Abstract
Background
We have recently shown that curcumin (a diferuloylmethane, the yellow pigment in turmeric) enhances apoptosis-inducing potential of TRAIL in prostate cancer PC-3 cells, and sensitizes TRAIL-resistant LNCaP cells in vitro through multiple mechanisms. The objectives of this study were to investigate the molecular mechanisms by which curcumin sensitized TRAIL-resistant LNCaP xenografts in vivo.
Methods
Prostate cancer TRAIL-resistant LNCaP cells were implanted in Balb c nude mice to examine the effects of curcumin and/or TRAIL on tumor growth and genes related to apoptosis, metastasis and angiogenesis.
Results
Curcumin inhibited growth of LNCaP xenografts in nude mice by inducing apoptosis (TUNEL staining) and inhibiting proliferation (PCNA and Ki67 staining), and sensitized these tumors to undergo apoptosis by TRAIL. In xenogrfated tumors, curcumin upregulated the expression of TRAIL-R1/DR4, TRAIL-R2/DR5, Bax, Bak, p21/WAF1, and p27/KIP1, and inhibited the activation of NFκB and its gene products such as cyclin D1, VEGF, uPA, MMP-2, MMP-9, Bcl-2 and Bcl-XL. The regulation of death receptors and members of Bcl-2 family, and inactivation of NFκB may sensitize TRAIL-resistant LNCaP xenografts. Curcumin also inhibited number of blood vessels in tumors, and circulating endothelial growth factor receptor 2-positive endothelial cells in mice.
Conclusion
The ability of curcumin to inhibit tumor growth, metastasis and angiogenesis, and enhance the therapeutic potential of TRAIL suggests that curcumin alone or in combination with TRAIL can be used for prostate cancer prevention and/or therapy.
Introduction
The process of malignant transformation involves the sequential acquisition of a number of genetic and epigenetic alterations as a result of increasing genomic instability caused by defects in checkpoint controls [1,2]. These alterations allow cancer cells to acquire the capabilities to become self-sufficient in mitogenic signals, deregulate the control of cell cycle, escape from apoptosis, and obtain unlimited replication potential [3-5]. Within a growing tumor mass, the genetic changes during tumor progression also enable cancer cells to gain the ability to induce angiogenesis, invade neighboring tissues, and metastasize to distinct organs [6]. The new chemopreventive agents or therapeutic strategies that inhibit angiogenesis, metastasis and invasion can be considered for future clinical development.
Epidemiological data have demonstrated that curcumin is safe, non-toxic, and has long lasting beneficial effects on human health. Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-hepatadiene-3,5-dione; diferulolylmethane], a major constituent of the yellow spice turmeric, is derived from the rhizomes of Curcuma spp. [7]. It possesses antitumor, anti-inflammatory and anti-oxidant properties [7,8]. In addition, curcumin has been shown to inhibit tumor metastasis, invasion and angiogenesis [9-12]. We have recently shown that Bax and Bak genes completely inhibited curcumin-induced apoptosis in Bax-/- and Bax -/- mouse embryonic fibroblasts [13], and curcumin induced apoptosis in prostate cancer cells by inhibiting Akt activity upstream of mitochondria [14]. These data suggest that curcumin regulates multiple signaling pathways and possesses several therapeutic benefits.
Nuclear factor (NFκB) is a dimeric DNA binding protein consisting of members of the NFκB/Rel family [15]. Its expression is ubiquitous in mammalian cells. Normally, NFκB resides in the cytoplasm in an inactive form in association with inhibitory proteins. These inhibitory proteins, which belong to a family of proteins named inhibitor of NFκB [15], prevent NFκB nuclear translocation by masking the NFκB nuclear localization signal and thus, inhibit NFκB DNA binding and transactivational function [15,16]. Various stimuli activate a large number of distinct signaling pathways that eventually result in the phosphorylation of inhibitor of NFκB and its subsequent degradation by the proteasome or its dissociation from NFκB without additional degradation [15-17]. The released NFκB then translocates to the nucleus and binds to κB DNA motifs to initiate gene transcription. The putative target genes of NFκB are involved in immune and inflammatory responses, and in the control of cell proliferation, apoptosis, metastasis and angiogenesis [15,16]. Tumor cells usually express high levels of constitutively active NFκB [16,18]. Furthermore, curcumin inhibited NFκB activity in cancer cells [9,19] and sensitized cancer cells to chemotherapy and radiotherapy [20-25].
