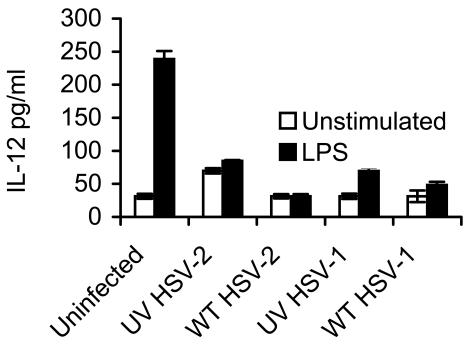
The induction of a Th-1 cytokine producing T-cell effectors has been shown to be essential for protection against intracellular pathogens like HSV. DC have recently been identified as the principal Th1-inducing cytokine in the primary T-cell response. Therefore, we compared the ability of wt HSV-infected DC to produce IL-12 with or without additional stimulation with LPS or CD40 ligation to the level produced by uninfected DC or DC infected with UV-inactivated HSV strains under the same conditions. CD40 ligation with MAb failed to produce detectable levels of IL-12, even by uninfected murine BMDC at 12, 24, 48, and 72 h p.i. (not shown). Shown in Fig. 10 are the levels of IL-12 in the supernatant after BMDC were infected and then incubated for 24 h, the time of optimal IL-12 production by uninfected, LPS-stimulated DC in our system (not shown). IL-12 production in the absence of stimuli in uninfected DC or after infection with wt HSV-1 or -2 was at the level of detection of the assay. Infection with either UV-inactivated HSV strain induced low levels of IL-12, which increased modestly when costimulated with LPS. Strikingly, infection of DC with either wt HSV strain suppressed LPS-induced DC IL-12 production to background levels (P < 0.01; Student's t test; wt HSV-1- or wt HSV-2-infected DC plus LPS versus uninfected DC plus LPS). IL-12 production by wt HSV-2-infected DC at earlier time points (12 and 18 h p.i.) showed similar suppression (not shown). Thus, wt HSV infection of BMDC reduced their ability to produce IL-12 in response to stimulation.
FIG. 10.
HSV impairs LPS-induced IL-12 secretion by murine BMDC. CD11c+ BMDC were infected with wt HSV-1 or -2 or the respective UV-inactivated HSV-2, all at an MOI of 4, or left untreated (Uninfected); washed; and then plated in 96-well plates and coincubated with LPS (50 ng/ml) for 24 h or in GM-CSF-supplemented medium alone (Unstimulated) (106 DC/well in triplicate per strain per treatment), and the level of IL-12 in the supernatant was determined by capture enzyme-linked immunosorbent assay. The level of sensitivity of the assay was 31.3 pg/ml. Shown are mean values ± standard errors of the means. The results shown are from one of five independent experiments with similar results. P < 0.01 (Student's t test; wt HSV-2- or wt HSV-1-infected and LPS-treated DC compared to uninfected and LPS-treated DC).
DISCUSSION
Here, we describe the novel finding that HSV induces rapid cell death in murine BMDC at a serotype-dependent rate, with HSV-2 being more potent than HSV-1 in this regard. The type of HSV-induced cell death was shown to be a mixed picture of necrosis and apoptosis. The phenomenon was not simply due to consumption of nutrients in the media, as uninfected DC remained viable throughout the period of observation. De novo HSV gene expression was required to induce BMDC cell death, as shown by the failure of UV-inactivated HSV strains (type 1 or 2) to reproduce this effect. Apoptosis was observed only in HSV-infected cells, but not all HSV-infected cells were apoptotic. It was also seen predominantly at early times, raising the possibility that many of the cells observed to be in the advanced stages of necrosis by 6 and 12 h p.i. by TEM and trypan blue may have progressed to this stage via apoptosis. Alternatively, there were two concurrent types of cell death occurring.
