Abstract
In contrast to simian immunodeficiency viruses (SIVs), which induce immunodeficiency over a 1- to 2-year period, highly pathogenic simian-human immunodeficiency viruses (SHIVs) cause an irreversible and systemic depletion of CD4+ T lymphocytes in macaque monkeys within weeks of inoculation. Nonetheless, the seemingly more aggressive SHIVs have proven to be easier to control by the same vaccine regimens which fail to contain SIV. Because early events during in vivo infections may determine both the pathogenic consequences of the challenge virus and its sensitivity to interventions that prevent disease, we have evaluated the effects of inoculum size and a potent antiretroviral drug on the development of disease in monkeys infected with SHIVDH12R. The results obtained show that in a majority of inoculated animals, suppression of SHIV replication during the first 2 weeks of infection, which prevents complete loss of CD4+ T cells, leads to very low to undetectable postpeak viremia and an asymptomatic clinical course for periods up to 4 years.
During the past few years, pathogenic simian-human immunodeficiency viruses (SHIVs) have largely supplanted simian immunodeficiency viruses (SIVs) as the primate lentivirus of choice for the challenge of macaque monkeys in vaccine experiments (1, 4, 6, 35, 40, 49). This has occurred for two principal reasons: (i) SHIVs bear the human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein, thereby permitting an assessment of anti-HIV-1 neutralizing antibody (NAb) induction, and (ii) SHIVs cause an unusually rapid, irreversible, and systemic elimination of CD4+ T lymphocytes within 3 to 4 weeks of inoculation (17, 19, 33). Although the latter pathogenic phenotype permits an early assessment of vaccine efficacy against disease, it is profoundly different from the clinical course commonly associated with SIV and HIV-1 infections, which are characterized by more-moderate depletions of CD4+ T cells and the development of clinical immunodeficiency over a much longer time frame (1 to 2 years and 10 years, respectively) (8, 21, 28, 31). Despite their seemingly more aggressive pathogenicity in vivo, SHIVs have proven to be easier to control by the same vaccination regimens that fail to protect rhesus monkeys from challenges with pathogenic SIV strains such as SIVmac239 and SIVE660 (15, 32). Because these discrepancies in vaccine sensitivity might reflect fundamental differences in the mechanisms underlying the diseases induced by SIV and SHIVs, we have examined how a directed intervention (administration of a potent reverse transcriptase [RT] inhibitor) during the first 2 weeks of the acute infection or the conditions of initiating the primary infection by varying the inoculum size might modulate the natural history of pathogenic SHIV infections over a 2- to 4-year observation period. The results obtained have been compared with those previously reported for SIV.
In the present study, we used uncloned SHIVDH12R stock (13, 17) and found that the complete and irreversible depletion of CD4+ T cells in infected rhesus monkeys could be abolished, following a single 4-week course of anti-retroviral therapy (using 9-[2-(R)-(phosphonomethoxy) propyl]adenine [PMPA]) initiated on day 5 postinfection. Three of four animals remain alive and healthy, with low to undetectable levels of plasma viral RNA, 1.5 to 3 years after cessation of treatment. Furthermore, one of two monkeys receiving similar 4-week PMPA regimens beginning on day 14 postinoculation (when plasma viral RNA levels were quite high [5.1 × 107 RNA copies/ml] and the number of CD4+ T lymphocytes had started to decline) was also asymptomatic for more than 3 years postinfection. Similar benign long-term clinical outcomes were observed in several naïve monkeys inoculated with very low doses (1 to 25 tissue culture infective doses [TCID50]) of SHIVDH12R even though they had experienced high levels (107 to 108 RNA copies/ml) of peak viremia between weeks 2 and 3. Thus, blunting of the acute SHIVDH12R infection, which prevents the complete elimination of CD4+ T cells, resulted in an uneventful and durably maintained (2 to 4 years) clinical course in the majority of inoculated rhesus monkeys.
MATERIALS AND METHODS
Animal experiments.
Rhesus macaques (Macaca mulatta) were maintained in accordance with the guidelines of the Committee on Care and Use of Laboratory Animals (Guide for the Care and Use of Laboratory Animals, NIH 85-23, Department of Health and Human Services, 1985) and were housed in a biosafety level 2 facility; biosafety level 3 practices were followed. Animals were anesthetized with intramuscular injections of ketamine hydrochloride (Ketaject; Phoenix Pharmaceutical, Inc. St. Joseph, Mo.) and acepromazine maleate (Fermenta Animal Health Co. Kansas City, Mo.) during phlebotomies or SHIV inoculations.
Virus and plasma viral RNA measurements.
The emergence, characterization, and preparation of the highly pathogenic SHIVDH12R stock have been described previously (13, 16, 17). In the experiments described, the indicated amounts of SHIVDH12R were inoculated into the saphenous veins of anesthetized rhesus monkeys. Plasma viral RNA levels were determined by real-time PCR (ABI Prism 7700 sequence detection system; Applied Biosystems, Foster City, Calif.) utilizing reverse-transcribed viral RNA from macaque plasma samples as templates as previously described (13).
Lymphocyte immunophenotyping.
EDTA-treated blood samples were stained with fluorochrome-conjugated monoclonal antibodies (MAbs) (CD3-fluorescein isothiocyanate [Serotec Ltd., Oxford, England, or BD Biosciences Pharmingen, San Diego, Calif.], CD4-allophycocyanin, CD8-peridinin chlorophyll protein, and CD20-phycoerythrin [BD Biosciences Immunocytometry Systems, San Diego, Calif.]) and analyzed by flow cytometry (FACSort; BD Biosciences Immunocytometry Systems) as previously described (39).
PMPA treatment of SHIVDH12R-infected macaques.
PMPA was generously provided by Norbert Bischofberger, Gilead Sciences, Inc. (Foster City, Calif.). An aqueous solution (60 mg/ml) of PMPA was prepared after adjusting the pH to 7.0 with NaOH and filtration through a membrane filter (pore size, 0.22 μm) as previously described (44). PMPA was administered intramuscularly (30 mg/kg of body weight) every 24 h for 4 weeks starting on day 5 (for monkeys N056, N077, BC74, and BI36) or on day 14 (for monkeys 96N130 and 96N135) postinfection with 103 TCID50 of the SHIVDH12R stock.
Virus isolation from SHIVDH12R chronically infected animals.
Peripheral blood mononuclear cells (PBMC) (107 cells) from infected monkeys (or a mixture of PBMC, lymph node, and bone marrow cells [∼107 cells total] from macaque RhH520) were resuspended in 10 ml of RPMI 1640 medium (Cambrex Bio Science, Walkersville, Md.) supplemented with 10% fetal bovine serum (HyClone, Logan, Utah) and 20 U of recombinant human interleukin-2 (IL-2) (Roche Diagnostics Corporation, Indianapolis, Ind.)/ml. These specimens were cocultivated with 107 naïve monkey PBMC which had been previously stimulated with concanavalin A (Amersham Biosciences Corp., Piscataway, N.J.) (25 μg/ml) for 24 h and were maintained an additional 48 h in the presence of IL-2. All of the medium was replaced daily during 14 days of cocultivation, while the cell concentration of 2 × 106 cells/ml was maintained. Virus production was assessed by RT assays of the daily culture supernatant samples, and the specimen with the highest level of RT activity was used as stock virus after titration in MT-4 cells (39).
