Abstract
Human cytomegalovirus (HCMV) infections in immunocompromised patients are associated with impaired immunological functions. Blood monocytes, which can differentiate into dendritic cells upon cytokine stimulation, play a central role in adequate immune reactivity and are believed to carry latent HCMV. We demonstrate here that HCMV infection of monocytes results in a block in the cytokine-induced differentiation of monocytes into functionally active CD1a-positive dendritic cells, which exhibited severely depressed immunological functions in vitro. The HCMV-infected cells exhibited a significantly reduced ability to endocytose fluorescein isothiocyanate-labeled dextran particles as well as a more than 90% reduced ability to migrate in response to the chemoattractant factors RANTES, MIP-1α, and MIP-3β. Interestingly, HCMV-infected cells expressed high levels of the costimulatory molecule CD86, in contrast to the low levels of expression that was observed on uninfected monocytes and uninfected immature dendritic cells. Furthermore, HCMV-infected CD1a-negative cells were unable to induce a T-cell response. Thus, these observations suggest that HCMV infection of monocytes in vitro blocks cytokine-induced dendritic cell differentiation, and since dendritic cells play a central role in initiating immune responses, these findings suggest a powerful tactic to avoid immune recognition and to blunt the immune response at early phases of infection.
Immunosuppression is a common outcome of several virus infections, where human immunodeficiency virus, measles virus, and human cytomegalovirus (HCMV) are the main human pathogens that are able to depress the immune system in different ways. The general immunosuppression caused by these viruses often leads to an impaired ability of the host to combat the virus infection in particular, but also appears to impair the ability of the immune system to fight infections by unrelated pathogens such as bacteria and fungi, as has often been observed in transplant patients (28, 30, 53). Since immunosuppression may also be associated with the onset of tumors that can utilize immune evasion strategies for their survival, an understanding of the mechanisms involved in virus-induced immunosuppression is critical for the development of better immunotherapies. In this study, we focused on examining HCMV's ability to affect the immune system by focusing on the ability of the virus to affect the most important functions of the professional antigen-presenting dendritic cells.
HCMV is an opportunistic pathogen that mainly causes serious health problems in neonates and in immunosuppressed patients, such as bone marrow and solid organ transplant patients as well as AIDS patients (7). The virus has also been linked to the development of atherosclerosis, restenosis after coronary angioplasty, chronic rejection in organ transplant patients, and chronic graft-versus-host disease in bone marrow transplant patients (14, 25, 47). HCMV belongs to the herpesvirus family, and between 60 and 100% of the population are persistently infected with and carry the virus (7). In immunocompetent hosts, the virus appears to be able to cause a life-long latent and persistent infection without causing overt disease. The latency phase is characterized by viral genome persistence without replication of the viral genome (6), which enables the virus to avoid immune recognition. Several viral genes inhibit the expression of HLA class I and II molecules, and the virus inhibits peptide presentation and T-cell activation, inhibits NK lysis of infected cells, upregulates cellular Fc receptor levels, and encodes a viral Fc receptor homologue (reviewed in reference 23). HCMV also induces the production of chemokines and chemokine receptor homologues and interferes with cytokine signaling. It is therefore not surprising that the virus can cause a transient but profound immunosuppression during acute infections.
Dendritic cells are the most potent professional antigen-presenting cells and play a central and unique role in the generation of primary T-cell responses against microorganisms (reviewed in reference 3). The importance of dendritic cells for viral infections in particular stems from their superiority over other antigen-presenting cells in stimulating T-lymphocyte responses and maintaining protective antiviral immunity. Circulating precursor dendritic cells are believed to enter tissues as immature cells that exhibit a high endocytotic capacity. Immature dendritic cells can then develop into mature dendritic cells by a process that is driven by inflammatory stimuli or microenviromental factors such as bacterial products, lipopolysaccharides, and locally produced cytokines such as tumor necrosis factor alpha and interleukin-1β (IL-1β) (3).
Dendritic cells can be generated from two sources: from histiocytic precursor cells, and from monocytes that can differentiate to immature dendritic cells in vitro upon stimulation with granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 (38). Interestingly, monocytes are primary HCMV target cells in vivo and are responsible for dissemination of the virus throughout the body during the latent, acute, and late phases of infection. Thus, the monocyte represents a key cell type in the pathogenesis of HCMV infection, since this cell type represents an important cellular reservoir for latent virus (45, 50, 51). A number of studies have shown that HCMV infection in monocytes is nonpermissive (11, 34) and that cellular differentiation is a prerequisite for HCMV replication (13, 18, 42, 46, 52).
While HCMV infection of monocytes and progenitor cells is nonpermissive, infection is permissive in differentiated macrophages, which can produce new viral progenies. Immune activation followed by production of proinflammatory cytokines appears to play an essential role in HCMV reactivation and replication in infected individuals. For example, we and others have previously shown that both gamma interferon (IFN-γ) and tumor necrosis factor alpha appear to play important roles in differentiating monocytes to HCMV-permissive macrophages (31, 45, 46), and elevated levels of tumor necrosis factor alpha and IFN-γ have also been observed in HCMV-infected patients (10, 21). Recent evidence also suggests that clinical or endothelial cell-adapted HCMV strains (TB40/E) can be used to permissively infect immature dendritic cells in vitro (35). In addition, HCMV has been implied to affect dendritic cell functions by driving the differentiation of immature dendritic cells into mature dendritic cells (32). However, in contrast to these observations, both HCMV and murine CMV appear to inhibit lipopolysaccharide (LPS)-induced maturation of dendritic cells (2, 27).
Since the dendritic cell, due to its role in initiating antiviral immune responses, represents an ideal target for virus immune evasion strategies, it is not surprising that several viruses, including HCMV, measles virus, vaccinia virus, and herpes simplex virus type 1, have been found to interfere with different dendritic cell functions (12, 15, 26). We recently found that HCMV infection of monocytes results in a transient inhibition of the cells′ ability to differentiate into macrophages (S. Gredmark, T. Tilburgs, and C. Söderber-Naclér, unpublished data). In this study, we aimed to examine HCMV's ability to inhibit the differentiation of monocytes into monocyte-derived dendritic cells. We found that HCMV infection of monocytes almost completely inhibited IL-4- and GM-CSF-induced differentiation into CD1a-positive dendritic cells. The HCMV-infected cells exhibited a significantly reduced ability for endocytosis, phagocytosis, and migration and a reduced ability to activate T cells. These observations suggest that HCMV infection inhibits dendritic cell differentiation and suggest a viral strategy to avoid immune recognition and to interfere with early functions of the immune system that may contribute to HCMV's ability to impair immunological functions in infected patients.
MATERIALS AND METHODS
Establishment of dendritic cell cultures.
Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated as previously described (44), and cells were washed and cultured in cell culture flasks at a concentration of 5 × 106 cells/ml in RPMI medium supplemented with 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml (Gibco BRL, Grand Island, N.Y.), and 10% fetal bovine serum and incubated at 37°C for 2 h. The nonadherent cells were removed, the cultures were washed extensively, and the monocyte-enriched cells were further cultured in RPMI 1640 with glutamine supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, 1% sodium pyruvate, 1% nonessential amino acids, and 5 × 10−5 M 2-β-mercaptoethanol, representing complete medium (complete medium), with cytokine stimulation IL-4 (1000 U/ml) and GM-CSF (50 ng/ml) (both from R&D Systems, Minneapolis, Minn.). Cells were cultured for 7 days, with change of medium at day 3 as previously described (38). Nonadherent dendritic cells were collected and used for the experiments after 7 days in culture.
Maturation of dendritic cells was induced after 7 days in culture by addition of 50 ng of LPS (Sigma, St. Louis, Mo.) per ml in complete medium for 48 h.
Restimulation experiments were performed by treating HCMV-infected monocytes for 3 days with complete medium and IL-4 and GM-CSF at 7 days or 10 days postinfection. The cells were examined by flow cytometry 7 days after the second stimulation.
Establishment of monocyte-enriched cultures.
Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated as previously described (44), and the cells were washed and cultured in 96-well plates at a concentration of 6 × 106 cells/ml in Iscove′s medium supplemented with 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml (Gibco BRL, Grand Island, N.Y.), and 10% AB serum and incubated at 37°C for 2 h. The nonadherent cells were removed, the cultures were washed extensively, and the monocyte-enriched cells were further cultured in Iscove’s medium until use.
Virus infection of monocytes.
At the time of cytokine stimulation with IL-4 and GM-CSF, the cells were infected with HCMV at a multiplicity of infection (MOI) of 0.1 to 5 for 3 days at 37°C. After 3 days, the medium was replaced with fresh complete medium. Three different HCMV strains were used for infection of monocytes, AD169, Towne, and a clinical isolate (PO). Experiments were also performed with UV-irradiated Towne and with filtered virus-cleared virus inoculum (filtered through a 0.1-μm-pore-size filter), free of infectious virus particles. The efficiency of the filtration procedure and UV-irradiation treatment of virus was tested on human lung fibroblasts (HL) and resulted in >95% inhibition of viral infectivity.