TNF-related apoptosis-inducing ligand (TRAIL) binds to TRAIL-R1/DR4 and TRAIL-R2/DR5. TRAIL induces apoptosis in cancer cells of various origins [26-30]. Data on experimental animals and primates led us to believe that TRAIL has great promise as a selective anticancer agent [27,28,31]. We have recently demonstrated that TRAIL induces apoptosis in several prostate cancer cells lines, but it was ineffective in inducing apoptosis in LNCaP cells [27,28,32]. Furthermore, curcumin sensitizes TRAIL-resistant prostate cancer cells to growth inhibition by TRAIL in vitro [33-35]. However, the ability of curcumin to sensitize TRAIL-resistant prostate cancer cells in vivo has not yet been demonstrated.
The purpose of our studies was to investigate the molecular mechanisms by which curcumin sensitized TRAIL-resistant prostate cancer cells in vivo. Our results indicated that curcumin inhibited growth, metastasis, and angiogenesis of TRAIL-resistant LNCaP xenografts in nude mice through regulation of NFκB and its gene products, and sensitized these xenografts to TRAIL treatment. Thus, curcumin can be used alone or combined with TRAIL for prostate cancer prevention and/or therapy.
Results
Curcumin sensitizes TRAIL-resistant tumor cells in vivo
We have recently shown that curcumin sensitizes TRAIL-resistant prostate cancer LNCaP cells in vitro [35]. Therefore in the present study, we examined the ability of curcumin to sensitize TRAIL-resistant LNCaP cells in vivo. LNCaP cells were xenografted in Balb c nude mice. After tumor formation, these mice were treated with curcumin, TRAIL, or curcumin plus TRAIL for 6 weeks to examine their effects on tumor growth and markers of proliferation, apoptosis, metastasis, invasion and angiogenesis. While TRAIL was ineffective in inhibiting the tumor growth, curcumin inhibited the growth of LNCaP xenografts in nude mice (Fig. 1A). Interestingly, curcumin sensitized TRAIL-resistant xenografts in nude mice, which was evident in reduction in tumor volume starting from week 2.
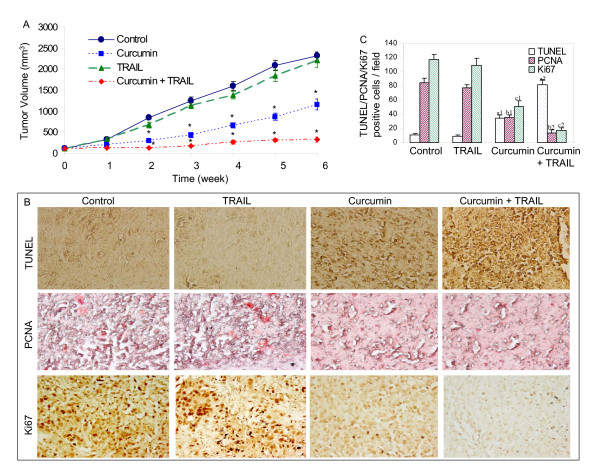
Figure 1.
Curcumin sensitizes TRAIL-resistant LNCaP xenografts. (A), LNCaP cells were injected into the right flanks of Balb c nude mice. After tumor formation (about 100 mm3), mice were treated with saline, curcumin (30 mg/kg, three days per week), TRAIL (15 mg/kg, four times during first three weeks) or curcumin and TRAIL. Tumor volume was calculated weekly. Data represent mean ± SE. (B), Effects of curcumin and/TRAIL on apoptosis (TUNEL staining) and cell proliferation (PCNA and Ki67 staining). Immunohistochemistry was performed to measure apoptosis (TUNEL assay), and expression of PCNA and Ki67 in tumor tissues derived from control and treated mice on week 6. (C), Quantification of TUNEL, PCNA and Ki67 positive tumor cells. Tumor slides of different treatment groups were visualized under microscope, and TUNEL, PCNA and Ki67 positive cells were quantified. Data represent mean ± SE. a1, a2; b1, b2 and c1, c2 are significantly different from their respective controls or TRAIL treated group (P < 0.05).