Effect of coreceptor-specific inhibitors on SIV and SHIV replication.
TAK-779 and AMD3100 were obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. AD101 was provided by Bahige Baroudy (Schering Plough Research Institute, Bloomfield, N.J.). Chemokine coreceptor usage was determined as described previously (51), with minor modifications. Briefly, uninfected rhesus PBMC were prepared as described above. On day 3, 5 × 104 cells were dispensed in 96-well round-bottom plates. Various concentrations (0, 0.05, 0.1, 0.5, 1, 5, and 10 μM) of small-molecule CCR5- or CXCR4-specific inhibitors (AMD3100 [11], TAK-779 [3], and AD101 [41]) were added to duplicate wells and incubated for 1 h at 37°C. After this incubation, each test virus was spinoculated (30) at 1,200 × g for 1 h at a multiplicity of infection of 0.1. On day 5 postinfection, virus replication was assessed by RT assays of the culture supernatants.
RESULTS
SHIVDH12R-induced disease is rapid, irreversible, and complete.
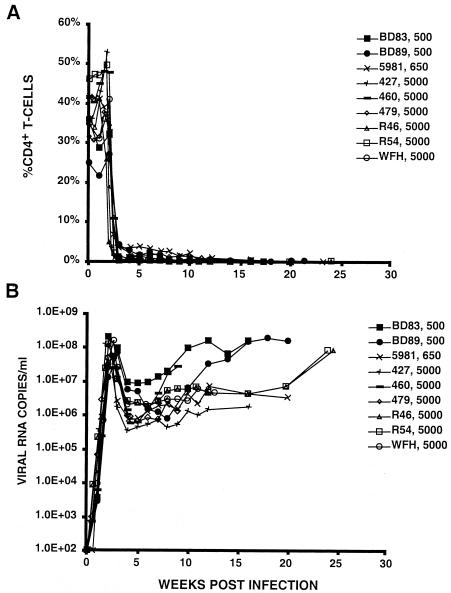
SIVmac/SIVsm infection of rhesus macaques typically causes a gradual decline of CD4+ T cells in the peripheral blood and the induction of immunodeficiency over a 1- to 2-year period (21, 31). As is the case for HIV-1, the development of disease by SIV does not require the complete elimination of the CD4+ T-lymphocyte subset. In contrast, highly pathogenic SHIVs, including SHIVDH12R, cause a rapid, systemic, and complete depletion of CD4+ T cells in rhesus macaques within 3 to 4 weeks of virus inoculation and death from immunodeficiency during the ensuing 3 to 7 months (17, 19, 33). As shown in Fig. 1, nine animals inoculated intravenously with moderate to high (500 to 5,000 TCID50) levels of SHIVDH12R experienced the characteristic CD4+ T-cell loss within several weeks (Fig. 1A) and were euthanized 15 to 30 weeks postinfection due to uncontrollable diarrhea, marked weight loss, or the onset of opportunistic infections. Plasma viral RNA levels in SHIVDH12R-infected rhesus macaques typically reached 107 to 108 copies/ml at 2 to 3 weeks postinoculation, coinciding with the rapid loss of CD4+ T lymphocytes. After declining 20- to 400-fold from the initial peak of viremia, the plasma viral loads gradually increased to the 107 RNA copies/ml level. Of the 28 monkeys inoculated with 500 TCID50 or more of SHIVDH12R, 26 exhibited the pattern shown in Fig. 1. The other two monkeys were the only recipients of SHIVDH12R (5,000 TCID50) from the same thawed vial of stock virus, and both experienced a delayed and transient depletion of their CD4+ T cells. Each has remained asymptomatic for more than 3 years. We presently have no explanation for the unusual course of infection in these two monkeys except that they were the only animals inoculated with virus from the same vial of SHIVDH12R.
FIG. 1.
Peripheral blood CD4+ T-cell profiles (A) and plasma viral RNA loads (B) of SHIVDH12R-infected monkeys. Each animal was inoculated intravenously with the indicated amount (500, 650, or 5,000 TCID50) of SHIVDH12R. Peripheral blood CD4+ T-cell numbers and plasma viral RNA levels were measured at the indicated times.
SHIVDH12R induces disease in an inoculum size-dependent manner.
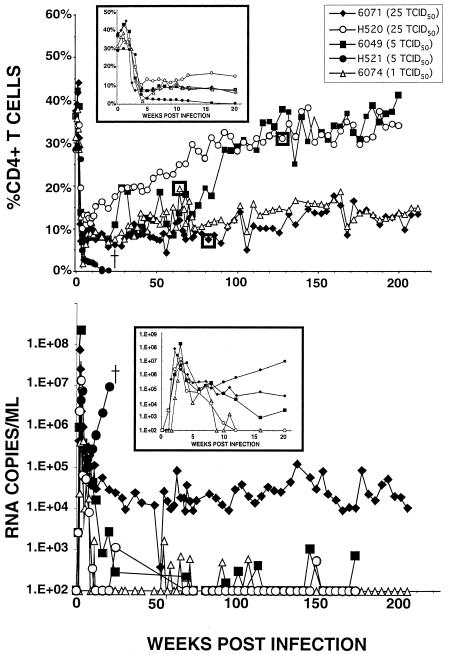
Unlike the immunodeficiency caused by SIVmac/SIVsm, the results that Endo et al. previously reported showed that the disease induced by SHIVDH12R (characterized by rapid, systemic, and irreversible loss of CD4+ T cells) is dose dependent (13). As shown in Fig. 2 (upper panel inset), all five animals inoculated intravenously with 25 TCID50 or less of SHIVDH12R experienced significant depletions of CD4+ T lymphocytes during the first 4 weeks of infection. However, in contrast to macaques inoculated with larger amounts of SHIVDH12R (500 TCID50 or more), the CD4+ T-cell levels did not fall to baseline values in any of these animals but stabilized at the 3 to 12% range over the first 10 weeks of infection. Each of these macaques still generated peak plasma virus loads of 107 to 108 RNA copies/ml within 2 to 3 weeks of infection. The plasma viral RNA loads subsequently fell in all five of the macaques but, starting at week 10, began to rise in one monkey (H521) and were accompanied by a further decline of its CD4+ T cells to less than 1% at week 16. Macaque H521 was subsequently euthanized at week 24 because of anorexia and marked weight loss.
FIG. 2.
Long-term effects of small intravenous SHIVDH12R inocula on disease development and control of viremia. Five macaques were inoculated intravenously with 1 to 25 TCID50 of SHIVDH12R. The insets in each panel highlight percent CD4+ T-cell levels (upper panel) and plasma viral RNA concentrations (lower panel) during the first 20 weeks of infection. The boxed data points in the upper panel indicate the times when virus was recovered from animal 6074 (week 64), animal 6071 (week 75), and animal H520 (week 137) by cocultivation of PBMC from each animal with PBMC from a naïve macaque.
The remaining four monkeys inoculated with low doses of SHIVDH12R were alive and clinically asymptomatic at 4 years after their initial exposure to virus. Two distinct patterns of circulating CD4+ T cells were observed. For two of these chronically infected monkeys (6071 and 6074), the CD4+ T-lymphocyte numbers have remained depressed (10 to 14%) for nearly 4 years whereas, for animals 6049 and H520, they have climbed back to near preinoculation levels (Fig. 2, upper panel). The recovery or continued maintenance of CD4+ T-cell numbers following their initial loss is clearly different from the results observed with animals inoculated with 500 TCID50 or more of SHIVDH12R, whose depleted CD4+ T-lymphocyte levels never increased over baseline levels (Fig. 1A).