Experiments were also performed with purified ultracentrifuged virus (pelleted at 10,000 rpm at 4°C overnight in a Beckman ultracentrifuge [rotor JA-10] and dissolved in phosphate-buffered saline). Cell-free virus stocks were prepared from supernatants of infected HL cells, frozen, and stored until use at −70°C. Virus titers were determined by plaque assays as previously described (54). Virus infection was also performed with measles virus, virus titer 1/20 (kindly provided by Annika Lindhe, Karolinska Institutet).
Treatment with recombinant protein, mitogens, and LPS.
At the time of cytokine stimulation with IL-4 and GM-CSF, the cells were also treated with recombinant IL-6 (100 to 1,000 ng/ml), recombinant IL-10 (1 μg/ml), or the recombinant HCMV IL-10 homologue (1 μg/ml), antibodies against IL-10 (10 μg/ml) and the IL-10 receptor (IL-10R) (10 μg/ml) (all from R&D Systems). The virus inoculum was treated for 30 min with anti-IL-10 and/or the cells were treated for 30 min with anti-IL-10R before challenge with the virus inoculum. LPS was used at 10 ng/ml or 50 ng/ml, and phorbol myristate acetate (Sigma) was used at 15 ng/ml or 5 ng/ml.
Flow cytometric analyses of dendritic cells and HCMV-infected monocytes.
A fluorescence-activated cell sorter (FACSort; Becton Dickinson, San Jose, Calif.) was used to analyze dendritic cells and HCMV-infected monocytes for expression of the dendritic cell marker CD1a and the monocyte marker CD14 (antibodies were purchased from Becton Dickinson, San Jose, Calif.) as well as for the expression of other cellular markers. The dendritic cells were harvested and stained with antibodies recognizing different HLA class II molecules (HLA DR, DQ, and DP; all from Becton Dickinson, San Jose, Calif.), HLA class I molecules (Dakopatts, Glostrup, Danmark), and the cell surface markers CD13, CD14, CD40, CD64, CD80, CD83, CD86, ICAM-1, and HCMV pp52, with isotype control antibodies (immunoglobulin G1 and G2a; Dakopatts) followed by appropriate fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (Dakopatts). The level of expression of the different cellular markers was measured for dendritic cells and HCMV-infected monocytes as the mean channel fluorescence value compared to the isotype control. The difference in the histogram mean channel value for uninfected and HCMV-infected cells was calculated on a linear scale, and a difference of more than 10 channels between uninfected and HCMV-infected cells was considered a positive or negative change, based on variations from the controls.
Detection of HCMV replication in HCMV-infected monocytes and dendritic cells.
RNA samples were prepared from dendritic cells and HCMV-infected monocytes at 7 days postinfection by adding Trizol (Gibco BRL) to the cell cultures, followed by RNA purification as previously described (44). cDNA was synthesized with a first-strand cDNA kit (Amersham Biosciences, Buckinghamshire, United Kingdom) according to the manufacturer's instructions and used as templates for HCMV-specific primer pairs for the major immediate-early and pp150 genes in a nested reverse transcription-PCR assay as previously described (44). DNA was prepared from the cells in the cultures as previously described (44). As a positive control for the detection of DNA or RNA, primers specific for the glucose-6-phosphatese dehydrogenase gene were used for each sample (44). DNA and cDNA samples from uninfected and HCMV-infected HL cells were included as positive and negative controls, respectively, in the PCR assays. The PCR products were visualized on a 2% agarose gel.
Measurement of cytokine production.
Dendritic cells and HCMV-infected monocytes were established as described above. Supernatants were collected by two different strategies. In the first, cells were cultured in complete medium with cytokine stimulation with IL-4 (1,000 U/ml) and GM-CSF (50 ng/ml) for the whole period of time, and cell supernatants were collected from 0 to 6, 0 to 12, 0 to 24, 0 to 48, and 0 to 72 h. In the second, cells were cultured in complete medium with cytokine stimulation for 0 to 6 h, supernatants were collected, and complete medium without cytokines was added for 6 to 12 and 12 to 24 h. The supernatants were collected at these time points, cleared by centrifugation, and stored at −70°C. The concentrations of IFN-α and IFN-β were measured with the Quantikine human IFN-α and IFN-β colorimetric sandwich enzyme-linked immunosorbent assay (ELISA) (PBL Biomedical Laboratories, Piscataway, N.J.), the concentration of IL-12 was measured with the Quantikine human IL-12 sandwich ELISA (R&D Systems), and the concentrations of IL-6, IL-10, and IL-1β were measured with the Quantikine human colorimetric sandwich ELISAs for IL-6, IL-10 and IL-1β (BD Biosciences) according to the manufacturer's instructions.
T-cell proliferation assays.
CD4+ T cells were isolated from PBMCs obtained from healthy blood donors as previously described (44) and by using CD4 magnetic beads (Dynal ASA, Oslo, Norway) according to the manufacturer's instructions. The beads were allowed to detach from the CD4+ cells by incubating the cells at 37°C overnight in RPMI medium supplemented with 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml (Gibco BRL, Grand Island, N.Y.), and 10% fetal bovine serum.
The dendritic cells or HCMV-infected monocytes (0.5 × 105/well) and the CD4+ cells (105/well) were incubated in 96-well round-bottomed plates for 5 days; the cells were pulsed with 1 μCi of [3H]thymidine (Amersham Biosciences) for the last 16 h of a 5-day in vitro culture. The cells were harvested onto filters with a plate harvester (Harvester 996; Tomtec, Hamden, Conn.) according to the manufacturer′s instructions and counted in an automated counter (1450 MicroBeta Trilux; Wallac, Upplands Väsby, Sweden). The results were expressed as counts per minute (cpm) per well. All means of cpm values were obtained from triplicate cultures.
Mixed leukocyte reaction.
Responder cells (105) from an unrelated individual were isolated from PBMCs obtained from healthy blood donors as previously described (44) and incubated with dendritic cells or HCMV-infected monocytes (0.5 × 105), monocytes, and HCMV-infected monocytes from the same donor in 96-well round-bottomed plates for 5 days. Responder cells (105) were also incubated with dendritic cells or HCMV-infected monocytes (0.5 × 105) together with the mitogen phytohemagglutinin (90 ng/ml) (Boehringer, Mannheim, Germany) in 96-well round-bottomed plates for 3 days. The cells were pulsed with 1 μCi of [3H]thymidine (Amersham Biosciences) for the last 16 h of the in vitro culture, and the cells were harvested as described above. Restimulation of T cells was performed by adding 30 ng of Orthoclone OKT3 (Cilag AG Int., Zug, Switzerland) per ml to PBMCs, dendritic cells, or HCMV-infected monocytes for the last 72 h of the 5-day in vitro culture. The cells were thereafter pulsed with [3H]thymidine and harvested as described above.
Collection of supernatants from mixed leukocyte reaction and dendritic cell/HCMV-infected monocyte cultures.
Supernatants from responder cells (106/ml) and dendritic cell or HCMV-infected monocyte (0.5 × 106/ml) cultures were collected at 1, 3, and 5 days, cleared by centrifugation, and stored at −70°C until use. The supernatants were used at a dilution of 1:10 in the mixed leukocyte reaction assay or phytohemagglutinin stimulation of responder cells purified from PBMC. The assay was performed as described above.
The supernatants were tested on human lung fibroblasts (HL) for HCMV content, which resulted in <1% viral infectivity by staining with immediate-early antibody.
Endocytosis assay.
Dendritic cells or HCMV-infected monocytes (200 μl of 106 cells/ml) were incubated with dextran-FITC (Molecular Probes, Eugene, Oreg.) at a final concentration of 1 mg/ml for 1 h at 37°C. As a negative control, cells were incubated under the same conditions at 4°C. Thereafter, the cells were washed four times in phosphate-buffered saline supplemented with 0.01% NaN3 as previously described (39). The levels of endocytosed FITC-dextran in dendritic cells and HCMV-infected monocytes were measured as the mean channel fluorescence value by flow cytometric analyses. The difference in the histogram mean channel value for dendritic cells and HCMV-infected monocytes was calculated on a linear scale.
Determination of phagocytosis.
Yeast particles were labeled with FITC as previously described (41) and incubated with dendritic cells and HCMV-infected monocytes at 7 days postinfection for 30 min at 37°C. Then 106 yeast particles were used in each tube with 105 cells in a total volume of 200 μl of RPMI medium. After 30 min, phagocytosis was arrested by transferring the tubes to 4°C. Thereafter, phosphate-buffered saline containing 5% trypan blue was added to quench the extracellular bound particles. Phagocytosis was monitored and quantified in a fluorescence microscope, and the results are presented as the number of cells that had phagocytosed at least one yeast particle at 30 min. The number of cells which had ingested yeast particles was determined in duplicate in two independent experiments, and at least 100 cells per well were counted.
Chemotaxis assay.
