Abstract
Objective
Physical activity (PA) begins to decline in adolescence with concomitant increase in weight. We hypothesized that a vicious circle may arise between decreasing physical activity and weight gain from adolescence to early adulthood.
Research Methods and Procedures
PA and self-perceived physical fitness assessed in adolescence (16-18 years) were used to predict the development of obesity (BMI≥30 kg/m2) and abdominal obesity (waist≥88 cm in females and ≥102 cm in males) at age 25 in 4240 twin individuals (90% of twins born in Finland 1975-1979). Ten 25-year-old monozygotic (MZ) twin pairs discordant for obesity (16 kg weight difference) were then carefully evaluated for current PA (triaxial accelerometer), total energy expenditure (TEE, doubly labeled water), and basal metabolic rate (BMR, indirect calorimetry).
Results
Physical inactivity in adolescence strongly predicted the risk of obesity (OR 3.9, 95%CI 1.4-10.9) and abdominal obesity (4.8, 1.9-12.0) at age 25, even after adjusting for baseline and current BMI. Poor physical fitness in adolescence also increased the risk of overall (5.1, 2.0-12.7) and abdominal obesity (3.2, 1.5-6.7) in adulthood. Physical inactivity was both causative and secondary to the development of obesity discordance in the MZ pairs. TEE did not differ between the MZ co-twins. PA levels were lower whereas BMR was higher in the obese co-twins.
Discussion
Physical inactivity in adolescence strongly and independently predicts total and especially abdominal obesity in young adulthood, favoring the development of a self-perpetuating vicious circle of obesity and physical inactivity. Physical (in)activity should be a major target of obesity prevention in the young.
Keywords: longitudinal, twin studies, body mass index, waist circumference, energy expenditure
Introduction
It is well documented that physical activity begins to decline in adolescence (1) with concomitant increase in weight (2). Whether these trends continue in the transitional period from adolescence to adulthood, a time critical for the development of obesity (3), is not clear (4). The relationship between physical inactivity in adolescence and obesity in adulthood has been weak or non-existent in the few published longitudinal studies (4-8). Very little is known about the role of physical activity on abdominal obesity. A long-term follow up of 5700 Finnish men and women failed to show a relationship between adolescent activity and later obesity, but an increased risk of abdominal obesity, independent of current BMI, was documented for women who became inactive after adolescence (6). Because both physical activity (9) and body size (10) are influenced by genetic factors, it is possible that individuals not undertaking exercise and then gaining weight are genetically predisposed to do so. Disentangling genetic effects from life-style has not been possible in previous studies.
Once obesity is established, energy balance changes. Data on 319 adults from the UK indicate that total energy expenditure (TEE, assessed by the doubly-labelled water method), basal metabolic rate (BMR), and AEE (activity-induced energy expenditure) are increased in obesity due to the larger body size (11). According to these results, physical activity is not changed in obesity (11). Other studies suggest the contrary: obese subjects are less active and expend less energy in physical activity than do lighter subjects (12,13). About 40% of the inter-individual variation of the various components of energy expenditure in humans is explained by genetic factors (14), in part because of a shared genetic background of energy expenditure with body size (15). To better understand the relationships between obesity and energy balance, it is important to distinguish genetic effects from those that are acquired by weight gain.
In the present study, we assessed the role of persistent physical activity vs. inactivity in adolescence (16-18 years) on the development of obesity and abdominal obesity in adulthood at 25 years in a longitudinal population-based sample of twins. Further, we chose from this sample a rare group of monozygotic (MZ) twin pairs discordant for obesity in young adulthood to determine the effects of acquired obesity on various components of energy expenditure.
Subjects and Methods
Study Populations
The Twin Cohort Study
Participants were recruited from a population-based, longitudinal study of five consecutive birth cohorts (1975-1979) of Finnish twins (the FinnTwin16 cohort) (16). All twins had been sent a questionnaire in adolescence at 16, 17, 18, and again as adults at 22-27 (mean 25) years of age. Response rates were high (83-97%) on all occasions. We included healthy twins who had responded to all four questionnaires (4240 individuals, including 1870 twin pairs).
MZ Twin Study
Fourteen of the 658 MZ pairs in the FinnTwin16 cohort reported in the adult questionnaire a BMI difference of at least 4 kg/m2, with one twin being non-obese (BMI approximately 25 kg/m2), and the other obese (BMI approximately 30 kg/m2) (17,18). Five male and five female pairs (measured BMI differences from 3.8 to 10.1 kg/m2) agreed to participate in the clinical protocol described below. We also studied nine concordant MZ pairs with a reported BMI difference of less than 2 kg/m2 (measured BMI differences from 0.0 to 2.3 kg/m2 as a reference MZ twin sample (17,18). All twins were healthy and weight-stable. Females were scheduled to attend during the follicular phase of their menstrual circle.