It is worth noting that all of the macaques inoculated with 25 TCID50 or less of SHIVDH12R generated peak plasma viral RNA levels comparable to those of the recipients of 500 TCID50 or more of SHIVDH12R (compare Fig. 2, lower panel inset, with Fig. 1). Following the initial peak, the plasma viral RNA levels eventually became undetectable in three of the monkeys (6049, 6074, and H520), with only sporadic bursts of viremia that never exceeded 2 × 103 RNA copies/ml. The plasma viral RNA loads stabilized in the range of 104 to 105 RNA copies/ml in the fourth chronically infected macaque (6071).
Animals experiencing the complete and irreversible depletion of CD4+ T cells following inoculation of SHIVDH12R are unable to mount humoral immune responses (13). In contrast, rhesus monkeys infected with the nonpathogenic SHIVDH12, which carries the env gene from the HIV-1DH12 primary isolate, typically experience a very modest and short-lived loss of CD4+ T lymphocytes and generate robust titers of anti-virus NAbs (25). This is illustrated in Table 1 for macaque TPP, which was originally inoculated intravenously with a large SHIVDH12 inoculum (1.6 × 106 TCID50) yet experienced only a transient decline of its CD4+ T lymphocytes from 41% (prechallenge) to 20% at week 2; by week 5 postinfection, its percent CD4+ T-cell level had climbed to 35%, returning to preinoculation values by week 20. This animal produced detectable NAbs by week 3 postinoculation and titers greater than 1:1,000 by week 10, using an assay that measured complete neutralization of virus (38). The levels of NAbs in the four monkeys originally inoculated with 25 TCID50 or less of the highly pathogenic SHIVDH12R were similarly determined. As shown in Table 2, all of these chronically infected animals generated NAbs but at titers which were significantly lower than that measured in animal TPP. When the long-term profiles of NAb production in the four recipients of low-dose SHIVDH12R were analyzed, two distinct patterns (which paralleled those of the levels of CD4+ T lymphocytes) became evident. Monkeys H520 and 6049, whose CD4+ T-cell numbers eventually returned to preinoculation levels, produced higher titers of NAbs between weeks 30 and 50 postinfection than animals 6071 and 6074, which maintained persistently low levels of CD4+ T cells (Table 2, Fig. 2, upper panel). Thus, although the SHIVDH12R-induced CD4+ T-lymphocyte depletion was partial in the chronically infected monkeys, antibody production seemed to be significantly impaired.
TABLE 1.
NAbs elicited against nonpathogenic SHIVDH12a
| Plasma sample taken at (wk) | Neutralizing titer against SHIVDH12 | |||||
|---|---|---|---|---|---|---|
| Preinfection | <1.7 | |||||
| 2 | <1.7 | |||||
| 3 | 4 | |||||
| 4 | 48 | |||||
| 6 | 125 | |||||
| 8 | 431 | |||||
| 10 | 1,078 | |||||
| 12 | 359 |
NAb elicited in animal TPP following inoculation with 1.6 × 106 TCID50.
TABLE 2.
NAbs elicited against pathogenic SHIVDH12R
| Plasma sample taken (wk) | Neutralizing titer against SHIVDH12R for animal:
|
||||
|---|---|---|---|---|---|
| 6071 (25 TCID50 | H520 (25 TCID50) | H521 (5 TCID50) | 6049 (5 TCID50) | 6074 (1 TCID50) | |
| Preinfection | <0.8 | <3.1 | <3.1 | <3.1 | <0.8 |
| 10 | <0.8 | 16 | <3.1 | <3.1 | <0.8 |
| 20 | 4 | 21 | <1.6 | 7 | 11 |
| 30 | 12 | 21 | 63 | 13 | |
| 40 | 7 | 44 | 76 | 8 | |
| 50 | 5 | 34 | ND | 7 | |
| 60 | 7 | ND | ND | 8 | |
| 70 | 8 | ND | ND | 8 | |
| 92 | NDa | 4 | ND | ND | |
| 126 | 67 | ND | ND | 12 | |
| 184 | ND | 12 | ND | ND | |
| 212 | 7 | ND | ND | 12 | |
ND, not determined.
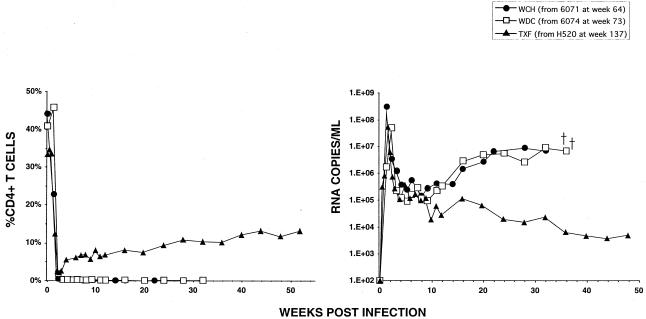
The appearance of distinct long-term CD4+ T-cell profiles in monkeys inoculated with low doses of SHIVDH12R suggested the possible emergence in vivo of nonpathogenic variants that had been present in the original uncloned SHIVDH12R stock. This possibility was investigated by examining the pathogenic phenotype of the SHIVs recovered from three of the four chronically infected macaques between years 2 and 4 postinoculation from PBMC by cocultivation (Fig. 2). The viruses isolated from animals 6071 and 6074, both of which had persistently low CD4+ T-cell levels, were inoculated intravenously (approximately 105 TCID50) into naïve monkeys WCH and WDC (Fig. 3). WCH and WDC rapidly and irreversibly lost CD4+ T lymphocytes and died from immunodeficiency at weeks 35 and 36, respectively. This result indicates that chronically infected, but asymptomatic, monkeys 6071 and 6074 still harbored virus with a pathogenic phenotype indistinguishable from that of the original SHIVDH12R. In contrast, the SHIV recovered at week 137 from animal H520 induced a significant but incomplete decline in CD4+ T lymphocyte levels following inoculation of macaque TXF (Fig. 3). By week 51, the level of CD4+ T cells in animal TXF had risen to more than 13% and its plasma viremia level had fallen to 4 × 103 RNA copies/ml.
FIG. 3.
Virus recovered from monkeys persistently infected with SHIVDH12R exhibited divergent pathogenic phenotypes. SHIVs isolated from animals 6074, 6071, and H520 (Fig. 2) were propagated in vitro and inoculated intravenously into monkeys WDC (7 × 105 TCID50), WCH (2.9 × 104 TCID50), and TXF (3.9 × 105 TCID50), respectively.
Although one could argue that the dependence of disease development on inoculum size simply reflects the dilution of highly pathogenic variants present in the uncloned, genetically heterogeneous SHIVDH12R stock, the same phenomenon has been observed with the recently obtained molecular clone SHIVDH12R-Cl-7, which consistently induces the prototypic rapid and complete CD4+ T-cell depletion following the intravenous inoculation of 500 TCID50 or more of virus (R. Sadjadpour, unpublished data).