A chemotaxis assay was performed as previously described (24). Briefly, Transwell cell culture chambers (48 wells) with polycarbonate filters with 8-μm pores (Costar, Cambridge, Mass.) were used. The filters were coated with 5 μg of gelatin in a volume of 50 μl, and dried overnight at room temperature. The coated filters were washed once in phosphate-buffered saline and dried immediately before use. Then 100 000 cells in 100 μl were added to the upper compartment in serum-free RPMI supplemented with 2 mM glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml (Gibco-BRL, Grand Island, N.Y.). Then 600 μl of serum-free medium containing 100 ng of either RANTES, MIP-1α, or MIP-3β (R&D Systems, Minneapolis, Minn.) per ml were added to the lower compartment. The cells were incubated for 2 h at 37°C. The filters were washed three times in phosphate-buffered saline, fixed in methanol for 5 min, and then washed once in phosphate-buffered saline, and the wells were stained in Mayer's hematoxylin and eosin (Histolab Products SB, Gothenburg, Sweden). Cells still present on the upper side of the filter were scraped off with a cotton swab, which allowed the cells on the lower side of the filter to be quantified under a light microscope (40× lens). The cells in five representative fields for each filter were quantified, and the experiments were always performed in triplicate.
Transendothelial migration assay.
The transendothelial migration assay was performed as described before (22) with some modifications. Briefly, Transwell cell culture chambers (48 wells) with polycarbonate filters with 5-μm pores (Costar, Cambridge, Mass.) were used. An endothelial cell monolayer was established on the filter by first incubating the filters with fibronectin (Boehringer, Mannheim, Germany) according to the manufacturer's protocol before human pulmonary aortic endothelial cells (Clonetics, Cambrex Bio Science, Walkersville, Md.) were seeded and allowed to grow on the filter to confluency. The monolayer integrity was tested by adding 40 μl of 20-mg/ml FITC-dextran to each insert, and thereafter the mean fluorescence intensity was tested with a Fluoroscan II (Thermo Labsystems, Vantaa, Finland). We added 100,000 cells in 100 μl to the upper compartment in serum-free RPMI supplemented with 2 mM glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml (Gibco BRL, Grand Island, N.Y.). Then 600 μl of serum-free medium containing 100 ng of RANTES per ml was added to the lower compartment. The cells were incubated for 2 h at 37°C. After 2 h, the Transwell inserts were removed and the bases were rinsed twice into the lower chamber. Cells in the lower chamber were then collected and pelleted, and the number of cells that had migrated (migrating cells) was quantified in a Bürker chamber under a light microscope. Results are presented as the total number of migrating cells per Transwell and are from duplicate assays with cells from five different donors.
RESULTS
HCMV inhibits the differentiation of monocytes into dendritic cells.
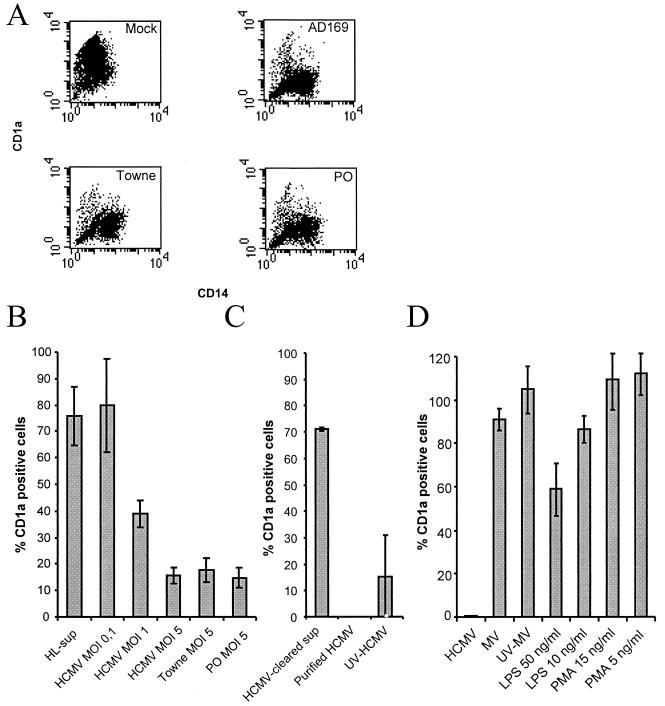
To examine whether HCMV could affect the differentiation of monocytes into dendritic cells, we mock infected or HCMV infected monocytes at the same time point as cytokine stimulation. After 7 days in culture, mock-infected monocytes stimulated with the cytokines IL-4 and GM-CSF differentiated into dendritic cells that uniformly expressed high levels of the dendritic cell marker CD1a, as detected by flow cytometry (Fig. 1A). Furthermore, the expression level of CD14 was either low or absent in this cell population (Fig. 1A). In contrast, HCMV-infected monocytes stimulated with IL-4 and GM-CSF did not express CD1a but retained the expression of CD14, which suggested that these cells remained at a monocytic stage and did not differentiate into CD1a-expressing dendritic cells (Fig. 1A). The experiments were performed with different viral strains and titers (MOI 0.1 to 5) and showed that HCMV inhibited dendritic cell differentiation in a dose-dependent fashion (Fig. 1B) and that different HCMV strains (AD169, Towne, and a clinical isolate, PO) exhibited similar phenotypes after infection (Fig. 1B).
FIG. 1.
HCMV infection inhibits cytokine-induced differentiation of monocytes into dendritic cells. (A) HCMV was tested for its ability to inhibit differentiation of monocytes into dendritic cells obtained by IL-4 and GM-CSF stimulation by infecting monocytes with the laboratory strain AD169 or Towne or a clinical isolate (PO) at an MOI of 5. The figure presents a representative sample of a flow cytometric analysis of the expression of the cell surface molecules CD1a (y axes represent the mean fluorescent intensity of CD1a) and CD14 (x axes represent the mean fluorescent intensity of CD14) on mock-infected dendritic cells and HCMV-infected monocytes. (B) HCMV was tested for its ability to affect cellular differentiation by infecting cells with the laboratory strain AD169 at an MOI of 0.1 to 5, laboratory strain Towne at an MOI of 5, and a clinical isolate (PO) at an MOI of 5. Supernatants from mock-infected fibroblasts (HL-sup) were also used for infection. (C) To test whether the HCMV-induced block in cellular differentiation was dependent on soluble factors, filtered virus-free supernatants were used for infection of cells. To test if the HCMV-induced block in cellular differentiation was directly dependent on the virus particle, ultracentrifuged pure virus particles were used for infection. To test if the inhibition was dependent upon active virus replication or virus uptake by the cell, we used UV-inactivated (replication-deficient) virus for infection of cells. (D) To determine if the inability of the cells to differentiate into dendritic cells was an HCMV-specific phenomenon, monocytes were infected with measles virus or treated with phorbol myristate acetate and LPS. The percentage of CD1a-positive cells in the individual sample compared to the uninfected control was determined. The data represent mean and standard error of the mean values for six separate experiments.
To further examine whether the effect on dendritic cell differentiation was caused by soluble factors present in the virus inoculum, we cleared viral preparations from virus particles by filtration. Virus-free supernatants from mock-infected and HCMV-infected fibroblast cultures did not affect dendritic cell differentiation (Fig. 1B and 1C), which suggests that the virus rather than soluble factors produced by infected cells mediated the effect. In addition, in experiments with purified HCMV particle preparations, we also observed a complete block in dendritic cell differentiation.
To further examine whether active HCMV replication was required to block dendritic cell differentiation, we used UV-inactivated and nonreplicating HCMV to infect monocytes and found that inactivated virus inhibited dendritic cell differentiation to an extent similar to that in untreated wild-type virus (Fig. 1C). We also tested whether active HCMV replication could be detected in HCMV-infected cells. DNA and RNA samples were prepared from uninfected and infected cultures, and reverse transcription-PCR and PCR assays were performed with primers specific for the HCMV major immediate-early and pp150 genes and the cellular gene for glucose-6-phosphate dehydrogenase. While all HCMV-infected samples were positive for both HCMV DNA as well as HCMV RNA in the major immediate-early and pp150 PCR assays, all the uninfected dendritic cell samples were negative for HCMV DNA and HCMV RNA (data not shown). These results imply that HCMV infection of monocytes at the same time as stimulation with IL-4 and GM-CSF resulted in detectable levels of HCMV replication. However, the level of replication appeared to be very low, since we could not detect the major immediate-early HCMV protein by immunofluorescence or pp52 by flow cytometry in the infected cells (data not shown).
To determine if the inability of the cells to differentiate into dendritic cells was an HCMV-specific phenomenon, we infected the monocytes with measles virus but could not observe a difference in dendritic cell differentiation, which suggests that virus-induced inhibition of dendritic cell differentiation was not a general event following virus infection (Fig. 1D). To further examine whether other stimuli that are known to affect cellular differentiation pathways could affect dendritic cell differentiation, we tested the ability of the mitogen phorbol myristate acetate and LPS to affect dendritic cell differentiation. While high concentrations of LPS decreased the levels of CD1a-positive cells by 40%, phorbol myristate acetate did not affect dendritic cell differentiation (Fig. 1D).
HCMV transiently inhibits dendritic cell differentiation.