The study protocols were approved by the institutional review boards of Indiana University, Bloomington and the University & the University Central Hospital of Helsinki. All participants in the clinical study gave written informed consent.
Obesity
Weight (kg) and height (cm) were self-reported in all questionnaires and used to compute body mass index (BMI, kg/m2). In adolescents up to age 18 years, obesity was classified using age- and sex-specific BMI reference data (19), and in adults, using a BMI cut point of 30 kg/m2. At age 25, waist circumference was self-measured using a tape measure supplied with the questionnaire. Abdominal obesity was defined by cut points of 88 cm for women and 102 cm for men (20). The comparability of self-reported and measured data was ascertained in 566 twins on average 663 days after the completion of the questionnaire. The intraclass correlation for BMI was 0.94 and for waist circumference 0.73. The kappa value for obesity was 0.66 (95% CI 0.58 to 0.74) and for abdominal obesity 0.60 (0.52 to 0.69).
In the clinical study, adolescent growth charts were obtained from health records (17) and adult weight, height and waist measured. Fat mass, fat-free mass, and percent whole body fat was assessed by dual-energy x-ray absorptiometry (DEXA) (21) (Lunar Prodigy, Madison, WI, software version 2.15).
Physical Activity
The frequency of leisure-time physical activity was assessed by a structured question included in all four mailed questionnaires with the following response alternatives: “not at all, less than once a month, 1-2 times a month, about once a week, 2-3 times a week, 4-5 times a week, and every day” (22). Those reporting the two highest alternatives in all adolescent questionnaires at age 16 to 18 were defined as persistently active, and those who exercised 1-2 times a month or less as persistently inactive, and others as occasionally active at baseline (22). The second question asked for the subject's own perception of his or her physical fitness. Alternatives were “very good, fairly good, satisfactory, rather poor, very poor” (22). The two first and two last alternatives were combined to yield good, satisfactory and poor fitness classes. Persistency of fitness was grouped into five classes: (1) those reporting good fitness at age 16 to 18 were defined as persistently fit, (2) those reporting satisfactory fitness during these baseline time points as persistently satisfactory fit, and (3) those reporting poor fitness as persistently unfit. In addition, if the subject's fitness improved or declined over the baseline, from 16 to 18 years, (s)he was grouped into (4) increasing or (5) decreasing fitness classes, respectively. Validity of the questionnaire-reported physical activity and fitness was tested in a separate sub-sample (16 male pairs) (22). The frequency of leisure-time physical activity from the questionnaires correlated with leisure time physical activity during past 12 months in an interview (r=0.52, p=0.002) and with maximal oxygen uptake (VO2max) (r=0.45, p=0.010). The respective correlations for self-perceived physical fitness were (r=0.62, p<0.001) and (r=0.47, p=0.006).
For the MZ twins participating in the clinical protocol, the frequency of leisure-time physical activity from the questionnaires at 16-25 years was additionally converted into a semi-continuous measure describing days per year with physical activity, with values of 0, 6, 18, 52, 130, 230 & 360 days per year. The original fitness question grouped into good, satisfactory or poor was used to assess self-perceived fitness prospectively from 16 to 25 years. As part of the clinical protocol at 25 years, physical activity of the MZ twins was ascertained by several methods. Their current physical activity was assessed by tri-axial accelerometers (23), a questionnaire (Baecke et al (24)) and an interview (Kriska et al (25)) which also provides physical activity assessment retrospectively between 12 and 18 years. In the interview, each activity was weighted by its relative metabolic cost, thereby deriving MET-hours (one-hour energy expenditure for an individual at rest) per week as the final unit of expression (25). Activity counts of a tri-axial accelerometer for movement registration (Tracmor, Philips Research, Eindhoven, The Netherlands) were used for 7 consecutive days during waking hours, except during bathing (23). The movement was expressed as counts per day by adding body accelerations in anterioposterior, mediolateral and vertical axes. The tri-axial accelerometer has been validated against doubly-labeled water (DLW) (23). Accelerometers were used by the eight discordant pairs who also were measured by DLW (vide infra). Unfortunately, accelerometer data for 4 individuals (from 3 full pairs) was lost during transportation, leaving 5 discordant twin pairs for this analysis.
Energy Expenditure
In the clinical study, the basal metabolic rate (BMR) was measured in all pairs after an overnight fast using the Deltatrac Metabolic Monitor (Datex, Helsinki, Finland) (26). Total energy expenditure (TEE) was measured in eight (five male, three female) discordant pairs with DLW for 14 consecutive days following the Maastricht protocol (27). Activity-induced energy expenditure (AEE) was calculated as 0.9 * TEE − BMR, with a diet-induced thermogenesis of 10% assumed. The physical activity level (PAL) was calculated as TEE/BMR (28).