Taken together, these results indicate that rhesus monkeys inoculated with small amounts of the highly pathogenic SHIVDH12R experienced a marked but incomplete loss of CD4+ T cells and remained clinically asymptomatic over a nearly 4-year observation period. However, the capacity of these seemingly healthy animals (with depressed levels of CD4+ T lymphocytes) to produce high titers of NAbs was impaired compared to that of infected animals with minimal or no loss of this T-cell subset. In one of the chronically infected macaques whose CD4+ T-cell numbers had returned to preinoculation levels, the only virus recovered (at nearly 3 years postinfection) failed to induce the characteristic rapid and irreversible depletion of CD4+ T cells. This attenuated SHIV variant, presumably present in the original uncloned SHIVDH12R inoculum, emerged following long-term replication in vivo.
Administration of a single course of PMPA during the first weeks of SHIVDH12R infection can prevent virus-induced disease.
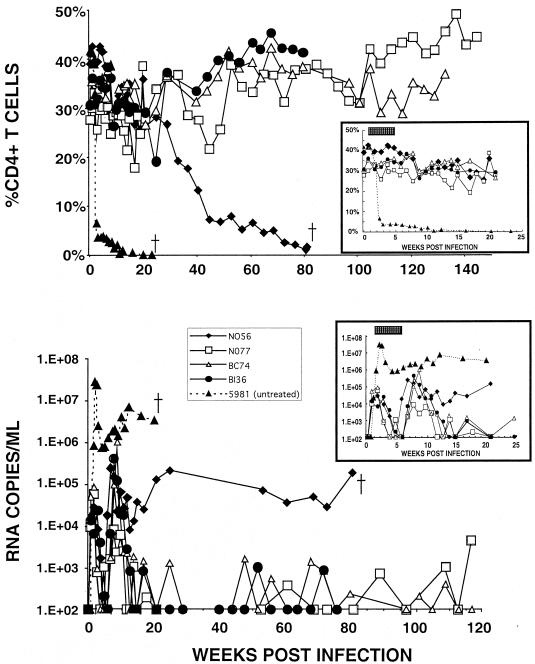
The potent RT inhibitor PMPA has previously been shown to block both acute and chronic SIV infections in vivo (42, 43). When administered at 24 but not 72 h postinoculation with SIVsmE660, a 28-day course of therapy prevented the establishment of infection (23). In an initial experiment to assess the efficacy of PMPA to contain highly pathogenic SHIVs, the initiation of treatment was deliberately delayed until 5 days following virus inoculation because of numerous reports showing that SHIVs can be contained by vaccine approaches that fail to control SIVs (1, 5, 6, 15, 32, 40). Four rhesus monkeys were inoculated intravenously with 1,000 TCID50 of SHIVDH12R and received single daily intramuscular injections of PMPA (30 mg/kg of body weight) for 4 weeks beginning on day 5 postinoculation with virus. At the time of PMPA therapy initiation, SHIVDH12R had caused no loss of CD4+ T lymphocytes, although ∼104 copies/ml of plasma viral RNA had already become detectable (Fig. 4, right inset in lower panel). Compared to the results for untreated acutely SHIVDH12R-infected monkeys (Fig. 1), which experienced a rapid depletion of their CD4+ T cells and symptoms of immunodeficiency requiring euthanasia, the four recipients of PMPA experienced no appreciable loss of CD4+ T lymphocytes during the first 20 weeks postinfection (Fig. 4, upper panel inset). Peak plasma virus loads (ranging from 1.4 ×104 to 8.8 ×104 RNA copies/ml) rapidly fell below the detection threshold (<200 RNA copies/ml) during the period of PMPA administration and rebounded to somewhat higher levels following the cessation of treatment. In three of the four macaques (N077, BC74, and BI36), plasma viral RNA loads gradually declined to background levels by week 20 postinoculation (Fig. 4, lower panel). In the fourth animal (N056), the plasma viremia level fell approximately 10-fold between weeks 8 and 13 but, by week 20, had climbed to 1.4 × 105 RNA copies/ml. The CD4+ T-cell levels in this monkey also began to decline between weeks 20 and 40, reaching 10 cells/mm3 on week 81, at which time the animal was euthanized because of anorexia, diarrhea, and marked weight loss. Nonetheless, three of the four rhesus monkeys receiving a single 4-week course of PMPA beginning on day 5 postinfection with SHIVDH12R maintained their CD4+ T cells at preinoculation levels for nearly 3 years and readily controlled their virus loads during this period (Fig. 4). This clinical outcome is clearly different from that reported for SIV-infected monkeys in a study in which PMPA treatment was started more than 24 h postinoculation with virus (23).
FIG. 4.
A single 4-week course of PMPA treatment initiated on day 5 postinfection prevented irreversible CD4+ T-cell depletion and durably controlled virus replication in three of four treated animals. Four rhesus monkeys (N056, N077, BC74, and BI36) were inoculated intravenously with 103 TCID50 of the SHIVDH12R stock and treated for 4 weeks with PMPA (30 mg administered intramuscularly per day/kg of body weight) beginning on day 5 postinfection. An untreated monkey (5981) was inoculated intravenously with 650 TCID50 of SHIVDH12R. The insets in each panel highlight percent CD4+ T-lymphocyte (upper panel) and the plasma viral RNA (lower panel) levels during the initial 25 weeks of infection. The stippled bar in each inset indicates the 4-week period of PMPA administration.
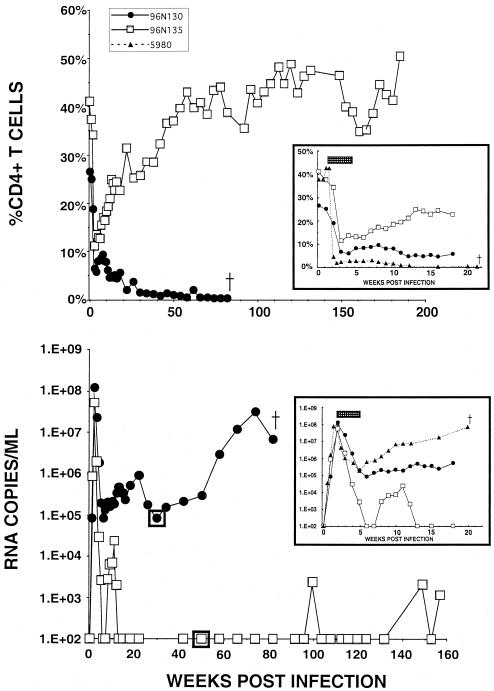
In view of the success at maintaining CD4+ T-lymphocyte levels and containing viremia (both acute and chronic) when PMPA treatment was started prior to the peak of virus production and before any loss of CD4+ T cells was detectable, we next inoculated two rhesus monkeys (96N130 and 96N135) intravenously with 1,000 TCID50 of SHIVDH12R but did not initiate daily PMPA administration until day 14 postinoculation with virus. At this point in the infection, the CD4+ T-lymphocyte levels had begun to fall and the plasma virus loads (1.2 × 108 and 5.2 × 107 RNA copies/ml [Fig. 5 insets]) were comparable to those measured for the group of untreated animals (Fig. 1). The subsequent 4-week course of PMPA therapy potently repressed the plasma viremia to background levels in animal 96N135 and partially controlled virus loads in macaque 96N130. During the first 18 weeks of infection, both of the PMPA-treated monkeys experienced severe but partial depletions of their CD4+ T cells, avoiding the irreversible and complete loss of this T-lymphocyte subset suffered by the untreated animals (compare Fig. 5 insets with Fig. 1). Although the CD4+ T-cell levels stabilized at the 4 to 6% range in macaque 96N130, they declined even further after week 18 as plasma virus loads began to rise. Ultimately, this animal had to be euthanized at week 85 because of marked weight loss and intractable diarrhea (Fig. 5). This clinical course contrasted with that of monkey 96N135, which maintained excellent control of its plasma viremia over a 3-year observation period and whose CD4+ T cells returned to their preinoculation levels.