Since tumor growth is determined by the balance of cell proliferation and apoptosis, mechanisms that promote cell survival or prevent apoptosis of cancer cells would favor the establishment of tumor colonies. To test this, we examined the extent of cellular apoptosis in those tumors. Examination of tumor tissues by TUNEL assay revealed that curcumin alone induced apoptosis (Fig. 1B and 1C). A very few apoptotic cells were found in the highly vascularized tumors derived from control mice. TRAIL treatment of mice had no significant effect on apoptosis compared to control mice. On the other hand, treatment of mice with a combination of curcumin and TRAIL significantly showed more apoptosis than that of mice treated with curcumin alone. Immunohistochemical studies also demonstrated that curcumin inhibited cell proliferation (PCNA and Ki67 staining) in xenogrfated tumors (Fig. 1B and 1C). TRAIL had no effect on tumor cell proliferation because the staining of PCNA and Ki67 was not affected. The combination of curcumin plus TRAIL demonstrated slightly less tumor cell proliferation than curcumin alone. These data are in agreement with our recently published in vitro data where curcumin sensitized TRAIL-resistant LNCaP cells [35].
In vivo regulation of Bcl-2 family members and death receptors by curcumin and/or TRAIL
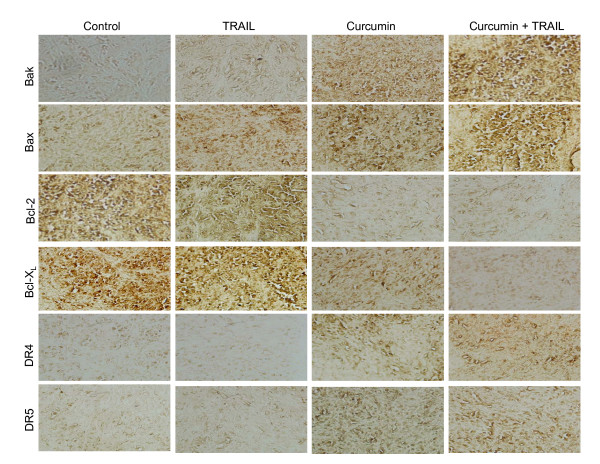
Since curcumin sensitized TRAIL-resistant cells in vivo, we sought to examine the molecular mechanisms by which curcumin sensitized TRAIL-resistant LNCaP cells. We next examined the effects of curcumin and/or TRAIL on the expression of Bcl-2 family members (Bax, Bak, Bcl-2, and Bcl-XL) and death receptors (TRAIL-R1/DR4 and TRAIL-R2/DR5) by immunohistochemistry in tumor tissues derived from in vivo experiment (Fig. 2). Treatment of mice with curcumin enhanced the expression of Bax, Bak, DR4 and DR5, and inhibited the expression of antiapoptotic Bcl-2 and Bcl-XL proteins. TRAIL has no significant effect on the expression of Bak, Bax, Bcl-2, Bcl-XL, DR4 and DR5. On the other hand, treatment of mice with a combination of curcumin and TRAIL significantly showed more expression of Bax, Bak, DR4 and DR5, and less expression of Bcl-2 and Bcl-XL proteins than that of mice treated with curcumin alone or TRAIL alone.
Figure 2.
Immunohistochemical examination of Bcl-2 family members and death receptors. Immunohistochemistry was performed to measure the expression of Bak, Bax, Bcl-2, Bcl-XL, TRAIL-R1/DR4 and TRAIL-R2/DR5 in tumor tissues derived from control and/or treated mice on week 6.