Statistics
In the whole cohort, the relationship between adolescent physical activity and adult obesity outcome was quantified using logistic regression models with adjustment for sex and baseline BMI at 16-18 years. Both genders were combined, as there were no significant sex effects in the models. The odds ratio for adult abdominal obesity was further adjusted for adult BMI. Confidence intervals were corrected for clustered sampling of co-twins within pairs (29).
In the clinical substudy, Wilcoxon matched pairs signed ranks test was used to compare the obese and the non-obese co-twins. ANOVA for repeated measures in matched individuals was used in longitudinal analyses of the frequency of physical activity (Figure 1). Symmetry test was used to test the longitudinal trends in the fitness categories between 16 and 25 years. Spearman's correlation and multiple linear regression analyses of intra-pair differences (all MZ pairs included) were used to assess the relationships between physical activity, body composition and energy expenditure independent of genetic influences. For individual twins, Pearson's correlations and linear regressions were corrected for clustered sampling of co-twins within pairs (29). Data are mean ± SE.
Figure 1.
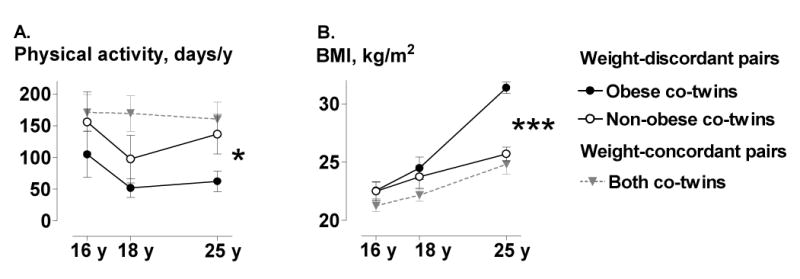
A. The frequency of leisure-time physical activity (mean (SE) days per year from prospective questionnaires) and B. mean (SE) BMI (from health registries) from 16 to 25 years in 10 weight-discordant and 9 weight-concordant MZ twin pairs. *p<0.05, ***p<0.001
Results
Physical Inactivity as a Predictor of Obesity in the Twin Cohort Study
Obesity in adolescence was rare (0.7% at 16 y, 0.7% at 17 y and 1.1% at 18 years). At the age of 25 years, four percent of the twins were obese and eight percent had abdominal obesity. The risk of becoming obese in adulthood was 3.9 (95%CI 1.4-10.9) fold higher among the physically inactive adolescents compared to those who were physically active (Table 1). The risk for abdominal obesity in adulthood was almost five-fold (4.8, 1.9-12.0) even after adjusting for adult BMI (Table 1). Those who perceived themselves as persistently unfit in adolescence had a marked risk for adult overall (5.1, 2.0-12.7) and abdominal obesity (3.2, 1.5-6.7) (Table 2). Adult obesity risk was also increased in those whose fitness declined from 16 to 18 years (Table 2).
Table 1.
Odds ratios (OR and 95% confidence intervals (CI)) of obesity and abdominal obesity at 25 years by physical activity at 16-18 years in the Twin Cohort Study
| Obesity* at 25 y
(n=4068) |
Abdominal obesity† at 25 y
(n=3982) |
|||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Physical activity at 16-18 y | ||||
|
| ||||
| Persistently active | 1.0 | 1.0 | ||
|
| ||||
| Occasionally active | ||||
| Adjusted for sex and BMI at 16-18 y | 2.04 | 0.98, 4.26 | 3.00 | 1.72, 5.24 |
| Additionally adjusted for BMI at 25 y | 2.72 | 1.31, 5.65 | ||
|
| ||||
| Persistently inactive | ||||
| Adjusted for sex and BMI at 16-18 y | 3.86 | 1.37, 10.89 | 5.71 | 2.83, 11.53 |
| Additionally adjusted for BMI at 25 y | 4.79 | 1.92, 12.00 | ||
body mass index ≥ 30 kg/m2
waist circumference ≥ 88 cm in females and ≥ 102 cm in males
Table 2.