FIG. 5.
Administration of PMPA to two rhesus monkeys beginning on day 14 postinfection. Two monkeys (96N130 and 96N135) were inoculated intravenously with 103 TCID50 of the SHIVDH12R stock and treated for 4 weeks with PMPA (30 mg administered intramuscularly per day/kg of body weight) beginning on day 14 postinfection. An untreated monkey (5980) was inoculated intravenously with 1.6 × 104 of SHIVDH12R. The insets in each panel highlight percent CD4+ T-lymphocyte (upper panel) and the plasma viral RNA (lower panel) levels during the initial 20 weeks of infection. The stippled bar in each inset indicates the 4-week period of PMPA administration. The boxed data points in the lower panel indicate the times when virus was recovered from animal 96N130 (week 30) or 96N135 (week 46) by cocultivation of its PBMC with PBMC from a naïve macaque.
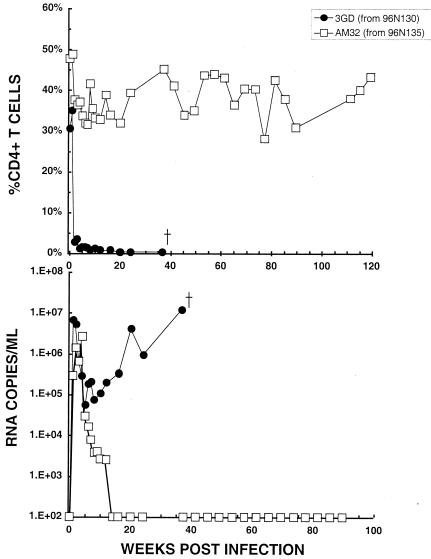
To investigate the possible emergence of nonpathogenic variant virus in animal 96N135, whose CD4+ T cells had returned to preinoculation levels, virus was isolated at weeks 30 and 46 from animals 96N130 and 96N135, respectively (Fig. 5), and used to inoculate (at 104 TCID50) two naïve macaques. As shown in Fig. 6, the virus recovered from animal 96N130, which had been unable to effectively control its viremia and ultimately died from immunodeficiency, caused the characteristic rapid and irreversible loss of CD4+ T cells in monkey 3GD and death at week 36. In contrast, animal AM32, the recipient of virus isolated from healthy monkey 96N135, experienced only a modest and transient depletion of its CD4+ T lymphocytes, which remained at near preinoculation levels for more than 2 years. Furthermore, the viremia in this monkey was rapidly suppressed and became undetectable by week 14 postinfection.
FIG. 6.
Virus recovered from monkeys treated with PMPA starting on day 14 postinfection exhibited divergent pathogenic phenotypes in vivo. The SHIV isolates recovered from animal 96N130 at week 30 and animal 96N135 at week 46 (boxed data points in Fig. 5) were propagated in vitro, and 104 TCID50 of each isolate was inoculated intravenously into macaques 3GD and AM32, respectively.
Taken together, these experiments indicate that antiretroviral therapy, when begun prior to peak viremia and before demonstrable depletion of CD4+ T lymphocytes, was able to durably control virus replication and prevent disease induction in three of four monkeys inoculated with the highly pathogenic SHVDH12R. Starting PMPA administration at the time of peak viremia or after some loss of CD4+ T lymphocytes had occurred was less effective but did block the characteristic SHIV-induced disease in one of two infected macaques. In the latter case, the healthy animal harbored virus exhibiting an attenuated pathogenic phenotype.
SHIVDH12R utilized CXCR4, not CCR5, to enter monkey PBMC.
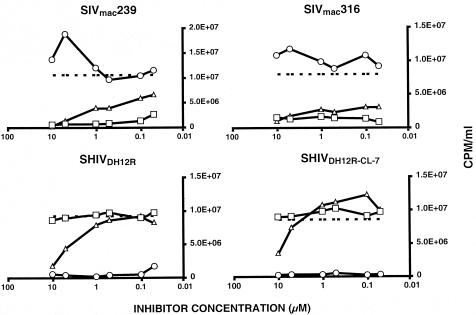
The distinctive patterns of disease induction and sensitivity to intervention by both antiviral agents and vaccines raised the possibility that SHIVDH12R and SIVs target different subsets of T lymphocytes during the initial phase(s) of in vivo infection. We therefore examined the chemokine coreceptor dependence of both for replication in rhesus monkey PBMC. In these experiments, virus was spinoculated (30) onto PBMC in the presence of small-molecule coreceptor-targeted inhibitors specific for CCR5 or CXCR4. The production of progeny virus was measured as levels of RT activity released into the medium on day 5 postinfection. As shown in Fig. 7, infections of monkey PBMC by the T-cell-tropic SIVmac239 and the macrophage-tropic SIVmac316 were both blocked by two different CCR5 inhibitors (TAK-779 and AD-101) but not by the CXCR4 inhibitor AMD3100. For SIVmac239, this result is in agreement with a previous report showing that CCR5 is the coreceptor used to infect macaque PBMC (51). The same assay was also performed with two different DH12-related SHIVs (SHIVDH12R and SHIVDH12R-CL-7, a recently obtained molecular clone that reproducibly induces a rapid, complete, and irreversible depletion of CD4+ T cells in inoculated rhesus monkeys) (18) (R. Sadjadpour et al., unpublished data). For both SHIVs, the opposite outcome was observed: infection of PBMC was completely blocked by AMD 3100 and not by the CCR5 inhibitors. Some competition was observed with TAK-779 but only at the highest concentrations tested (5 to 10 μM), perhaps reflecting nonspecific effects of this compound. These results indicate that both SIVs utilized CCR5 and SHIVDH12R used CXCR4 to enter and spread through cultured rhesus PBMC.
FIG. 7.
Coreceptor usage by SHIVs and SIVs. SHIVDH12R, SHIVDH12R-CL-7, SIVmac239, and SIVmac316 were spinoculated onto rhesus PBMC in the presence of the indicated amounts of small-molecule chemokine receptor inhibitors. RT activity released into the medium on day 5 postinfection was determined in the absence (dashed line) or presence of inhibitor. Lines with circles represent AMD 3100; lines with triangles represent TAK-779; lines with squares represent AD-101.
DISCUSSION
The results of this study show that (i) the immunodeficiency caused by highly pathogenic SHIVDH12R is dependent on inoculum size; (ii) SHIV-induced disease can be prevented by antiretroviral drug therapy initiated several days following exposure to virus; (iii) when the rapid and complete depletion of CD4+ T cells induced by SHIVDH12R fails to occur, a majority of infected animals experience an asymptomatic clinical course; and (iv) highly pathogenic SHIVDH12R exclusively uses CXCR4 to enter macaque PBMC, whereas SIVmac239 and SIVmac316 utilize CCR5 to infect the same cells. When considered together with the strikingly different patterns of CD4+ T-lymphocyte depletion and the temporal development of disease, these results suggest that the immunodeficiencies caused by SIV and highly pathogenic SHIVs are mechanistically dissimilar.