In contrast to our observations in BMDC, HSV has been shown to strongly inhibit apoptosis induced by the virus itself in a number of cell types, including HEp-2 cells (7), Jurkat cells (11), PC-12 cells (21), and murine neurons (2, 24). The virus can also protect infected cells against apoptosis induced by external triggers, including Fas, C2-ceramide, and both osmotic and thermal shock, although the degree of protection varies with the cell type studied (8). Consistent with our observations in murine DC, spontaneous induction of apoptosis by HSV has been reported in activated human cord blood T cells (10), in activated murine and human T cells (25), and in freshly isolated murine macrophages after infection in vitro with HSV (5). Thus, cells of hematological origin may reflect one end of the spectrum in which the balance between virus-induced apoptosis and virus-induced inhibition of apoptosis at times favors the former. This is not specific to DC of murine origin, as shown by similar observations made in human MoDC (L. Bosnjak, M. Miranda-Saksena, C. A. Jones, R. A. Boadle, Z. Mikloska, and A. L. Cunningham, unpublished data), although the effect was much more rapid in mice.
Intertypic differences in the ability of HSV to protect against apoptosis have been reported. HSV-1 has been shown to be a more potent inhibitor of apoptosis induced by UV light or by anti-Fas antibody than HSV-2 (11). Although this effect was seen for both low-passage clinical isolates and laboratory-adapted HSV-1 strains, only laboratory-adapted HSV-2 strains protected cells against externally triggered apoptosis. Importantly, neither laboratory-adapted strains nor clinical HSV-2 isolates were by themselves shown to induce apoptosis in other cell types up to 48 h p.i. (16). Consistent with these earlier reports, we found that HSV-2 was a more potent trigger for apoptosis than HSV-1. However, we observed no difference between the abilities of clinical and laboratory-adapted isolates of HSV-2 (or HSV-1) to induce apoptosis at 12 h p.i. in murine BMDC.
Infection with increasing doses of wt HSV or UV-inactivated virus of either HSV type resulted in an increased percentage of infected DC until a plateau was reached, similar to an earlier observation with HSV-1 infection of human MoDC (19). Whereas the previously reported human MoDC were a largely immature population, our DC population contained ∼10 to 15% mature DC (CD11c+ MHCIIhi), which may be synonymous with the group that was refractory to infection in our population. All DC in our study rapidly died after exposure to HSV of either type, suggesting that the uninfected subpopulation did not die directly from viral infection but from a bystander mechanism. A further study is under way to explore this possibility.
HSV underwent de novo expression of early and late proteins in murine BMDC after both HSV-2 and HSV-1 infection. However, for reasons that remain to be elucidated, the kinetics of HSV-2 expression in murine DC was more rapid than previously observed in cells of primate origin (34). Infection of the murine BMDC with either strain appeared to be abortive, as we did not detect any significant increase in the titer of infectious virus released into the supernatant during prolonged culture. A likely explanation is that the rapid induction of cell death by both HSV-2 and HSV-1 in the murine BMDC inhibits completion of the viral replication cycle and release of infectious progeny. This is supported by the findings that the single-stranded DNA binding protein, ICP8, remained predominantly cytoplasmic and did not exhibit the characteristic localization to the nucleus (13) and that early and late protein expression was markedly reduced at 16 h p.i. instead of being strong. Using the same clinical HSV-1 isolate (19), HSV-1 has been shown to productively infect immature MoDC of human origin, although the levels of infectious virus produced were very low. The different observations could be explained by the different rate of induction of apoptosis between the two species. The permissiveness of human DC for HSV replication also appears to differ with the maturation state of the DC, with nonproductivity reported after incubation of mature MoDC with the laboratory-adapted HSV-1 strain, Ang (14).
Intertypic differences were also observed in both the extent to which HSV affects the DC immunophenotype and the kinetics of this effect. Although a proportion of DC were apoptotic at the time point studied, only viable cells were included in the analysis, of which 80% were HSV infected. We observed that wt HSV-2 induced moderate downregulation of MHC class I expression, in contrast to no detectable change after infection with UV-HSV-2. This suggests that the mechanism by which wt HSV-2 exerted this effect was not due to viral entry. MHC class I expression has been shown to be reduced in a variety of human cell types by HSV ICP47 (type 1 and type 2) inhibition of TAP. However, as HSV ICP47 has been shown to be only a weak inhibitor of murine TAP (6, 9, 32), it is possible that an alternative mechanism exists in the mouse to impair MHC class I presentation. The effect of the clinical HSV-1 isolate on the adult murine DC immunophenotype by 12 h p.i. was minimal, consistent with previous observations using the same strain in human MoDC at the same time point (19).