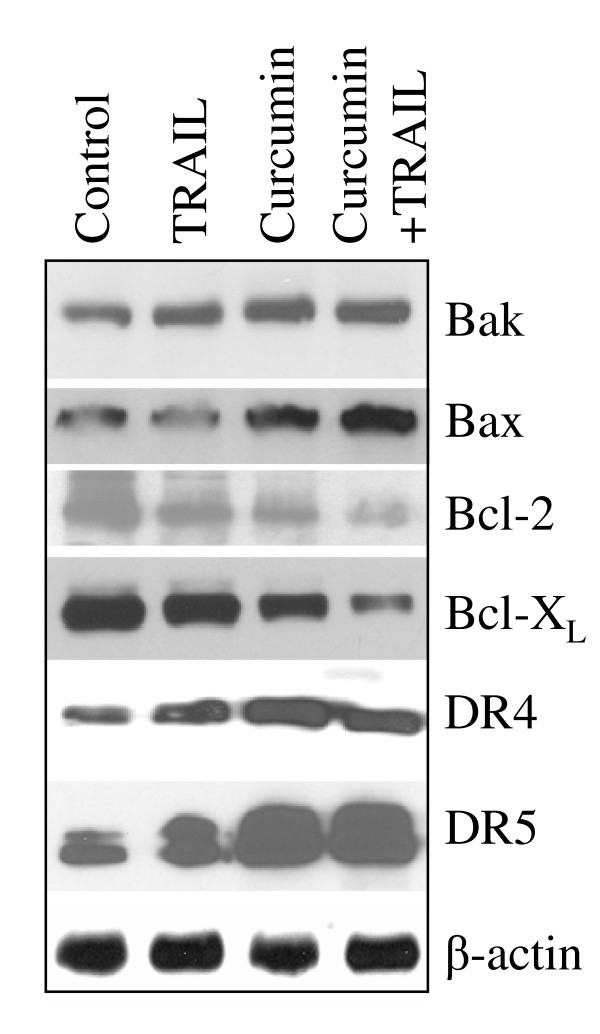
We confirmed the immunohistochemistry data by examining the expression of these proteins by the Western blot analysis (Fig. 3). TRAIL has no effect on the expression of Bcl-2 family members (Bax, Bak, Bcl-2, and Bcl-XL) and death receptors (DR4 and DR5). By comparison, curcumin induced the expression of Bak, Bax, DR4 and DR5, and inhibited the expression of Bcl-2 and Bcl-XL. These data are in agreement with immunohistochemistry data where the expression of proapoptotic Bak, Bax, DR4 and DR5 proteins were induced and the expression of antiapoptotic Bcl-2 and Bcl-XL proteins were inhibited.
Figure 3.
Western blot analysis of Bcl-2 family members and death receptors. Protein expression of Bak, Bax, Bcl-2, Bcl-XL, TRAIL-R1/DR4, TRAIL-R2/DR5 and β-actin in tumor tissues derived from control and/or treated on week 6 was measured by the Western blot analysis.
In vivo regulation of cell cycle inhibitors (p21/WAF1/CIP1 and p27/KIP1) and cyclin D1 by curcumin and/or TRAIL
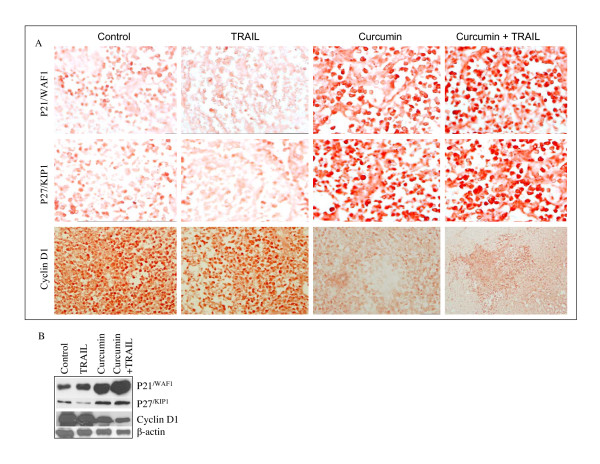
Since curcumin caused growth arrest by inducing expression of cell cycle inhibitors p21/WAF1/CIP1 and p27/KIP1 in vitro, we sought to validate this phenomenon in vivo in xenografted tumors. Treatment of mice with curcumin resulted in the induction of cell cycle inhibitors p21/WAF1/CIP1 and p27/KIP1 and inhibition of cyclin D1 (Fig. 4A). By comparison, TRAIL had no effect on the expression of p21, p27 and cyclin D1 compared to control group. However, the combination of curcumin and TRAIL showed upregulation of p21 and p27, and inhibition of cyclin D1 compared to that of curcumin or TRAIL treated group. We next confirmed the immunohistochemistry data by examining the expression of these proteins by the Western blot analysis (Fig. 4B). TRAIL has no significant effect on the expression of p21, p27 and cyclin D1. By comparison, curcumin induced the expression of p21 and p27, and inhibited the expression of cyclin D1. These mice data are in agreement with in vitro data where curcumin upregulated the expression of p21 and p27, and inhibited the expression of cyclin D1 [35].