For SHIVs, clinical outcomes can be readily and durably modulated by therapeutic and vaccine interventions. In one of the longitudinal studies described in the present report, three of four macaques remained free of disease for 2 to 3 years following a single 28-day course of treatment (initiated on day 5 postinoculation with virus) with a nonnucleoside RT inhibitor. Similarly, long-term monitoring of several animals exposed to 25 TCID50 or less of SHIVDH12R indicated that they remained disease-free, with low to undetectable plasma virus loads, during a 4-year observation period. The simplest model consistent with all of the data is that a threshold level of systemic virus production which causes massive and systemic killing of all CD4+ T-lymphocyte subsets in lymphoid tissues must be reached within the first week of a SHIVDH12R infection. A delay (caused by, e.g., anti-retroviral drugs, vaccines, or a low level of inoculum) of only a few days in achieving this threshold total body virus burden effectively abolishes the irreversible loss of CD4+ T cells, presumably by allowing time for an immune response to develop and control virus replication (22). If the elimination of CD4+ T lymphocytes is not complete, systemic viral loads will be contained and a majority of infected animals will remain asymptomatic for extended periods of time. While the prevention of SHIV-induced disease by PMPA treatment initiated on day 5 can be attributed to the direct suppression of virus replication by a potent RT inhibitor, the attenuating effect of low inoculum size is less understandable. Infections initiated with small amounts of pathogenic SHIVs are likely to be intrinsically slower because they require several additional replication cycles to catch up to those started with high virus inputs. The correspondingly slower kinetics of virus-induced cell killing during the critical first week of infection may therefore preserve sufficient numbers of CD4+ T-cell activity to control virus replication.
The clinical features of SIV-induced immunodeficiency are quite different. First, although SIV can cause reductions in peripheral blood CD4+ T-cell levels during acute infections, the depletions observed are relatively modest and never approach the complete and systemic loss observed in SHIV-infected monkeys (20, 36). More importantly, the clinical symptoms of immunodeficiency in SIV-infected animals do not require the total elimination of CD4+ T lymphocytes but can become apparent at levels of 200 to 300 cells/μl or higher (10, 24). Second, the induction of AIDS in SIV-infected macaques does not appear to be dose dependent. Animals inoculated with 1:50- or 1:1,000-fold dilutions of a SIVmac251 stock died from immunodeficiency within similar time frames (an average of 218 or 275 days, respectively) (9). In a separate study, several pig-tailed macaques infected with SIVsmE660 (0.3 to 30,000 50% monkey infective doses) or SIVsm543 (0.6 to 600 50% monkey infective doses) developed disease in 5 to 25 or 3 to 19 months, respectively, independent of inoculum size (14). Finally, anti-retroviral agents appear to be less effective in the SIV-macaque system. When the most widely employed and effective agent, PMPA, was used and treatment was started at 24 h postinoculation with SIVsmE660, plasma viremia became undetectable during and following the cessation of therapy (23). However, when PMPA administration was delayed until day 3 of the SIV infection, plasma virus loads increased significantly after treatment was stopped, reaching the levels measured in control monkeys. Although no information pertaining to final clinical outcomes was provided in that study, the results reported contrast with those observed for the SHIV-infected-PMPA-treated animals, three of four of which remain disease-free, with undetectable levels of plasma viral RNA, even though the single course of PMPA treatment was delayed until 5 days postinoculation with virus.
The results obtained with inhibitors that target specific chemokine receptors may be key in explaining the different disease phenotypes elicited by SHIVs and SIVs: SHIVs use CXCR4 for entry of rhesus monkey PBMC, while SIVs use CCR5. These chemokine receptors are differentially expressed on leukocyte subsets and in individual body compartments. In monkey and human PBMC, for example, 80 to 95% of CD4+ T cells express CXCR4 while only 5 to 10% produce detectable CCR5 (2, 46, 50) (Y. Nishimura, unpublished observation). A similar pattern of chemokine receptor expression has been reported for CD4+ T lymphocytes in lymph nodes and spleen (46, 47). The vast majority of these CXCR4+ CD4+ cells share surface molecule expression patterns with naïve CD4+ T lymphocytes (29). In contrast, a large fraction of CD4+ T cells in other organs, including the gut-associated lymphoid tissue, express CCR5 and memory lymphocyte markers (37, 46); many of these tissue-infiltrating memory CD4+ T cells are also activated (26, 48). Rhesus monkeys inoculated with SIVmac typically experience a profound and selective loss of CD4+ CCR5+ memory T lymphocytes in the gut-associated lymphoid tissue during the first few weeks of infection and only modest depletions of CD4+ T cells in the blood and peripheral lymphoid organs, the bulk of which are CXCR4+ CCR5− naïve cells (12, 45, 46, 48). In contrast, the expression of CXCR4 on virtually all naïve and a significant fraction of memory CD4+ T cells (29) (Y. Nishimura, unpublished observation) makes them prime targets of highly pathogenic SHIVs and could explain the unrelenting and rapid pattern of their depletion in SHIV, but not, SIV infections.
Thus, instead of causing the irreversible elimination of CD4+ T cells in the blood and lymphoid tissues, SIV selectively and systemically depletes the CD4+ CCR5 memory subset in infected monkeys. The loss of these cells results in a reduced capacity to produce gamma interferon, tumor necrosis factor alpha, and IL-2, following in vitro stimulation with phorbol myristate acetate plus ionomycin, within 2 to 3 weeks postinfection (27). This memory CD4+ T-cell dysfunction persists for several months and worsens in those animals developing clinical disease.
When considered in the context of HIV-1 infections of humans, it is tempting to speculate that most infected individuals are exposed to HIV-1 inocula containing both syncytium-inducing CXCR4- and non-syncytium-inducing CCR5-utilizing variants. The X4 component may be responsible for the modest depletion of CD4+ T cells that has been reported to accompany most acute HIV-1 infections (36). However, as CCR5-using variants become predominant, these CXCR4-utilizing HIV-1 strains may be readily suppressed and rapidly become undetectable in recently infected individuals (34, 52). Such a scenario is consistent with a report showing that syncytium-inducing variants of HIV-1 (presumably X4 virus), which were recovered during primary infections of two individuals following accidental parenteral virus transmissions, were subsequently supplanted in both patients by non-syncytium-inducing (presumably R5) virus (7). The slowly developing immune dysfunction (associated with chronic HIV-1 infection of several years' duration) could eventually permit archival CXCR4-utilizing virus to emerge in some infected individuals and contribute to the accelerated decline of CD4+ T cells during the late stages of disease.
Acknowledgments
Tatsuhiko Igarashi and Yasuyuki Endo contributed equally to this work. We thank Bahige Baroudy and Jayaram Tagat, Schering-Plough Research Institute, for providing AD101, Russ Byrum, Sekou Savane, Wes Thornton, and Carol Clarke for devoted animal care, Randy Elkins for invaluable assistance in the animal experiments, and John P. Moore for advice on coreceptor experiments.