Odds ratios (OR and 95% confidence intervals (CI)) of obesity and abdominal obesity at 25 years by participant's own perception of physical fitness at 16-18 years in the Twin Cohort Study
| Obesity* at 25 y
(n=3947) |
Abdominal obesity† at 25 y
(n=3866) |
|||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Physical fitness at 16-18 y | ||||
|
| ||||
| Persistently fit | 1.0 | 1.0 | ||
|
| ||||
| Persistently unfit | ||||
| Adjusted for sex and BMI at 16-18 y | 5.07 | 2.03, 12.67 | 3.00 | 1.47, 6.00 |
| Additionally adjusted for BMI at 25 y | 3.16 | 1.48, 6.73 | ||
|
| ||||
| Persistently satisfactory fitness | ||||
| Adjusted for sex and BMI at 16-18 y | 1.3 | 0.63, 2.67 | 1.43 | 0.92, 2.22 |
| Additionally adjusted for BMI at 25 y | 1.49 | 0.91, 2.44 | ||
|
| ||||
| Increasing fitness | ||||
| Adjusted for sex and BMI at 16-18 y | 1.42 | 0.74, 2.76 | 1.35 | 0.89, 2.06 |
| Additionally adjusted for BMI at 25 y | 1.27 | 0.75, 2.15 | ||
|
| ||||
| Decreasing fitness | ||||
| Adjusted for sex and BMI at 16-18 y | 2.73 | 1.56, 4.78 | 2.22 | 1.54, 3.21 |
| Additionally adjusted for BMI at 25 y | 1.50 | 0.98, 2.23 | ||
body mass index ≥ 30 kg/m2
waist circumference ≥ 88 cm in females and ≥ 102 cm in males
Physical Inactivity and Obesity Independent of Genetic Effects in the MZ Twins
In the MZ twin substudy, the co-twins who ultimately became obese (Figure 1, Table 2) had been less physically active in adolescence than their non-obese co-twins (Figure 1) (ANOVA F = 5.40, p = 0.025). During the development of obesity, physical activity declined further. According to the Kriska interview (25), physical activity decreased by 51% from age 12-18 to age 25 years in the subsequently obese MZ co-twins (Figure 2). Prospectively measured self-perceived fitness declined from 16 to 25 years in the obese co-twins with a borderline significance (p=0.065) but remained unchanged in the non-obese co-twins (p=0.4) (Figure 3). In the adulthood, the obese co-twins (2.6 ± 0.2) were significantly less active than the non-obese co-twins (3.1 ± 0.2, p = 0.01) by the Baecke questionnaire indices (24). This was also confirmed objectively by the accelerometers. The obese co-twins had less than half the activity (4.2 ± 0.5 × 109 counts per day) of that of their non-obese co-twins (9.7 ± 2.0 × 109 counts per day, p = 0.04) throughout the study week (Figure 4). An example in Figure 5 illustrates that the obese co-twins had low overall and virtually no high-intensity exercise. Activity counts correlated negatively with fat mass (r = −0.63, p = 0.059), but not with fat-free mass (r = 0.19, p = 0.27) in the twin individuals. In the weight-concordant reference MZ twin pairs, physical activity patterns and fitness changed little during adolescence and were similar in the co-twins, as was the development of BMI (Figures 1, 2 and 3).
Figure 2.
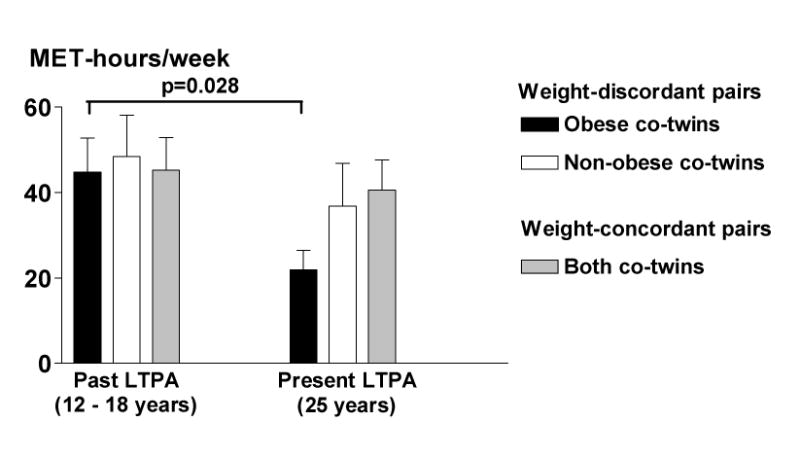
Past and present leisure-time physical activity (LTPA) (from the Kriska Interview (25)) in 10 weight-discordant and 9 weight-concordant MZ twin pairs. Data are mean (SE).
Figure 3.
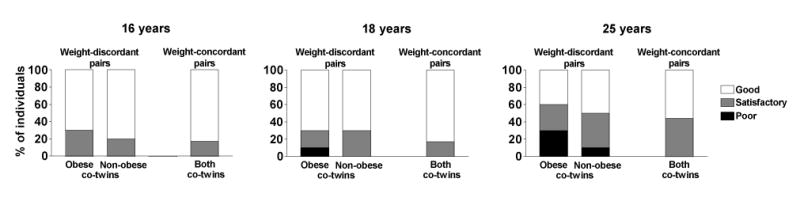
Self-perceive physical fitness from 16 to 25 years in 10 weight-discordant and 9 weight-concordant twin pairs.