We observed an age-related difference in the effects of HSV-2 on the DC immunophenotype, with greater wt HSV-2-induced downregulation of MHC class I and CD86 in neonatal BMDC than was observed in infected adult counterparts. Both the CD28/CD80-86 and the CD40/CD40 ligand (CD154) costimulatory pathways have been shown to be important for the induction of the primary T-cell response to HSV in mice, using reagents that block the pathway, and mice with genetic defects in the relevant receptors (3). Neonatal T cells have been shown to require increased costimulatory signals to achieve the adult levels of activation to a predetermined stimulus. Supporting this notion, neither wt HSV-2 or UV-HSV-2 induced the same degree of maturation in newborn DC that was observed with adult DC, suggesting that BMDC derived from neonatal mice do not mature to the same extent as their adult counterparts in response to the same signal. Thus, it is possible that HSV-induced reduction in surface DC costimulatory molecule expression may further reduce the ability of naive neonatal T cells to become activated in the mucosa or in the draining lymph nodes.
Infection of the BMDC with wt HSV of either serotype significantly reduced their ability to stimulate an alloproliferative response or to secrete IL-12, in contrast to DC that had been infected with a UV-inactivated strain. T-cell activation occurs rapidly after T-cell receptor engagement with the DC; therefore, although wt HSV-2 had induced cell death in most of the BMDC by the time the alloproliferation assay was completed, these functional impairments were likely due to “apoptosis in progress,” resulting in disarranged cell architecture. However, reproduction of the results of the alloproliferation assays with HSV-2 DC set up 1 h p.i. also suggests the possibility of an additional virus-induced block to function (C. A. Jones, M. Fernandez, and K. Herc, unpublished observations). IL-12 is thought to be the principal Th-1-driving cytokine during a primary immune response to a pathogen (18). As HSV induces a predominantly CD4 Th1 response early in infection, this suggests that bystander DC primed with HSV antigen play a greater role in the induction of the HSV-specific CD4 T-cell response than HSV-infected DC. Our findings are in contrast to the observations that wt HSV-1 (KOS) infection of murine “splenocyte-enriched DC” at a comparable MOI upregulates IL-12 p40 mRNA expression by 12 h p.i (12). In the same study, UV-inactivated HSV-1 induced only minor IL-12 mRNA upregulation at the same time points. Low-level induction of IL-12 p40 mRNA expression was also demonstrated in the draining lymph nodes after footpad inoculation with wt HSV-1. It is possible that the observed differences in HSV-induced production of IL-12 by DC are due to differences in the types of cells being studied (i.e., a highly purified population of BMDC compared to splenocyte-enriched DC that were derived by passing splenocytes through a metrizamide gradient, which, as acknowledged by the authors, also contained many macrophages and neutrophils, without further purification). Alternatively, it may relate to the types of assays used to measure IL-12 production. Reverse transcription-PCR determination of IL-12 p40 mRNA expression (12) would fail to detect a posttranscriptional HSV-induced block to IL-12 secretion, which could be detected by determination of the level of IL-12 heterodimer in infected DC supernatant, as used in our experiments. Further studies to test the effect HSV infection on IL-12 secretion and expression in DC derived directly ex vivo, or draining lymph node cells after infection in vivo, are under way.
These experiments indicate that HSV-2 has differential effects on DC immunobiology in mice in vitro compared to HSV-1. Whether this translates into important functional effects in the natural history of HSV infection in vivo in mice or humans has yet to be determined. HSV-2 replication-defective vectors expressing simian immunodeficiency virus genes have been shown to be less potent inducers of cytolytic T-cell responses in monkeys than HSV-1 expressing the same gene product (D. Knipe, personal communication). Exploration of the intertypic differences in the effects of HSV on DC structure and function may provide valuable insights into the molecular mechanisms behind the induction of a protective anti-HSV immune response.
Acknowledgments
This work was supported in part by the March of Dimes Basil O'Connor Research Scholar Award (2000), the International Herpesvirus Medical Forum G. S. K. Elion Award (2001), and the Children's Hospital at Westmead Research Career Development Award (all to C.A.J.).