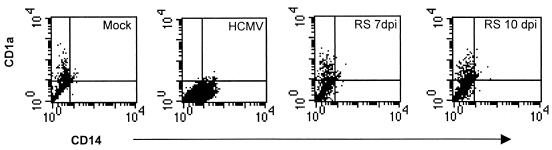
Our observations suggested that HCMV affected the ability of the monocytes to differentiate into dendritic cells upon stimulation with IL-4 and GM-CSF. We therefore asked whether HCMV-infected monocytes could be restimulated into CD1a-positive dendritic cells at a later time point by stimulating HCMV-infected monocytes with IL-4 and GM-CSF at either 7 days or 10 days postinfection. We found that a subpopulation of the HCMV-infected monocytes could indeed differentiate into CD1a-positive cells, but approximately 70% of the HCMV-infected monocytes that were restimulated at 7 or 10 days postinfection did not appear to differentiate into dendritic cells (Fig. 2 and data not shown). However, while HCMV-infected monocytes still expressed CD14 after more than 2 weeks in culture, the expression of CD14 almost completely disappeared on restimulated HCMV-infected monocytes.
FIG. 2.
HCMV-induced inhibition of dendritic cell differentiation is partly reversible. To test whether HCMV-infected monocytes, which appear to be arrested in their ability to differentiate, were able to differentiate into dendritic cells upon secondary stimulation with cytokines, we treated HCMV-infected monocytes for 3 days with IL-4 and GM-CSF at 7 days or at 10 days postinfection (dpi). The cells were examined by flow cytometry 7 days after the second stimulation. The figure shows a representative example of flow cytometry data for the expression of the cell surface molecules CD1a (y axes represent the mean fluorescent intensity) and CD14 (x axes represent the mean fluorescent intensity) on dendritic cells and HCMV-infected monocytes (RS, restimulated).
Phenotypic analysis of HCMV-infected monocyte populations.
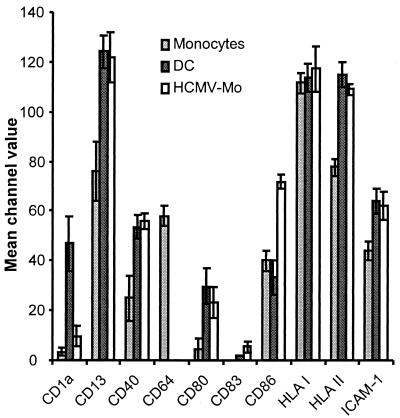
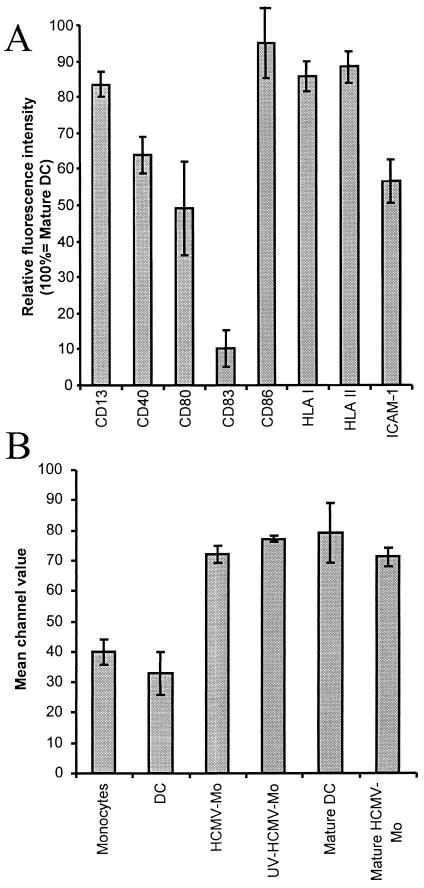
The dendritic cell is one of the most potent professional antigen-presenting cells that can initiate the adaptive immune response against microorganisms. To be able to initiate an immune response, dendritic cells must express microbial peptides in the context of HLA class II molecules on the cell surface and provide secondary signals through costimulatory molecules. To investigate whether HCMV infection affected the expression of molecules involved in antigen presentation, we compared the expression of different surface markers on monocytes, HCMV-infected monocytes, and dendritic cells by flow cytometry. We found that both HCMV-infected monocytes and dendritic cells exhibited increased levels of expression of the cellular markers CD13, ICAM-1, and the HLA class II molecules CD40 and CD80 compared to uninfected monocytes (Fig. 3). However, while the levels of expression of HLA class I molecules were equally high on all cell types examined, expression of CD64 could only be detected on fresh monocytes (Fig. 3). Interestingly, we found that the expression of CD86 was induced on HCMV-infected monocytes, but not on immature dendritic cells, on which the level of expression of CD86 remained at levels similar to those on uninfected monocytes. Hence, in regard to the expression of surface markers, these results imply that HCMV-infected monocytes appeared to constitute a specific cell phenotype.
FIG. 3.
Phenotypic analyses of HCMV-infected monocyte populations. To investigate whether HCMV infection affected the expression of cell surface molecules involved in antigen presentation, we compared the expression of the cell surface molecules CD1a, CD13, CD40, CD64, CD80, CD83, CD86, HLA classes I and II, and ICAM-1 on fresh monocytes, HCMV-infected monocytes (HCMV-Mo), and dendritic cells (DC) by flow cytometry. The cells were analyzed after 7 days in culture, and the data obtained represent mean channel values for six separate experiments.
Dendritic cell differentiation is inhibited by IL-10 and the HCMV IL-10 homologue.
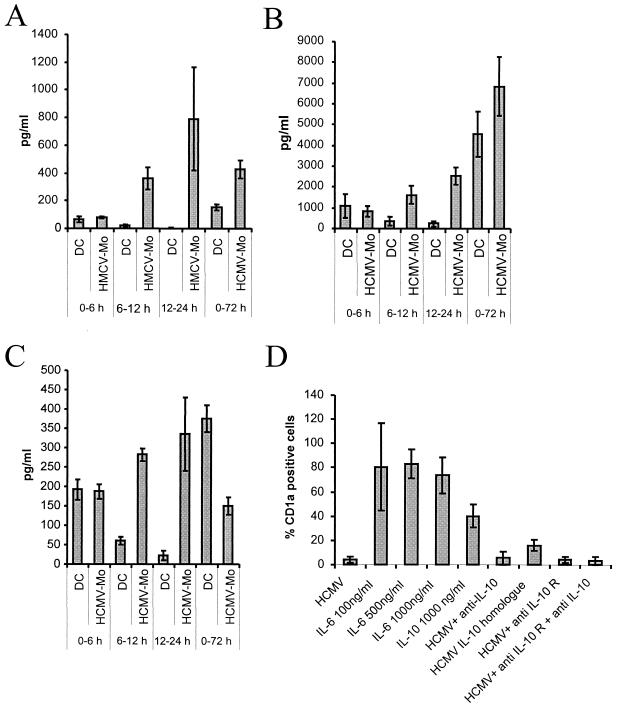
A number of previous studies have shown that HCMV infection of different cell types can alter cytokine production profiles, which could possibly explain why HCMV-infected monocytes fail to differentiate into dendritic cells. We therefore examined whether HCMV infection of monocytes could alter the secretion of cytokines. We collected supernatants from HCMV-infected monocytes and dendritic cells at different time points after infection and tested these for the presence of IFN-α, IFN-β, IL-1β, IL-6, IL-10, and IL-12. We found that HCMV infection of monocytes resulted in an increased production of IL-1β, IL-6, and IL-10 (Fig. 4A to C) but not IFN-α, IFN-β, and IL-12 (data not shown). While the accumulated levels of IL-1β and IL-6 were higher in HCMV-infected monocytes at 72 h postinfection, the levels of IL-10 decreased at this time point.
FIG. 4.
Recombinant IL-10 and HCMV-IL-10 inhibit dendritic cell differentiation. To examine whether HCMV infection of monocytes alters the secretion of cytokines, supernatants were collected from HCMV-infected monocytes (HCMV-Mo) and dendritic cell (DC) cultures. The levels of cytokines (A) IL-1β, (B) IL-6, and (C) IL-10 in the cultures were determined at the time points indicated. Recombinant IL-6, recombinant IL-10, or HCMV-IL-10 was added to the cultures at the same time as cytokine stimulation, and antibodies against IL-10 and the IL-10 receptor (IL-10 R) were added to test if the HCMV effect could be reversed.
Since previous data suggest that IL-6 altered the differentiation of monocytes from a dendritic cell phenotype to a macrophage phenotype (8), we added an excess of recombinant IL-6 to the cultures but did not observe an inhibition of dendritic cell differentiation (Fig. 4D). Furthermore, since previous studies also suggested that recombinant IL-10 and the HCMV IL-10 homologue (HCMV-IL-10) can inhibit the proliferation of mitogen-stimulated PBMCs (48), we tested recombinant IL-10 and HCMV-IL-10 in this assay. We found that IL-10 inhibited dendritic cell differentiation by 60% and that HCMV-IL-10 completely inhibited the differentiation of dendritic cells. However, we were not able to reverse the effect by HCMV on dendritic cell differentiation by using antibodies specific for IL-10 and the IL-10 receptor that have been described to neutralize the effect IL-10 and HCMV-IL-10 (Fig. 4D).
HCMV-infected monocytes exhibit a decreased ability for endocytosis, phagocytosis, and migration.