Figure 4.
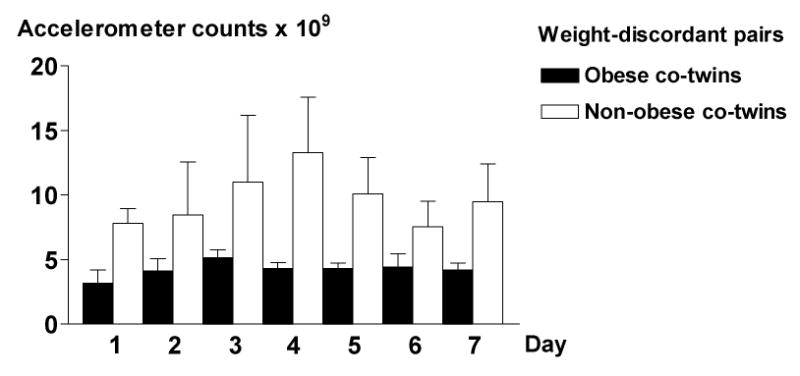
Daily mean (SE) accelerometer counts from the one-week measuring period in five weight-discordant MZ twin pairs.
Figure 5.
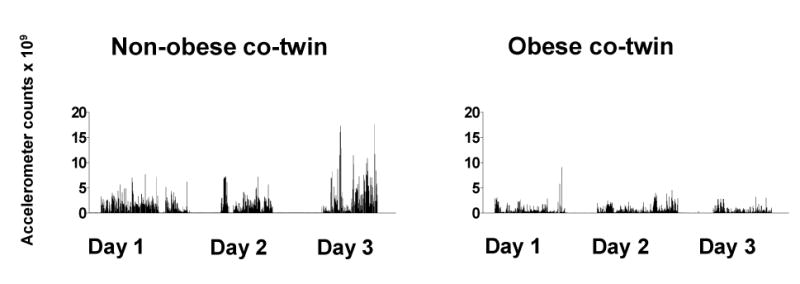
Accelerometer measurements during three days in a male MZ obesity-discordant pair illustrating low overall and lack of high-intensity exercise in the obese co-twin.
Energy Balance and Obesity Independent of Genetic Effects in the MZ Twins
Due to the significantly higher physical activity levels of the non-obese co-twins, they reached similar levels of total energy expenditure (11 541 ± 687 kJ/d) than the much heavier obese co-twins (12 404 ± 351 kJ/d, p = 0.21). AEE and PAL were also similar between the co-twins (data not shown), whereas AEE/kg body weight showed a non-significant trend for lower activity in the obese (45 ± 19 kJ/kg) as compared with the non-obese co-twins (54 ± 14 kJ/kg). BMR was 605 kJ higher in the obese (6821 ± 300 kJ/d) than in the non-obese co-twins (6217 ± 346 kJ/d, p = 0.009). However, when expressed per fat free mass, the daily BMRs of the co-twins were similar (133 ± 3 kJ/kg in the obese vs. 129 ± 5 kJ/kg in the non-obese, p = 0.24). The above BMR measures were similar in the co-twins of the weight-concordant twin pairs (data not shown).
Body weight correlated with BMR (r = 0.79, p = 0.0001) and TEE (r = 0.66, p = 0.003) in individual twins. Within twin pairs, weight differences were directly related to differences in BMR (r = 0.75, p = 0.002). In a multiple regression model, intra-pair differences in fat (β = 11.9 ± 4.6, p = 0.020) and fat-free mass (β = 25.3 ± 9.4, p = 0.017) together explained most (R2 = 57%, p = 0.0012) of the intra-pair difference in BMR. Differences in body composition did not explain the differences in TEE (R2 = 15%, p = 0.67), which was closely linked to accelerometer counts in individual twins (r = 0.56, p = 0.013). Accelerometer counts correlated also closely with AEE (r = 0.68, p = 0.004), AEE/kg body weight (r = 0.79, p = 0.002) and PAL (r = 0.62, p = 0.015).
Discussion
This study, covering the transition period from adolescence to young adulthood, shows that the risk of adult overall obesity (OR 3.9) and especially abdominal obesity (OR 4.8) was significantly increased in physically inactive adolescents. Self-perceived poor fitness in adolescence also captured the high risk for overall (5.1) and abdominal obesity (3.2) in adulthood. The risk excess was evident independent of genetic effects, as shown by the substudy in MZ twins discordant for adult obesity. Once obesity was established, habitual physical activity remained extremely low, denoting low energy expenditure. These findings provide cogent evidence that sedentary lifestyle in adolescence triggers the development of obesity, which may lead to a self-perpetuating vicious circle of less activity, low energy expenditure and increasing adiposity.