We thank Belinda Mercer, Rose Boutros, Ingrid Evans, and Cassandra Drury for their technical assistance and Lisa Sedger for thoughtful criticism of the data.
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, M., M. Lock, C. G. Miller, and N. W. Fraser. 2002. Regions of the herpes simplex virus type 1 latency-associated transcript that protect cells from apoptosis in vitro and protect neuronal cells in vivo. J. Virol. 76:717-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edelmann, K. H., and C. B. Wilson. 2001. Role of CD28/CD80-86 and CD40/CD154 co-stimulatory interactions in host defense to primary herpes simplex virus infection. J. Virol. 75:612-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans, I. A., and C. A. Jones. 2002. Protection against disseminated neonatal HSV disease by maternal immunisation with an HSV-2 replication-defective mutant in mice. J. Infect. Dis. 185:1550-1560. [DOI] [PubMed] [Google Scholar]
- 5.Fleck, M., J. D. Mountz, H. C. Hsu, J. Wu, C. K. Edwards III, and E. R. Kern. 1999. Herpes simplex virus type 2 infection induced apoptosis in peritoneal macrophages independent of Fas and tumor necrosis factor-receptor signaling. Viral Immunol. 12:263-275. [DOI] [PubMed] [Google Scholar]
- 6.Fruh, K., K. Ahn, H. Djaballah, P. Sempe, P. M. van Endert, R. Tampe, P. A. Peterson, and Y. Yang. 1995. A viral inhibitor of peptide transporters for antigen presentation. Nature 375:415-418. [DOI] [PubMed] [Google Scholar]
- 7.Galvan, V., R. Brandimarti, and B. Roizman. 1999. Herpes simplex virus 1 blocks caspase-3-independent and caspase-dependent pathways to cell death. J. Virol. 73:3219-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galvan, V., and B. Roizman. 1998. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc. Natl. Acad. Sci. USA 95:3931-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill, A., J. Jugovic, I. York, G. Russ, J. Bennink, J. Yewdell, H. Ploegh, and D. Johnson. 1995. Herpes simplex virus turns off the TAP to evade host immunity. Nature 375:411-415. [DOI] [PubMed] [Google Scholar]
- 10.Ito, M., W. Koide, M. Watanabe, H. Kamiya, and M. Sakurai. 1997. Apoptosis of cord blood T lymphocytes by herpes simplex virus type 1. J. Gen. Virol. 78:1971-1975. [DOI] [PubMed] [Google Scholar]
- 11.Jerome, K. R., R. Fox, Z. Chen, P. Sarkar, and L. Corey. 2001. Inhibition of apoptosis by primary isolates of herpes simplex virus. Arch. Virol. 146:2219-2225. [DOI] [PubMed] [Google Scholar]
- 12.Kanangat, S., J. Thomas, S. Gangappa, J. S. Babu, and B. T. Rouse. 1996. Herpes simplex virus type 1-mediated up-regulation of IL-12 (p40) mRNA expression. Implications in immunopathogenesis and protection. J. Immunol. 156:1110-1116. [PubMed] [Google Scholar]
- 13.Knipe, D. M., and A. E. Spang. 1982. Definition of a series of stages in the association of two herpesviral proteins with the cell nucleus. J. Virol. 43:314-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koyama, A. H., H. Irie, F. Ueno, M. Ogawa, A. Nomoto, and A. Adachi. 2001. Suppression of apoptotic and necrotic cell death by poliovirus. J. Gen. Virol. 82:2965-2972. [DOI] [PubMed] [Google Scholar]
- 15.Kruse, M., O. Rosorius, F. Kratzer, G. Stelz, C. Kuhnt, G. Schuler, J. Hauber, and A. Steinkasserer. 2000. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. J. Virol. 74:7127-7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leopardi, R., C. Van Sant, and B. Roizman. 1997. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc. Natl. Acad. Sci. USA 94:7891-7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutz, M. B., N. Kukutsch, A. L. Ogilvie, S. Rossner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol Methods 223:77-92. [DOI] [PubMed] [Google Scholar]
- 18.MacDonald, A. S., and E. J. Pearce. 2002. Polarized Th cell response induction by transferred antigen-pulsed dendritic cells is dependent on IL-4 or IL-12 production by recipient cells. J. Immunol. 168:3127-3130. [DOI] [PubMed] [Google Scholar]