REFERENCES
- 1.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 2.Anton, P. A., J. Elliott, M. A. Poles, I. M. McGowan, J. Matud, L. E. Hultin, K. Grovit-Ferbas, C. R. Mackay, I. S. Y. Chen, and J. V. Giorgi. 2000. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS 14:1761-1765. [DOI] [PubMed] [Google Scholar]
- 3.Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. [DOI] [PubMed] [Google Scholar]
- 5.Barouch, D. H., S. Santra, M. J. Kuroda, J. E. Schmitz, R. Plishka, A. Buckler-White, A. E. Gaitan, R. Zin, J.-H. Nam, L. S. Wyatt, M. A. Lifton, C. E. Nickerson, B. Moss, D. C. Montefiori, V. M. Hirsch, and N. L. Letvin. 2001. Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination. J. Virol. 75:5151-5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T.-M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M.-E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 7.Cornelissen, M., G. Mulder-Kampinga, J. Veenstra, F. Zorgdrager, C. Kuiken, S. Hartman, J. Dekker, L. van der Hoek, C. Sol, R. Coutinho, and J. Goudsmit. 1995. Syncytium-inducing (SI) phenotype suppression at seroconversion after intramuscular inoculation of a non-syncytium-inducing/SI phenotypically mixed human immunodeficiency virus population. J. Virol. 69:1810-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curran, J. W., H. W. Jaffe, A. M. Hardy, W. M. Morgan, R. M. Selik, and T. J. Dondero. 1988. Epidemiology of HIV infection and AIDS in the United States. Science 239:610-616. [DOI] [PubMed] [Google Scholar]
- 9.Daniel, M. D., N. L. Letvin, P. K. Sehgal, G. Hunsmann, D. K. Schmidt, N. W. King, and R. C. Desrosiers. 1987. Long-term persistent infection of macaque monkeys with the simian immunodeficiency virus. J. Gen. Virol. 68:3183-3189. [DOI] [PubMed] [Google Scholar]
- 10.Desrosiers, R. C. 2001. Nonhuman lentiviruses, p. 2095-2121. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 11.Donzella, G. A., D. Schols, S. W. Lin, J. A. Este, K. A. Nagashima, P. J. Maddon, G. P. Allaway, T. P. Sakmar, G. Henson, E. De Clercq, and J. P. Moore. 1998. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat. Med. 4:72-77. [DOI] [PubMed] [Google Scholar]
- 12.Douek, D. C., L. J. Picker, and R. A. Koup. 2003. T cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 21:265-304. [DOI] [PubMed] [Google Scholar]
- 13.Endo, Y., T. Igarashi, Y. Nishimura, C. Buckler, A. Buckler-White, R. Plishka, D. S. Dimitrov, and M. A. Martin. 2000. Short- and long-term clinical outcomes in rhesus monkeys inoculated with a highly pathogenic chimeric simian/human immunodeficiency virus. J. Virol. 74:6935-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirsch, V. M., and P. R. Johnson. 1994. Pathogenic diversity of simian immunodeficiency viruses. Virus Res. 32:183-203. [DOI] [PubMed] [Google Scholar]
- 15.Horton, H., T. U. Vogel, D. K. Carter, K. Vielhuber, D. H. Fuller, T. Shipley, J. T. Fuller, K. J. Kunstman, G. Sutter, D. C. Montefiori, V. Erfle, R. C. Desrosiers, N. Wilson, L. J. Picker, S. M. Wolinsky, C. Wang, D. B. Allison, and D. I. Watkins. 2002. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J. Virol. 76:7187-7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igarashi, T., C. R. Brown, R. A. Byrum, Y. Nishimura, Y. Endo, R. J. Plishka, C. Buckler, A. Buckler-White, G. Miller, V. M. Hirsch, and M. A. Martin. 2002. Rapid and irreversible CD4+ T-cell depletion induced by the highly pathogenic simian/human immunodeficiency virus SHIVDH12R is systemic and synchronous. J. Virol. 76:379-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igarashi, T., Y. Endo, G. Englund, R. Sadjadpour, T. Matano, C. Buckler, A. Buckler-White, R. Plishka, T. Theodore, R. Shibata, and M. Martin. 1999. Emergence of a highly pathogenic simian/human immunodeficiency virus in a rhesus macaque treated with anti-CD8 mAb during a primary infection with a nonpathogenic virus. Proc. Natl. Acad. Sci. USA 96:14049-14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imamichi, H., T. Igarashi, T. Imamichi, O. K. Donau, Y. Endo, Y. Nishimura, R. L. Willey, A. F. Suffredini, H. C. Lane, and M. A. Martin. 2002. Amino acid deletions are introduced into the V2 region of gp120 during independent pathogenic simian immunodeficiency virus/HIV chimeric virus (SHIV) infections of rhesus monkeys generating variants that are macrophage tropic. Proc. Natl. Acad. Sci. USA 99:13813-13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joag, S. V., Z. Li, L. Foresman, E. B. Stephens, L.-J. Zhao, I. Adany, D. M. Pinson, H. M. McClure, and O. Narayan. 1996. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J. Virol. 70:3189-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaur, A., R. M. Grant, R. E. Means, H. McClure, M. Feinberg, and R. P. Johnson. 1998. Diverse host responses and outcomes following simian immunodeficiency virus SIVmac239 infection in sooty mangabeys and rhesus macaques. J. Virol. 72:9597-9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kestler, H., T. Kodama, D. Ringler, M. Marthas, N. Pedersen, A. Lackner, D. Regier, P. Sehgal, M. Daniel, N. King, and R. Desrosiers. 1990. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science 248:1109-1112. [DOI] [PubMed] [Google Scholar]
- 22.Kuroda, M. J., J. E. Schmitz, W. A. Charini, C. E. Nickerson, M. A. Lifton, C. I. Lord, M. A. Forman, and N. L. Letvin. 1999. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J. Immunol. 162:5127-5133. [PubMed] [Google Scholar]
- 23.Lifson, J. D., J. L. Rossio, R. Arnaout, L. Li, T. L. Parks, D. K. Schneider, R. F. Kiser, V. J. Coalter, G. Walsh, R. J. Imming, B. Fisher, B. M. Flynn, N. Bischofberger, M. Piatak, Jr., V. M. Hirsch, M. A. Nowak, and D. Wodarz. 2000. Containment of simian immunodeficiency virus infection: cellular immune responses and protection from rechallenge following transient postinoculation antiretroviral treatment. J. Virol. 74:2584-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masur, H., F. P. Ognibene, R. Yarchoan, J. H. Shelhamer, B. F. Baird, W. Travis, A. F. Suffredini, L. Deyton, J. A. Kovacs, J. Falloon, et al. 1989. CD4 counts as predictors of opportunistic pneumonias in human immunodeficiency virus (HIV) infection. Ann. Intern. Med. 111:223-231. [DOI] [PubMed] [Google Scholar]