Previous results on the relationship between physical activity and weight trajectories in youth have been inconsistent. A decline in physical activity during adolescence (1) has been related to increase in adiposity at 18-19 years in a cohort of black and white girls from the USA (2). Females from the 1958 British cohort who were physically active at 16 years showed a slower gain in BMI between 16 and 45 years than less active females, whereas the reverse was found for males (4). In previous Finnish studies, decreasing activity between adolescence and adulthood rather than adolescent inactivity has been associated with adult obesity risk (5,6). In the present study, it was clear that persistent physical activity in adolescence prevents adult obesity.
We were also able to show, for the first time, that physical activity in adolescence helps prevent adult abdominal obesity above and beyond its effect on overall obesity. The risk of abdominal obesity in the persistently inactive group was substantial after adjustment for both adolescent (OR 5.7) and adult BMI (OR 4.8). The previous Finnish study showed weaker similar trends (OR 1.8) with decreasing physical activity in females (6).
An observation that some people find it both easy to exercise and maintain a healthy weight, has led to a suggestion that physical activity and body composition share a common genetic background. The Quebec family study showed that habitual physical activity was more genetically determined than sports participation, and suggested that the propensity toward being spontaneously active could be partly influenced by the genotype (30). Levine et al (31) came to a similar conclusion with a completely different study design, in a study of 10 obese and 10 non-obese volunteers who wore activity sensors for 10 days to measure body postures and movements. Obese individuals were seated, on average, two hours longer daily than lean individuals. Posture allocation did not change when the obese individuals lost weight or when lean individuals gained weight, suggesting that the differences may be biologically determined. While not contradicting evidence that genes are important, we showed in an MZ co-twin control design that sedentary lifestyle leads to obesity, independent of genetic factors.
Obese and non-obese co-twins in the present study did not differ significantly for TEE or AEE. This may be explained by the fact that although obese people expend more energy in performing a given level of physical activity (11), they tend to move less and thus do not have increased total or activity energy expenditure (12). Accelerometer measurements demonstrated that the obese co-twins were only half as active overall as the non-obese co-twins (Figure 4) and avoided high intensity exercise bouts altogether (Figure 5)! We therefore suggest that obese persons already at a young age easily fall below a level of physical activity that might be needed to maintain cardiovascular health. With the low overall level of physical activity, it is easy to understand that obese persons are extremely vulnerable to continuous weight gain.
Despite no differences in TEE, BMR was significantly higher in twins with acquired obesity due to their larger body size. This is in line with previous studies showing that variations in resting metabolic rate following overfeeding or negative energy balance in MZ twins were accounted for primarily by the changes in body mass (32). Our study confirms that when expressed per its main determinant, i.e. fat-free mass (33), BMR does not change in acquired obesity.
The strengths of our study include prospective longitudinal measurements of physical activity and BMI over a critical period from adolescence to adulthood. In a cross-sectional setting at 25 years, detailed and objective measures of physical activity, energy expenditure and body composition were used to enrich the prospective questionnaire data. Within MZ twin pairs, the relationships are free of potentially confounding genetic effects, which is a major strength of the present study. The main limitations of the study include self-reported data in the entire population sample and limited number of discordant pairs in the MZ clinical protocol with just 10 highly discordant MZ pairs, despite a population-based screening of five full birth cohorts of young adult twins. This itself is consistent with the evidence that obesity is significantly influenced by genetic factors.
In summary, the present study underscores the causal role of low physical activity as a risk factor of obesity and especially abdominal obesity during the transition from adolescence to early adulthood. Further, our results show that physically inactive lifestyle triggers weight gain and vice versa independent of genetic effects. Thus, when it comes to the vicious circle of physical inactivity and obesity, genes confer dispositions, not destinies (34).
Table 3.
Characteristics of the weight-discordant and weight-concordant MZ twins.
| WEIGHT-DISCORDANT PAIRS
N=10 |
WEIGHT-CONCORDANT PAIRS
N=9 |
|||
|---|---|---|---|---|
| Non-obese co-twins | p | Obese co-twins | Both co-twins | |
| Age, years | 25.6 ± 0.3 | 25.6 ± 0.3 | 25.8 ± 0.4 | |
| Weight, kg | 73.5 ± 2.7 | 0.005 | 89.9 ± 2.7 | 74.8 ± 4.5 |
| BMI, kg/m2 | 25.7 ± 0.6 | 0.005 | 31.4 ± 0.5 | 24.8 ± 1.2 |
| Waist, cm | 90 ± 2 | 0.003 | 105 ± 1 | 86 ± 4 |
| Body fat, % | 30.5 ± 2.4 | 0.005 | 39.5 ± 1.9 | 25.9 ± 3.9 |
| Fat, kg | 22.2 ± 1.5 | 0.005 | 35.2 ± 1.3 | 20.0 ± 3.5 |
| Fat free mass, kg | 48.8 ± 3.2 | 0.02 | 51.7 ± 3.0 | 52.4 ± 3.4 |
Data are mean ± SE.