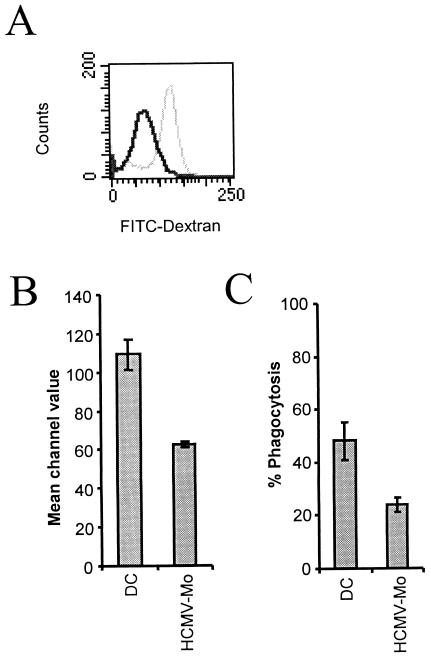
To examine whether the ability of HCMV-infected monocytes to capture antigens was affected by infection, we tested the endocytotic capacity of HCMV-infected monocytes and dendritic cells by measuring the uptake of FITC-conjugated dextran particles by flow cytometry. While uninfected dendritic cells exhibited a high endocytic capacity, HCMV-infected monocytes were significantly impaired (approximately 40 to 50% reduction) in their ability to endocytose FITC-labeled dextran particles (Fig. 5A and B). We also tested the ability of the cells to phagocytose FITC-labeled yeast particles and found that dendritic cells exhibited a significantly higher phagocytic activity than HCMV-infected monocytes (Fig. 5C).
FIG. 5.
HCMV-infected monocytes exhibit a decreased ability for endocytosis and phagocytosis. To test whether HCMV-infected monocytes exhibited altered endocytosis, we tested the ability of dendritic cells and HCMV-infected monocytes to endocytose FITC-labeled dextran particles for 1 h after 7 days in culture. The cells were thereafter examined by flow cytometry to obtain histogram plots of dendritic cells and HCMV-infected monocytes. (A) Representative example of a histogram plot of the fluorescence values of dextran-FITC of HCMV-infected monocytes (dark gray line) and dendritic cells (light gray line) obtained by flow cytometry. (B) Statistical analysis of the mean channel values obtained by flow cytometry of FITC-dextran in dendritic cells (DC) and HCMV-infected monocytes (HCMV-Mo). The results represent mean and standard error of the mean values obtained from six separate experiments performed in duplicate. (C) The ability of the cells to phagocytose FITC-labeled yeast particles was tested at 7 days postinfection for 30 min at 37°C. Phagocytosis was monitored and quantified in a fluorescence microscope, and the results are presented as the number of cells that phagocytosed at least one yeast particle.
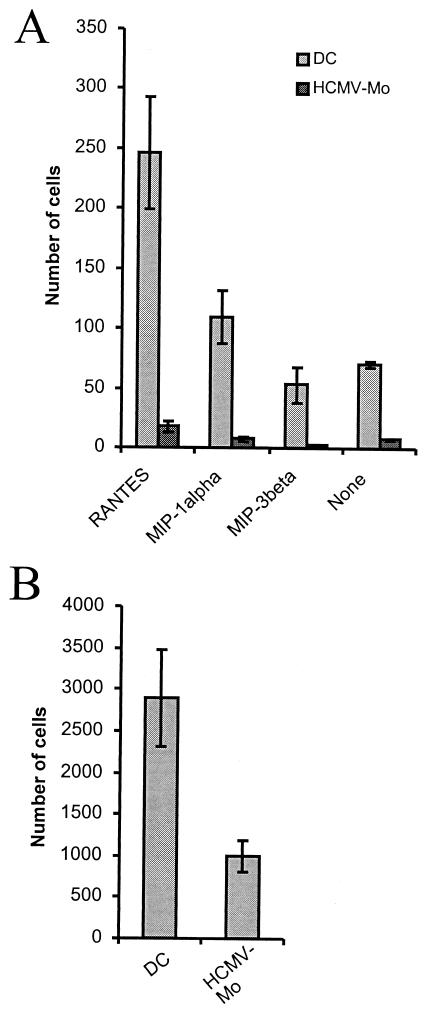
Since one of the major functions of immature dendritic cells is to migrate in response to chemoattractants, we further examined the ability of HCMV-infected monocytes to migrate in response to the chemoattractants RANTES, MIP-1α (ligands for both CCR1 and CCR5) and MIP-3β (ligand for CCR7) in a Transwell system. Interestingly, we found that the ability of HCMV-infected monocytes to migrate in response to these chemoattractants was almost completely blocked compared to that of uninfected dendritic cells (Fig. 6A). We also tested whether uninfected and infected cells could cross an endothelial barrier in a transendothelial migration assay. We found that the migration of HCMV-infected monocytes through this barrier was reduced by approximately 60% compared to that of dendritic cells (Fig. 6B). However, we were unable to detect a difference in the expression levels of CCR1, CCR5, or CCR7 (negative expression on both dendritic cells and HCMV-infected monocytes) between uninfected and infected cells (data not shown).
FIG. 6.
HCMV-infected monocytes exhibit a decreased ability to migrate in response to the chemoattractants RANTES, MIP-1α, and MIP-3β. (A) To test whether HCMV-infected monocytes (HCMV-Mo) exhibited a decreased ability to migrate in response to a chemotactic stimulus, we performed a migration assay on HCMV-infected monocytes and mock-infected dendritic cells (DC) after 7 days in culture. The cells were examined for their ability to migrate in a Transwell system in response to the chemoattractant factors RANTES, MIP-1α, and MIP-3β or no stimulus (which represents random migration). The number of cells that migrated through the filter pores was quantified by counting cells on the lower side of the filters under a light microscope in five representative fields for each well. The results are presented as the mean and standard error of the mean cell numbers obtained from three separate experiments performed in triplicate. (B) To test whether uninfected and infected cells could cross an endothelial barrier, we performed a transendothelial migration assay as described in Materials and Methods. The number of migrating cells was quantified in a Bürker chamber under a light microscope. Results are presented as the total number of migrating cells per Transwell and are from experiments performed in duplicate in five different donors.
HCMV-infected monocytes exhibit an impaired ability to mature in response to LPS.
We further examined whether the HCMV-infected monocytes could mature upon LPS stimulation and found that these cells failed to upregulate the costimulatory molecules CD40, CD80, and CD83 and the cell adhesion molecule ICAM-1 at 48 h poststimulation (Fig. 7A). The relative fluorescence intensity of these molecules on HCMV-infected monocytes was 64% for CD40, 49% for CD80, 10% for CD83, and 56% for ICAM-1. In contrast, other surface molecules such as CD13 and HLA class I and II molecules were expressed at similar levels on dendritic cells and HCMV-infected monocytes after LPS stimulation. Furthermore, we found that LPS-induced maturation of dendritic cells resulted in CD86 expression levels that were similar to the levels we observed on HCMV-infected monocytes that were not treated with LPS (Fig. 7B). Furthermore, infection of monocytes at the same time point as cytokine stimulation with UV-inactivated HCMV resulted in levels of CD86 expression similar to those on HCMV-infected monocytes. In addition, LPS treatment of HCMV-infected monocytes did not further induce expression of CD86. Since the costimulatory molecule CD86 is an important molecule that activates and induces T-cell responses, and the fact that HCMV-infected monocytes expressed CD86 at levels similar to those on mature dendritic cells, these results imply that HCMV-infected monocytes may constitute a distinct cell phenotype that possibly exhibits inhibitory properties for T-cell activation.
FIG. 7.
HCMV-infected monocytes express high levels of the costimulatory molecule CD86 and an impaired ability to mature in response to LPS treatment. (A) To examine whether the HCMV-infected monocytes (HCMV-Mo) could mature in response to activation by LPS, we stimulated HCMV-infected monocytes and mock-infected dendritic cells (DC) with LPS for 48 h as described in Materials and Methods. The relative fluorescence intensity of the cell surface molecules CD13, CD40, CD80, CD83, CD86, HLA classes I and II, and ICAM-1 was analyzed by flow cytometry. The relative fluorescence intensity of surface molecules on HCMV-infected monocytes following LPS stimulation is shown. Means and standard error of the mean values were calculated from five separate experiments. Relative fluorescence intensity was calculated as (mean fluorescent intensity on “mature” HCMV-infected monocytes/mean fluorescent intensity on mature dendritic cells) × 100. (B) Mean fluorescence intensity values obtained for the costimulatory molecule CD86 on monocytes, dendritic cells, HCMV-infected monocytes, UV-irradiated HCMV-infected monocytes, mature dendritic cells, and mature HCMV-infected monocytes obtained by flow cytometry. The results represent mean and standard error of the mean values obtained from five experiments. (B) We did not observe a difference in cell viability between dendritic cells and HCMV-infected monocytes, as demonstrated by trypan blue dye staining (data not shown), which suggests that the inability of the infected cells to upregulate cell surface molecules was not due to a difference in cell viability.
HCMV-infected monocytes exhibit a reduced capacity to stimulate alloreactive T cells.