- 25.Matano, T., R. Shibata, C. Siemon, M. Connors, H. C. Lane, and M. A. Martin. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72:164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattapallil, J. J., Z. Smit-McBride, P. Dailey, and S. Dandekar. 1999. Activated memory CD4+ T helper cells repopulate the intestine early following antiretroviral therapy of simian immunodeficiency virus-infected rhesus macaques but exhibit a decreased potential to produce interleukin-2. J. Virol. 73:6661-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKay, P. F., D. H. Barouch, J. E. Schmitz, R. S. Veazey, D. A. Gorgone, M. A. Lifton, K. C. Williams, and N. L. Letvin. 2003. Global dysfunction of CD4 T-lymphocyte cytokine expression in simian-human immunodeficiency virus/SIV-infected monkeys is prevented by vaccination. J. Virol. 77:4695-4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moss, A. R., and P. Bacchetti. 1989. Natural history of HIV infection. AIDS 3:55-61. [DOI] [PubMed] [Google Scholar]
- 29.Nicholson, J. K. A., S. W. Browning, R. L. Hengel, E. Lew, L. E. Gallagher, D. Rimland, and J. S. McDougal. 2001. CCR5 and CXCR4 expression on memory and naive T cells in HIV-1 infection and response to highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 27:105-115. [DOI] [PubMed] [Google Scholar]
- 30.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orandle, M. S., K. C. Williams, A. G. MacLean, S. V. Westmoreland, and A. A. Lackner. 2001. Macaques with rapid disease progression and simian immunodeficiency virus encephalitis have a unique cytokine profile in peripheral lymphoid tissues. J. Virol. 75:4448-4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ourmanov, I., C. R. Brown, B. Moss, M. Carroll, L. Wyatt, L. Pletneva, S. Goldstein, D. Venzon, and V. M. Hirsch. 2000. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J. Virol. 74:2740-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reimann, K. A., J. T. Li, R. Veazey, M. Halloran, I.-W. Park, G. B. Karlsson, J. Sodroski, and N. L. Letvin. 1996. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 70:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roos, M. T., J. M. Lange, R. E. de Goede, R. A. Coutinho, P. T. Schellekens, F. Miedema, and M. Tersmette. 1992. Viral phenotype and immune response in primary human immunodeficiency virus type 1 infection. J. Infect. Dis. 165:427-432. [DOI] [PubMed] [Google Scholar]
- 35.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 36.Schacker, T. W., J. P. Hughes, T. Shea, R. W. Coombs, and L. Corey. 1998. Biological and virologic characteristics of primary HIV infection. Ann. Intern. Med. 128:613-620. [DOI] [PubMed] [Google Scholar]
- 37.Shacklett, B. L., C. A. Cox, J. K. Sandberg, N. H. Stollman, M. A. Jacobson, and D. F. Nixon. 2003. Trafficking of human immunodeficiency virus type 1-specific CD8+ T cells to gut-associated lymphoid tissue during chronic infection. J. Virol. 77:5621-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shibata, R., T. Igarashi, N. Haigwood, A. Buckler-White, R. Ogert, W. Ross, R. Willey, M. W. Cho, and M. A. Martin. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5:204-210. [DOI] [PubMed] [Google Scholar]
- 39.Shibata, R., F. Maldarelli, C. Siemon, T. Matano, M. Parta, G. Miller, T. Fredrickson, and M. A. Martin. 1997. Infection and pathogenicity of chimeric simian-human immunodeficiency viruses in macaques: determinants of high virus loads and CD4 cell killing. J. Infect. Dis. 176:362-373. [DOI] [PubMed] [Google Scholar]
- 40.Shiver, J. W., T.-M. Fu, L. Chen, D. R. Casimiro, M.-E. Davies, R. K. Evans, Z.-Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 41.Trkola, A., S. E. Kuhmann, J. M. Strizki, E. Maxwell, T. Ketas, T. Morgan, P. Pugach, S. Xu, L. Wojcik, J. Tagat, A. Palani, S. Shapiro, J. W. Clader, S. McCombie, G. R. Reyes, B. M. Baroudy, and J. P. Moore. 2002. HIV-1 escape from a small molecule, CCR5-specific entry inhibitor does not involve CXCR4 use. Proc. Natl. Acad. Sci. USA 99:395-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai, C. C., K. E. Follis, T. W. Beck, A. Sabo, N. Bischofberger, and P. J. Dailey. 1997. Effects of (R)-9-(2-phosphonylmethoxypropyl)adenine monotherapy on chronic SIV infection in macaques. AIDS Res. Hum. Retrovir. 13:707-712. [DOI] [PubMed] [Google Scholar]
- 43.Tsai, C.-C., K. E. Follis, A. Sabo, T. W. Beck, R. F. Grant, N. Bischofberger, R. E. Benveniste, and R. Black. 1995. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science 270:1197-1199. [DOI] [PubMed] [Google Scholar]
- 44.Van Rompay, K. K. A., J. M. Cherrington, M. L. Marthas, C. J. Berardi, A. S. Mulato, A. Spinner, R. P. Tarara, D. R. Canfield, S. Telm, N. Bischofberger, and N. C. Pedersen. 1996. 9-[2-(Phosphonomethoxy)propyl]adenine therapy of established simian immunodeficiency virus infection in infant rhesus macaques. Antimicrob. Agents Chemother. 40:2586-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 46.Veazey, R. S., K. G. Mansfield, I. C. Tham, A. C. Carville, D. E. Shvetz, A. E. Forand, and A. A. Lackner. 2000. Dynamics of CCR5 expression by CD4+ T cells in lymphoid tissues during simian immunodeficiency virus infection. J. Virol. 74:11001-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veazey, R. S., P. A. Marx, and A. A. Lackner. 2003. Vaginal CD4+ T cells express high levels of CCR5 and are rapidly depleted in simian immunodeficiency virus infection. J. Infect. Dis. 187:769-776. [DOI] [PubMed] [Google Scholar]
- 48.Veazey, R. S., I. C. Tham, K. G. Mansfield, M. DeMaria, A. E. Forand, D. E. Shvetz, L. V. Chalifoux, P. K. Sehgal, and A. A. Lackner. 2000. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4+ T cells are rapidly eliminated in early SIV infection in vivo. J. Virol. 74:57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willey, R. L., R. Byrum, M. Piatak, Y. B. Kim, M. W. Cho, J. L. Rossio, Jr., J. Bess, Jr., T. Igarashi, Y. Endo, L. O. Arthur, J. D. Lifson, and M. A. Martin. 2003. Control of viremia and prevention of simian-human immunodeficiency virus-induced disease in rhesus macaques immunized with recombinant vaccinia viruses plus inactivated simian immunodeficiency virus and human immunodeficiency virus type 1 particles. J. Virol. 77:1163-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaitseva, M. B., S. Lee, R. L. Rabin, H. L. Tiffany, J. M. Farber, K. W. C. Peden, P. M. Murphy, and H. Golding. 1998. CXCR4 and CCR5 on human thymocytes: biological function and role in HIV-1 infection. J. Immunol. 161:3103-3113. [PubMed] [Google Scholar]
- 51.Zhang, Y.-J., B. Lou, R. B. Lal, A. Gettie, P. A. Marx, and J. P. Moore. 2000. Use of inhibitors to evaluate coreceptor usage by simian and simian/human immunodeficiency viruses and human immunodeficiency virus type 2 in primary cells. J. Virol. 74:6893-6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu, T., H. Mo, N. Wang, D. S. Nam, Y. Cao, R. A. Koup, and D. D. Ho. 1993. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science 261:1179-1181. [DOI] [PubMed] [Google Scholar]