Acknowledgments
We thank Erjastiina Heikkinen, Katja Tuominen and Mia Urjansson for their skillful assistance in the study. The study was supported by the National Institute on Alcohol Abuse and Alcoholism (grants AA-08315, AA-00145 and AA-12502), the European Union Fifth Framework Program (QLRT-1999-00916, QLG2-CT-2002-01254), the Academy of Finland (Grant 44069, 100499 and 201461), the Academy of Finland Centre of Excellence in Complex Disease Genetics, Helsinki University Central Hospital grants. K.H.P. is supported by the following foundations: Yrjö Jahnsson, Jalmari & Rauha Ahokas, Juho Vainio, Finnish Cultural, Finnish Medical Foundation, and Research Foundation of the Orion Coorporation.
AR initiated the concept of the study and AR, JK and KHP designed it. KHP conducted the trial and AR, JK, and HYJ supervised its progression. KRW provided the DLW and accelerometer protocols and together with GP analysed them. KHP, PB, UMK, and JK performed the statistical analyses. KHP and AR drafted the manuscript. RJR is the principal investigator of the FinnTwin16-25 study. All authors contributed to the conception of the paper and interpretation of data.
Footnotes
We declare that we have no conflict of interest.
Reference List
- 1.Kimm SY, Glynn NW, Kriska AM, et al. Decline in physical activity in black girls and white girls during adolescence. N Engl J Med. 2002;347:709–15. doi: 10.1056/NEJMoa003277. [DOI] [PubMed] [Google Scholar]
- 2.Kimm SY, Glynn NW, Obarzanek E, et al. Relation between the changes in physical activity and body-mass index during adolescence: a multicentre longitudinal study. Lancet. 2005;366:301–7. doi: 10.1016/S0140-6736(05)66837-7. [DOI] [PubMed] [Google Scholar]
- 3.Gordon-Larsen P, Adair LS, Nelson MC, Popkin BM. Five-year obesity incidence in the transition period between adolescence and adulthood: the National Longitudinal Study of Adolescent Health. Am J Clin Nutr. 2004;80:569–75. doi: 10.1093/ajcn/80.3.569. [DOI] [PubMed] [Google Scholar]
- 4.Parsons TJ, Manor O, Power C. Physical activity and change in body mass index from adolescence to mid-adulthood in the 1958 British cohort. Int J Epidemiol. 2006;35:197–204. doi: 10.1093/ije/dyi291. [DOI] [PubMed] [Google Scholar]
- 5.Yang X, Telama R, Viikari J, Raitakari OT. Risk of obesity in relation to physical activity tracking from youth to adulthood. Med Sci Sports Exerc. 2006;38:919–25. doi: 10.1249/01.mss.0000218121.19703.f7. [DOI] [PubMed] [Google Scholar]
- 6.Tammelin T, Laitinen J, Nayha S. Change in the level of physical activity from adolescence into adulthood and obesity at the age of 31 years. Int J Obes. 2004;28:775–82. doi: 10.1038/sj.ijo.0802622. [DOI] [PubMed] [Google Scholar]
- 7.Hasselstrom H, Hansen SE, Froberg K, Andersen LB. Physical fitness and physical activity during adolescence as predictors of cardiovascular disease risk in young adulthood. Danish Youth and Sports Study. An eight-year follow-up study. Int J Sports Med. 2002;23 1:S27–S31. doi: 10.1055/s-2002-28458. [DOI] [PubMed] [Google Scholar]
- 8.Wareham NJ, van Sluijs EM, Ekelund U. Physical activity and obesity prevention: a review of the current evidence. Proc Nutr Soc. 2005;64:229–47. doi: 10.1079/pns2005423. [DOI] [PubMed] [Google Scholar]
- 9.Beunen G, Thomis M. Genetic determinants of sports participation and daily physical activity. Int J Obes. 1999;23 3:S55–S63. doi: 10.1038/sj.ijo.0800885. [DOI] [PubMed] [Google Scholar]
- 10.Schousboe K, Willemsen G, Kyvik KO, et al. Sex differences in heritability of BMI: a comparative study of results from twin studies in eight countries. Twin Res. 2003;6:409–21. doi: 10.1375/136905203770326411. [DOI] [PubMed] [Google Scholar]
- 11.Prentice AM, Black AE, Coward WA, Cole TJ. Energy expenditure in overweight and obese adults in affluent societies: an analysis of 319 doubly-labelled water measurements. Eur J Clin Nutr. 1996;50:93–7. [PubMed] [Google Scholar]
- 12.Ferraro R, Boyce VL, Swinburn B, De Gregorio M, Ravussin E. Energy cost of physical activity on a metabolic ward in relationship to obesity. Am J Clin Nutr. 1991;53:1368–71. doi: 10.1093/ajcn/53.6.1368. [DOI] [PubMed] [Google Scholar]