- 19.Mikloska, Z., L. Bosnjak, and A. L. Cunningham. 2002. Immature monocyte-derived cells are productively infected with herpes simplex virus type 1. J. Virol. 75:5958-5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miranda-Saksena, M., P. Armati, R. A. Boadle, D. J. Holland, and A. L. Cunningham. 2000. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J. Virol. 74:1827-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moxley, M. J., T. M. Block, H. C. Liu, N. W. Fraser, G. C. Perng, S. L. Wechsler, and Y. H. Su. 2002. Herpes simplex virus type 1 infection prevents detachment of nerve growth factor-differentiated PC12 cells in culture. J. Gen. Virol. 83:1591-1600. [DOI] [PubMed] [Google Scholar]
- 22.Mueller, S. N., C. M. Jones, C. M. Smith, W. R. Heath, and F. R. Carbone. 2002. Rapid cytotoxic T lymphocyte activation occurs in the draining lymph nodes after cutaneous herpes simplex virus infection as a result of early antigen presentation and not the presence of virus. J. Exp. Med. 195:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palucka, K., and J. Banchereau. 2002. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr. Opin. Immunol. 14:420-431. [DOI] [PubMed] [Google Scholar]
- 24.Perkins, D., E. F. Pereira, M. Gober, P. J. Yarowsky, and L. Aurelian. 2002. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathway. J. Virol. 76:1435-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raftery, M. J., C. K. Behrens, A. Muller, P. H. Krammer, H. Walczak, and G. Schonrich. 1999. Herpes simplex virus type 1 infection of activated cytotoxic T cells: induction of fratricide as a mechanism of viral immune evasion. J. Exp. Med. 190:1103-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizzitelli, A., R. Berthier, V. Collin, S. M. Candeias, and P. N. Marche. 2002. T lymphocytes potentiate murine dendritic cells to produce IL-12. J. Immunol. 169:4237-4245. [DOI] [PubMed] [Google Scholar]
- 27.Salio, M., M. Cella, M. Suter, and A. Lanzavecchia. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur. J. Immunol. 29:3245-3253. [DOI] [PubMed] [Google Scholar]
- 28.Schon, E., A. M. Harandi, I. Nordstrom, J. Holmgren, and K. Eriksson. 2001. Dendritic cell vaccination protects mice against lethality caused by genital herpes simplex virus type 2 infection. J. Reprod. Immunol. 50:87-104. [DOI] [PubMed] [Google Scholar]
- 29.Spang, A. E., P. Godwoski, and D. M. Knipe. 1983. Characterization of herpes simplex virus type 2 temperature-sensitive mutants whose lesions map in or near the coding sequences for the major DNA-binding protein. J. Virol. 45:332-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sprecher, E., and Y. Becker. 1992. Detection of IL-1 beta, TNF-alpha, and IL-6 gene transcription by the polymerase chain reaction in keratinocytes, Langerhans cells and peritoneal exudate cells during infection with herpes simplex virus-1. Arch Virol. 126:253-269. [DOI] [PubMed] [Google Scholar]
- 31.Sousa, C. R., S. Hieny, T. Scharton-Kersten, D. Jankovic, H. Charest, R. N. Germain, and A. Sher. 1997. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp Med. 186:1819-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomazin, R., N. E. van Schoot, K. Goldsmith, P. Jugovic, P. Sempe, K. Fruh, and D. C. Johnson. 1998. Herpes simplex virus type 2 ICP47 inhibits human TAP but not mouse TAP. J. Virol. 72:2560-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu, X., S. Fournier, J. P. Allison, A. H. Sharpe, and R. J. Hodes. 2000. The role of B7 costimulation in CD4/CD8 T cell homeostasis. J. Immunol. 164:3543-3553. [DOI] [PubMed] [Google Scholar]
- 34.Zhu, H. Y., H. Yamada, Y. M. Jiang, M. Yamada, and Y. Nishiyama. 1999. Intracellular localization of the UL31 protein of herpes simplex virus type 2. Arch. Virol. 144:1923-1935. [DOI] [PubMed] [Google Scholar]