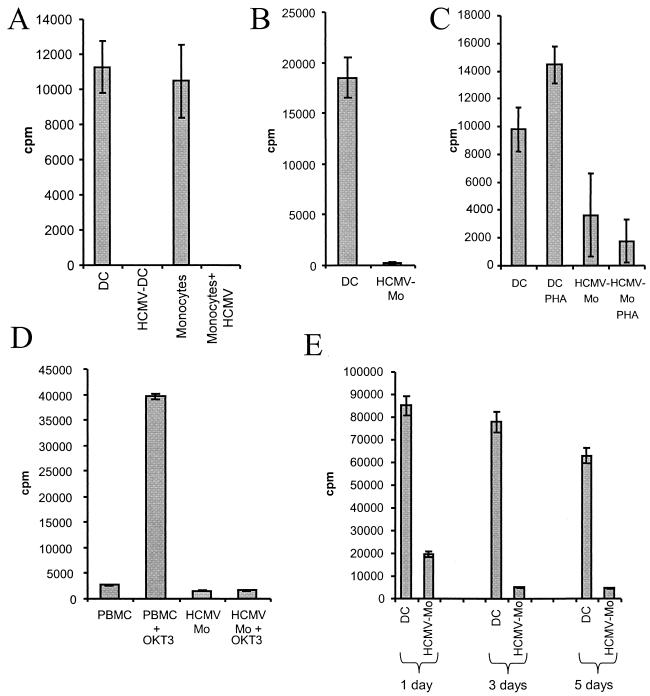
The presentation of antigenic peptides by dendritic cells is a crucial step in the initiation of specific immune responses and depends on the expression of HLA class II molecules as well as on costimulatory molecules. We therefore tested the ability of HCMV-infected monocytes and dendritic cells to induce the proliferation of naive allogeneic T cells in vitro (PBMCs or purified CD4-positive T cells). Interestingly, while the uninfected dendritic cells and monocytes triggered a substantial T-cell response, HCMV-infected monocytes could not induce a proliferative T-cell response under either condition (Fig. 8A and B).
FIG. 8.
HCMV-infected monocytes cannot induce a proliferative response in alloreactive T cells. To test the ability of dendritic cells and HCMV-infected monocytes to induce a proliferative response in alloreactive T cells in vitro, dendritic cells and HCMV-infected monocytes were tested in a mixed leukocyte reaction after 7 days in culture. (A) The data represent cpm values obtained from dendritic cells (DC), HCMV-infected monocytes (HCMV-Mo), monocytes, and HCMV-infected monocytes with PBMCs as responder cells. (B) The data represent cpm values obtained from dendritic cells and HCMV-infected monocytes with purified CD4-positive T cells as responder cells. (C) To examine if T-cell proliferation can be induced in cells through other pathways, dendritic cells and HCMV-infected monocytes were cocultured with PBMCs as responder cells and stimulated with phytohemagglutinin (PHA) as described in Materials and Methods. (D) To further examine whether T-cell proliferation could be induced in cells that had been in contact with HCMV-infected monocytes, anti-CD3 antibodies (OKT3) were added to cells in the mixed leukocyte reaction. (E) To examine if the inhibition of a T-cell response was dependent on cell-cell contact, supernatants were collected at 1, 3, and 5 days and used at a dilution of 1:10 in the mixed leukocyte reaction assay or in the phytohemagglutinin stimulation of responder cells purified from PBMCs. Mean [3H]thymidine uptake from triplicate wells is shown, and the results shown are mean values from three separate experiments performed in triplicate. T-cell viability was tested by adding propidium iodide to the cell cultures, which revealed that the T cells did not take up propidium iodide (data not shown).
To further examine whether T-cell proliferation could be induced in cells that had been in contact with HCMV-infected monocytes, phytohemagglutinin or anti-CD3 antibodies (OKT3) were added to the cultures. While both phytohemagglutinin and OKT3 treatment could induce T-cell proliferation in dendritic cell cultures, phytohemagglutinin and OKT3 treatment did not induce a proliferative T-cell response in HCMV-infected monocyte cultures (Fig. 8C and D). To test whether the inhibitory effect was mediated through cell-cell contact or a soluble factor, we cultured PBMC and dendritic cells or HCMV-infected monocytes together for different time intervals and used the culture supernatants in subsequent phytohemagglutinin stimulation assays. We found that while supernatants obtained from HCMV-infected monocyte/PBMC cultures inhibited phytohemagglutinin-induced T-cell proliferation, supernatants obtained from uninfected cultures did not affect T-cell proliferation (Fig. 8E). We also found that the observed inhibition of the T-cell response was not dependent on the presence of virus particles, since the supernatants did not contain infectious HCMV particles, as tested on fibroblast cultures (data not shown).
DISCUSSION
HCMV infections are frequently associated with a transient but often severe immunosuppression during the initial stages of the acute infection in transplant patients (16, 17, 36, 37). Other viruses such as human immunodeficiency virus and measles virus are also known to cause immunosuppression, and evidence has been presented that an interaction between viruses and dendritic cells may be important for the outcome of several infections (4). While some dendritic cell-tropic viruses such as influenza virus leave dendritic cell functions intact (5), other viruses, including HCMV, human immunodeficiency virus, measles virus, and herpes simplex virus type 1 have evolved different mechanisms to impair selected dendritic cell functions (15, 19, 26, 32).
Here, we examined HCMV's ability to affect differentiation of monocytes into dendritic cells and found that HCMV-infected monocytes could not differentiate into dendritic cells upon stimulation with GM-CSF and IL-4. HCMV-infected monocytes expressed sustained high levels of the monocytic cell marker CD14 but were negative for the dendritic cell-specific marker CD1a. Our results demonstrate that monocytes treated with virus-cleared supernatants differentiate into dendritic cells and that purified virus particle preparations that do not contain cytokines from the supernatant in the virus stocks completely inhibit dendritic cell differentiation. Furthermore, with UV-inactivated and nonreplicating virus, the differentiation of dendritic cells was inhibited to the same extent as was observed with untreated virus. Thus, these results demonstrate that virus particles but not active virus replication is required for the HCMV-mediated inhibition of dendritic cell differentiation. Furthermore, since measles virus was not able to inhibit dendritic cell differentiation, the observed phenomena suggest that this is not a general event following virus infection but rather appears to be HCMV specific.
Further evidence that the inhibition is HCMV specific was provided by the findings that neither LPS nor phorbol myristate acetate inhibited dendritic cell differentiation. In further support of the observation that the inhibition of dendritic cell differentiation could be directly dependent on the interaction with the monocyte and HCMV, Yurochko et al. have previously shown that binding of HCMV to monocytes induces a number of immunoregulatory genes and that HCMV initiates a signal transduction pathway that leads to monocyte activation that results in the production and release of IL-1β protein (55). We also observed an increased release of IL-1β in our HCMV-infected monocyte cultures. Furthermore, Noraz et al. found that PBMCs exposed to HCMV produced IFN-α within 4 to 10 h postexposure, which largely accounted for the observed HCMV-induced immunosuppression in vitro, as demonstrated by morphologically fewer differentiated monocytes in PBMCs exposed to HCMV and a decrease in monocyte oxidative activity in these cells (29). However, in our experimental system, we only observed low levels of released IFN-α and no differences between infected and uninfected cultures.
Yet another study has shown that IL-6, by an upregulation of the expression of functional macrophage colony-stimulating factor (M-CSF) receptors on monocytes allowed the monocytes to consume their autocrine M-CSF, which resulted in an alteration in the differentiation of monocytes from a dendritic cell phenotype to a macrophage phenotype (8). However, we could not alter the differentiation of monocytes from a dendritic cell phenotype to a macrophage phenotype even with an excess of IL-6 in our experimental system. Instead, we found a 10-fold increase in the levels of IL-10 in the HCMV-infected monocyte cultures compared to the dendritic cell cultures at 24 h postinfection. At 72 h, the IL-10 levels decreased in the HCMV-infected monocyte cultures, possibly suggesting that the HCMV-infected monocytes consumed the IL-10 produced by infected cells. Previous studies also suggest that recombinant IL-10 and the HCMV IL-10 homologue (20) can inhibit the proliferation of mitogen-stimulated PBMCs (48). Allavena et al. have also shown that IL-10 prevents the differentiation of monocytes into dendritic cells but promotes their maturation into macrophages (1). Here, we found that recombinant IL-10 inhibited dendritic cell differentiation by 60% and that the recombinant protein for the HCMV homologue IL-10 completely inhibited the differentiation of dendritic cells. However, we were not able to reverse the effect by using IL-10- and/or IL-10 receptor-specific antibodies, which suggests that a rapid autocrine loop may be at work or that other unidentified cytokines are involved.
Recently, it was demonstrated by Compton et al. that the pattern recognition receptors Toll-like receptor 2 (TLR-2) and CD14 recognize HCMV particles and trigger inflammatory cytokine production via TLR-2-dependent activation of NF-κB as a result of immune activation of innate pathways (9). Here, we found that HCMV but not LPS inhibited dendritic cell differentiation and that the costimulatory molecule CD86 is specifically upregulated on HCMV-infected monocytes. Interestingly, the CD86 molecule can be induced as a consequence of TLR signaling, which suggests that TLR-2 signaling may be involved in the inhibition of dendritic cell differentiation. The findings by Compton et al. do indeed suggest that the TLR system may be broadly capable of detecting virus during the early stage of infection. An early innate immune response during the initial phases of infection leading to inflammation may perhaps facilitate virus dissemination through the recruitment of mononuclear cells, which are primary targets for the virus, and thereby facilitate virus spread throughout the body. One could also speculate about the possible role of a virus-induced activation of the TLR system and an innate immune response in inflammatory diseases, such as cardiovascular diseases, which have been epidemiologically linked to HCMV infection (43, 49).