- 13.Rising R, Harper IT, Fontvielle AM, Ferraro RT, Spraul M, Ravussin E. Determinants of total daily energy expenditure: variability in physical activity. Am J Clin Nutr. 1994;59:800–4. doi: 10.1093/ajcn/59.4.800. [DOI] [PubMed] [Google Scholar]
- 14.Bouchard C, Perusse L, Deriaz O, Despres JP, Tremblay A. Genetic influences on energy expenditure in humans. Crit Rev Food Sci Nutr. 1993;33:345–50. doi: 10.1080/10408399309527631. [DOI] [PubMed] [Google Scholar]
- 15.Hewitt JK, Stunkard AJ, Carroll D, Sims J, Turner JR. A twin study approach towards understanding genetic contributors to body size and metabolic rate. Acta Genet Med Gemellol. 1991;40:133–46. doi: 10.1017/s0001566000002567. [DOI] [PubMed] [Google Scholar]
- 16.Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental factors in health-related behaviors: studies on Finnish twins and twin families. Twin Res. 2002;5:366–71. doi: 10.1375/136905202320906101. [DOI] [PubMed] [Google Scholar]
- 17.Pietiläinen KH, Rissanen A, Laamanen M, et al. Growth patterns in young adult monozygotic twin pairs discordant and concordant for obesity. Twin Res. 2004;7:421–9. doi: 10.1375/1369052042335368. [DOI] [PubMed] [Google Scholar]
- 18.Pietiläinen KH, Rissanen A, Kaprio J, et al. Acquired obesity is associated with increased liver fat, intra-abdominal fat, and insulin resistance in young adult monozygotic twins. Am J Physiol Endocrinol Metab. 2005;288:E768–E774. doi: 10.1152/ajpendo.00381.2004. [DOI] [PubMed] [Google Scholar]
- 19.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ. 1995;311:158–61. doi: 10.1136/bmj.311.6998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51:1106–12. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- 22.Aarnio M, Winter T, Kujala U, Kaprio J. Associations of health related behaviour, social relationships, and health status with persistent physical activity and inactivity: a study of Finnish adolescent twins. Br J Sports Med. 2002;36:360–4. doi: 10.1136/bjsm.36.5.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plasqui G, Joosen AM, Kester AD, Goris AH, Westerterp KR. Measuring free-living energy expenditure and physical activity with triaxial accelerometry. Obes Res. 2005;13:1363–9. doi: 10.1038/oby.2005.165. [DOI] [PubMed] [Google Scholar]
- 24.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 25.Kriska AM, Knowler WC, LaPorte RE, et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13:401–11. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 26.Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988;37:287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- 27.Westerterp KR, Wouters L, Marken Lichtenbelt WD. The Maastricht protocol for the measurement of body composition and energy expenditure with labeled water. Obes Res. 1995;3 1:49–57. doi: 10.1002/j.1550-8528.1995.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 28.Westerterp KR. Energy metabolism and body composition: general principles. Eur Respir Mon. 2003;24:1–10. [Google Scholar]
- 29.Rao JNK, Scott AJ. On chi-squared tests for multiway contingency tables with cell proportions estimated from survey data. Annals of Statistics. 1984;12:46–60. [Google Scholar]
- 30.Perusse L, Tremblay A, Leblanc C, Bouchard C. Genetic and environmental influences on level of habitual physical activity and exercise participation. Am J Epidemiol. 1989;129:1012–22. doi: 10.1093/oxfordjournals.aje.a115205. [DOI] [PubMed] [Google Scholar]
- 31.Levine JA, Lanningham-Foster LM, McCrady SK, et al. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307:584–6. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- 32.Bouchard C, Tremblay A. Genetic influences on the response of body fat and fat distribution to positive and negative energy balances in human identical twins. J Nutr. 1997;127:943S–7S. doi: 10.1093/jn/127.5.943S. [DOI] [PubMed] [Google Scholar]
- 33.Weyer C, Snitker S, Rising R, Bogardus C, Ravussin E. Determinants of energy expenditure and fuel utilization in man: effects of body composition, age, sex, ethnicity and glucose tolerance in 916 subjects. Int J Obes. 1999;23:715–22. doi: 10.1038/sj.ijo.0800910. [DOI] [PubMed] [Google Scholar]
- 34.Rose RJ. Genes and human behavior. Annu Rev Psychol. 1995;46:625–54. doi: 10.1146/annurev.ps.46.020195.003205. [DOI] [PubMed] [Google Scholar]