Dendritic cells are key cell types in the immune system, since they function as endocytosing and antigen-presenting cells and generate and maintain specific immune responses. In the absence of ongoing inflammatory and immune responses, dendritic cells constitutively patrol through the blood, peripheral tissues, lymph nodes, and secondary lymphoid organs. In peripheral tissues, dendritic cells take up self and nonself antigens by endocytosis, and efficient antigen internalization appears to be a specific attribute of immature dendritic cells, which downregulate their endocytotic activity during maturation and thereby limit the range of antigens that they are able to present to T cells after leaving the tissue (3).
Since dendritic cells play a crucial role in the generation of virus-specific T cells, targeting functions performed by this cell type during virus infection would be predicted to have a profound effect on the immune response. A number of previous studies have shown that immature and mature dendritic cells are permissive for HCMV infection but that certain endothelial cell-adapted strains grow significantly better in these cells than strains that have been passaged multiple times in fibroblasts. Here, using a fibroblast-adapted strain of the virus, we could detect low levels of HCMV replication by reverse transcription-PCR but did not detect major immediate-early or pp52 proteins in HCMV-infected monocytes. We did not observe strain variability with regard to the effect of the different viral strains on different HCMV-infected monocyte functions. In addition, while previous findings have shown that active virus replication and the use of a high MOI for infection are required to observe an effect on immature and mature dendritic cells, we observed an HCMV-induced inhibition of dendritic cell differentiation at very low MOIs.
Different results have been presented regarding HCMV's ability to influence the maturation of dendritic cells during an active infection. For example, one recent study suggested that HCMV can prevent maturation of dendritic cells and that immature dendritic cells fail to upregulate cell surface molecules such as major histocompatibility complex class I and II and costimulatory molecules upon LPS stimulation (27). HCMV infection of dendritic cells resulted in a slight downregulation of CD86 and major histocompatibility complex class II, and the authors observed impaired cytokine production induced by stimulation of the infected cells with CD40L or LPS. In contrast, other investigators have found that HCMV infection of immature dendritic cells results in an upregulation of costimulatory molecules and that infection promoted the maturation of immature dendritic cells (32). HCMV-infected dendritic cells have also been shown to exhibit enhanced expression of costimulatory molecules, which, along with decreased HLA class I molecule expression, may result in induction of anergy in T cells (33, 40).
Furthermore, other investigators who have examined the effect of murine cytomegalovirus (MCMV) infection on dendritic cells in vivo and in vitro found that MCMV infection appears to result in prevention of the delivery of the signals required for T-cell activation along with an impaired antigen uptake. When immature dendritic cells were infected with MCMV, two stages of phenotypic changes were observed; at 2 days after infection, major histocompatibility complex class I and II, CD40, CD54, and CD86 expression levels increased to levels observed after LPS stimulation (2). However, at 4 days after infection, the authors found decreased expression of all of the markers examined (2). Here, we found that HCMV infection of monocytes led to a failure of the cells to differentiate into dendritic cells, which was accompanied by a decreased ability to mature further upon LPS stimulation. We also found that the infected cells exhibited severely depressed immunological functions, such as reduced abilities for endocytosis, phagocytosis, migration, and induction of T-cell responses in vitro that was mediated by a soluble factor produced by in the coculture of T cells and HCMV-infected monocytes.
Interestingly, the HCMV-infected cells displayed a novel phenotype; they failed to upregulate CD1a expression upon IL-4 and GM-CSF stimulation. The cells retained the levels of CD14 expression and expressed high levels of the costimulatory molecule CD86, which is normally not present at high concentration on monocytes or on the surface of uninfected immature dendritic cells but is induced on mature dendritic cells. Such expression of costimulatory molecules in the absence of proper antigen presentation through major histocompatibility complex class I molecules could theoretically result in induction of T-cell anergy. We did indeed observe a profound negative effect on T-cell proliferation against cells that had been treated with virus that could not be rescued by OKT3 stimulation. A similar nondeletional suppression of T cells, where the cells did not proliferate after polyclonal restimulation in the absence of virus, has been observed by others in HCMV-infected mature dendritic cells (32).
Since dendritic cells play a crucial role in initiating and maintaining immune responses, our observations suggest an aggressive viral strategy that may blunt early antiviral defense mechanisms. Such a mechanism would indeed prevent dendritic cells from executing their tasks and would most likely contribute to immunosuppression and possibly enable the virus to persist in its host. Most likely, HCMV's ability to inhibit monocytes from differentiating into macrophages as well as dendritic cells may therefore have important clinical implications, particularly in the acute phase of disease in infected patients, since an inability of these cells to develop into functionally active professional antigen-presenting cells would have a profound negative influence on the immune response and would decrease the host's ability to combat other infections.
In summary, we propose that HCMV uses powerful tactics to evade immune recognition, since infection of monocytes appears to allow HCMV to evade the generation of a specific immune response early in the infectious process that may become clinically relevant by causing immunosuppression. An increased understanding of HCMV-induced immunosuppression will be of utmost importance to improve the currently available antiviral therapies by supporting immunological functions in HCMV-infected patients.
Acknowledgments
We thank Erna Möller for helpful discussions.
This work was supported by grants from the Swedish Medical Research Council (K98-06X-12615-01A), the Tobias Foundation (1313/98 and 20/01), the Swedish Children's Cancer Research Foundation (1998/065), the Heart-Lung Foundation (199941305, 200241138, and 20030055), Goljes (no. 520), and the Emil and Wera Cornells Foundation. C.S.-N. is a fellow of the Wenner-Gren Foundation, Sweden.
REFERENCES
- 1.Allavena, P., L. Piemonti, D. Longoni, S. Bernasconi, A. Stoppacciaro, L. Ruco, and A. Mantovani. 1998. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur. J. Immunol. 28:359-369. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, D. M., C. E. Andoniou, F. Granucci, P. Ricciardi-Castagnoli, and M. A. Degli-Esposti. 2001. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat. Immunol. 2:1077-1084. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 4.Bhardwaj, N. 1997. Interactions of viruses with dendritic cells: a double-edged sword. J. Exp. Med. 186:795-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhardwaj, N., A. Bender, N. Gonzalez, L. K. Bui, M. C. Garrett, and R. M. Steinman. 1994. Influenza virus-infected dendritic cells stimulate strong proliferative and cytolytic responses from human CD8+ T cells. J. Clin. Investig. 94:797-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolovan-Fritts, C. A., E. S. Mocarski, and J. A. Wiedeman. 1999. Peripheral blood CD14(+) cells from healthy subjects carry a circular conformation of latent cytomegalovirus genome. Blood 93:394-398. [PubMed] [Google Scholar]
- 7.Britt, W. A. A., C. A. 1996. Cytomegalovirus, p. 2493-2523. In B. N. Fields, D. M. Knipe, P. M. Howley, et al. (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 8.Chomarat, P., J. Banchereau, J. Davoust, and A. K. Palucka. 2000. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat. Immunol. 1:510-514. [DOI] [PubMed] [Google Scholar]
- 9.Compton, T., E. A. Kurt-Jones, K. W. Boehme, J. Belko, E. Latz, D. T. Golenbock, and R. W. Finberg. 2003. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J. Virol. 77:4588-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Docke, W. D., S. Prosch, E. Fietze, V. Kimel, H. Zuckermann, C. Klug, U. Syrbe, D. H. Kruger, R. von Baehr, and H. D. Volk. 1994. Cytomegalovirus reactivation and tumour necrosis factor. Lancet 343:268-269. [DOI] [PubMed] [Google Scholar]
- 11.Einhorn, L., and A. Ost. 1984. Cytomegalovirus infection of human blood cells. J. Infect. Dis. 149:207-214. [DOI] [PubMed] [Google Scholar]
- 12.Engelmayer, J., M. Larsson, M. Subklewe, A. Chahroudi, W. I. Cox, R. M. Steinman, and N. Bhardwaj. 1999. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J. Immunol. 163:6762-6768. [PubMed] [Google Scholar]
- 13.Fish, K. N., A. S. Depto, A. V. Moses, W. Britt, and J. A. Nelson. 1995. Growth kinetics of human cytomegalovirus are altered in monocyte-derived macrophages. J. Virol. 69:3737-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grattan, M. T., C. E. Moreno-Cabral, V. A. Starnes, P. E. Oyer, E. B. Stinson, and N. E. Shumway. 1989. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA 261:3561-3566. [PubMed] [Google Scholar]
- 15.Grosjean, I., C. Caux, C. Bella, I. Berger, F. Wild, J. Banchereau, and D. Kaiserlian. 1997. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J. Exp. Med. 186:801-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grundy, J. E., J. D. Shanley, and P. D. Griffiths. 1987. Is cytomegalovirus interstitial pneumonitis in transplant recipients an immunopathological condition? Lancet ii:996-999. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch, M. S., and D. Felsenstein. 1984. Cytomegalovirus-induced immunosuppression. Ann. N. Y. Acad. Sci. 437:8-15. [DOI] [PubMed] [Google Scholar]
- 18.Ibanez, C. E., R. Schrier, P. Ghazal, C. Wiley, and J. A. Nelson. 1991. Hum. cytomegalovirus productively infects primary differentiated macrophages. J. Virol. 65:6581-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knight, S. C., and S. Patterson. 1997. Bone marrow-derived dendritic cells, infection with human immunodeficiency virus, and immunopathology. Annu. Rev. Immunol. 15:593-615. [DOI] [PubMed] [Google Scholar]
- 20.Kotenko, S. V., S. Saccani, L. S. Izotova, O. V. Mirochnitchenko, and S. Pestka. 2000. Hum. cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10). Proc. Natl. Acad. Sci. USA 97:1695-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kutza, A. S., E. Muhl, H. Hackstein, H. Kirchner, and G. Bein. 1998. High incidence of active cytomegalovirus infection among septic patients. Clin. Infect. Dis. 26:1076-1082. [DOI] [PubMed] [Google Scholar]
- 22.Lin, C. L., R. M. Suri, R. A. Rahdon, J. M. Austyn, and J. A. Roake. 1998. Dendritic cell chemotaxis and transendothelial migration are induced by distinct chemokines and are regulated on maturation. Eur. J. Immunol. 28:4114-4122. [DOI] [PubMed] [Google Scholar]
- 23.Loenen, W. A., C. A. Bruggeman, and E. J. Wiertz. 2001. Immune evasion by human cytomegalovirus: lessons in immunology and cell biology. Semin. Immunol. 13:41-49. [DOI] [PubMed] [Google Scholar]
- 24.Loetscher, P., M. Seitz, M. Baggiolini, and B. Moser. 1996. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J. Exp. Med. 184:569-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lonnqvist, B., O. Ringden, B. Wahren, G. Gahrton, and G. Lundgren. 1984. Cytomegalovirus infection associated with and preceding chronic graft-versus-host disease. Transplantation 38:465-468. [DOI] [PubMed] [Google Scholar]
- 26.Mikloska, Z., L. Bosnjak, and A. L. Cunningham. 2001. Immature monocyte-derived dendritic cells are productively infected with herpes simplex virus type 1. J. Virol. 75:5958-5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moutaftsi, M., A. M. Mehl, L. K. Borysiewicz, and Z. Tabi. 2002. Hum. cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells. Blood 99:2913-2921. [DOI] [PubMed] [Google Scholar]
- 28.Mutimer, D., D. Mirza, J. Shaw, K. O'Donnell, and E. Elias. 1997. Enhanced (cytomegalovirus) viral replication associated with septic bacterial complications in liver transplant recipients. Transplantation 63:1411-1415. [DOI] [PubMed] [Google Scholar]
- 29.Noraz, N., J. L. Lathey, and S. A. Spector. 1997. Human cytomegalovirus-associated immunosuppression is mediated through interferon-alpha. Blood 89:2443-2452. [PubMed] [Google Scholar]
- 30.Paya, C. V., R. H. Wiesner, P. E. Hermans, J. J. Larson-Keller, D. M. Ilstrup, R. A. Krom, S. Rettke, and T. F. Smith. 1993. Risk factors for cytomegalovirus and severe bacterial infections following liver transplantation: a prospective multivariate time-dependent analysis. J. Hepatol. 18:185-195. [DOI] [PubMed] [Google Scholar]
- 31.Prosch, S., K. Staak, J. Stein, C. Liebenthal, T. Stamminger, H. D. Volk, and D. H. Kruger. 1995. Stimulation of the human cytomegalovirus immediate-early enhancer/promoter in HL-60 cells by TNFalpha is mediated via induction of NF-kappaB. Virology 208:197-206. [DOI] [PubMed] [Google Scholar]
- 32.Raftery, M. J., M. Schwab, S. M. Eibert, Y. Samstag, H. Walczak, and G. Schonrich. 2001. Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity 15:997-1009. [DOI] [PubMed] [Google Scholar]
- 33.Ragazzo, J. L., M. E. Ozaki, L. Karlsson, P. A. Peterson, and S. R. Webb. 2001. Costimulation via lymphocyte function-associated antigen 1 in the absence of CD28 ligation promotes anergy of naive CD4+ T cells. Proc. Natl. Acad. Sci. USA 98:241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rice, G. P., R. D. Schrier, and M. B. Oldstone. 1984. Cytomegalovirus infects human lymphocytes and monocytes: virus expression is restricted to immediate-early gene products. Proc. Natl. Acad. Sci. USA 81:6134-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riegler, S., H. Hebart, H. Einsele, P. Brossart, G. Jahn, and C. Sinzger. 2000. Monocyte-derived dendritic cells are permissive to the complete replicative cycle of human cytomegalovirus. J. Gen. Virol. 81:393-399. [DOI] [PubMed] [Google Scholar]
- 36.Rinaldo, C. R., Jr. 1990. Immune suppression by herpesviruses. Annu. Rev. Med. 41:331-338. [DOI] [PubMed] [Google Scholar]
- 37.Rubin, R. H., and N. E. Tolkoff-Rubin. 1988. Opportunistic infections in renal allograft recipients. Transplant. Proc. 20:12-18. [PubMed] [Google Scholar]
- 38.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sallusto, F., C. Nicolo, R. De Maria, S. Corinti, and R. Testi. 1996. Ceramide inhibits antigen uptake and presentation by dendritic cells. J. Exp. Med. 184:2411-2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz, R. H. 1990. A cell culture model for T lymphocyte clonal anergy. Science 248:1349-1356. [DOI] [PubMed] [Google Scholar]
- 41.Serrander, L., M. Fallman, and O. Stendahl. 1996. Activation of phospholipase D is an early event in integrin-mediated signalling leading to phagocytosis in human neutrophils. Inflammation 20:439-450. [DOI] [PubMed] [Google Scholar]
- 42.Sinclair, J. H., J. Baillie, L. A. Bryant, J. A. Taylor-Wiedeman, and J. G. Sissons. 1992. Repression of human cytomegalovirus major immediate early gene expression in a monocytic cell line. J. Gen. Virol. 73:433-435. [DOI] [PubMed] [Google Scholar]
- 43.Smieja, M., J. Gnarpe, E. Lonn, H. Gnarpe, G. Olsson, Q. Yi, V. Dzavik, M. McQueen, and S. Yusuf. 2003. Multiple infections and subsequent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation 107:251-257. [DOI] [PubMed] [Google Scholar]
- 44.Soderberg, C., S. Larsson, S. Bergstedt-Lindqvist, and E. Moller. 1993. Definition of a subset of human peripheral blood mononuclear cells that are permissive to human cytomegalovirus infection. J. Virol. 67:3166-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soderberg-Naucler, C., K. N. Fish, and J. A. Nelson. 1997. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 91:119-126. [DOI] [PubMed] [Google Scholar]
- 46.Soderberg-Naucler, C., D. N. Streblow, K. N. Fish, J. Allan-Yorke, P. P. Smith, and J. A. Nelson. 2001. Reactivation of latent human cytomegalovirus in CD14+ monocytes is differentiation dependent. J. Virol. 75:7543-7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Speir, E., R. Modali, E. S. Huang, M. B. Leon, F. Shawl, T. Finkel, and S. E. Epstein. 1994. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science 265:391-394. [DOI] [PubMed] [Google Scholar]
- 48.Spencer, J. V., K. M. Lockridge, P. A. Barry, G. Lin, M. Tsang, M. E. Penfold, and T. J. Schall. 2002. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J. Virol. 76:1285-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Streblow, D. N., S. L. Orloff, and J. A. Nelson. 2001. Do pathogens accelerate atherosclerosis? J. Nutr. 131:2798S-2804S. [DOI] [PubMed] [Google Scholar]
- 50.Taylor-Wiedeman, J., G. P. Hayhurst, J. G. Sissons, and J. H. Sinclair. 1993. Polymorphonuclear cells are not sites of persistence of human cytomegalovirus in healthy individuals. J. Gen. Virol. 74:265-268. [DOI] [PubMed] [Google Scholar]
- 51.Taylor-Wiedeman, J., J. G. Sissons, L. K. Borysiewicz, and J. H. Sinclair. 1991. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J. Gen. Virol. 72:2059-2064. [DOI] [PubMed] [Google Scholar]
- 52.Taylor-Wiedeman, J., P. Sissons, and J. Sinclair. 1994. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. J. Virol. 68:1597-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van den Berg, A. P., I. J. Klompmaker, E. B. Haagsma, P. M. Peeters, L. Meerman, R. Verwer, T. H. The, and M. J. Slooff. 1996. Evidence for an increased rate of bacterial infections in liver transplant patients with cytomegalovirus infection. Clin. Transplant. 10:224-231. [PubMed] [Google Scholar]
- 54.Wentworth, B. B., and L. French. 1970. Plaque assay of cytomegalovirus strains of human origin. Proc. Soc. Exp. Biol. Med. 135:253-258. [DOI] [PubMed] [Google Scholar]
- 55.Yurochko, A. D., and E. S. Huang. 1999. Human cytomegalovirus binding to human monocytes induces immunoregulatory gene expression. J. Immunol. 162:4806-4816. [PubMed] [Google Scholar]