Figure 4.
Effects of curcumin and/TRAIL on cell cycle inhibitors and cyclin D1. (A), Immunohistochemistry was performed to measure the expression of cell cycle inhibitors (p21/WAF1 and p27/KIP1) and cyclin D1 in tumor tissues derived from control and/or treated mice on week 6. (B), Expression of p21/WAF1, p27/KIP1, cyclin D1 and β-actin in tumor tissues derived on week 6 were measured by the Western blot analysis.
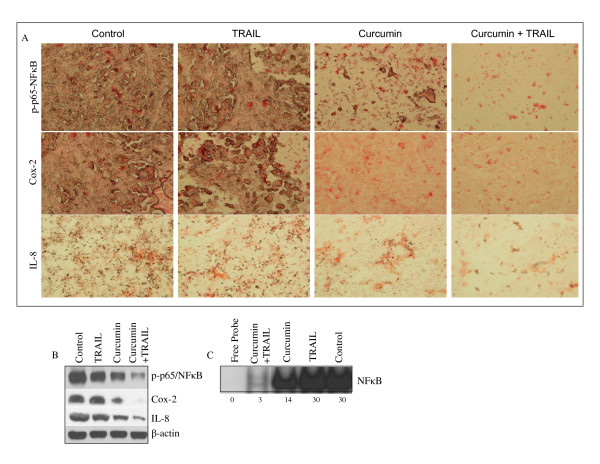
In vivo regulation of NFκB, Cox-2 and IL-8 by curcumin and/or TRAIL
The NFκB family of transcription factors has been shown to be constitutively activated in cancer cells and thus regulates genes involved in cell proliferation, apoptosis, metastasis and angiogenesis [36]. Since NFκB, Cox-2 and IL-8 play major role in cancer cell proliferation, and immune response, we sought to examine the effects of curcumin and/or TRAIL on the expression of these proteins by immunohistochemistry in tumor tissues derived from xenograft experiment (Fig. 5A). While TRAIL was ineffective, treatment of xenogrfated mice with curcumin resulted in inhibition of NFκB activation (as measured by phospho-p65 antibody), and cox-2 and IL-8 expression than control group. Furthermore, the combination of curcumin and TRAIL was more effective than curcumin alone in inhibiting NFκB activation and expression of cox-2 and IL-8.
Figure 5.
Effects of curcumin and/TRAIL on markers of phospho-p65NFκB, Cox-2, and IL-8. (A), Immunohistochemistry was performed to measure the expression of phospho-p65NFκB, Cox-2 and IL-8 in tumor tissues derived from control and/or treated mice on week 6. (B), Expression of phospho-p65NFκB, Cox-2, IL-8 and β-actin in tumor tissues derived on week 6 were measured by the Western blot analysis. (C), NFκB-DNA binding activity. Nuclear extracts were prepared from tumor tissues derived from different treatment groups on week 6. NFκB-DNA binding activity was measured by Gelshift assay as described in Materials and Methods. The relative nuclear NFκB-DNA binding activities were quantified by scanning densitometry.
We next confirmed the immunohistochemistry data by examining the expression of these proteins by the Western blot analysis (Fig. 5B). TRAIL has no significant effect on the phosphorylation of p-p65, and expression of cox-2 and IL-8. By comparison, curcumin inhibited the phosphorylation of p-p65, and expression of Cox-2 and IL-8. These data suggest that curcumin can inhibit NFκB activation and its gene products such as Cox-2 and IL-8.
Since curcumin inhibited the phosphorylation of p65 subunit of NFκB, we next measured the NFκB-DNA binding activity in tumor tissues derived from control, TRAIL, curcumin, and curcumin plus TRAIL treated groups. Nuclear extracts were prepared from tumor tissues derived from different treatment groups, and NFκB-DNA binding activity was measured by gelshift assay (Fig. 5C). TRAIL had no effect on NFκB-DNA binding activity compared to that of untreated mice. By comparison, curcumin inhibited NFκB-DNA binding activity by 53%. Interestingly, the combination of curcumin and TRAIL had more inhibitory effects on NFκB-DNA binding activity than curcumin alone. These data suggest that the inhibition of NFκB transcription factor by curcumin may play a major role in inducing sensitivity of TRAIL-resistant cells.
In vivo regulation of angiogenesis by curcumin and/or TRAIL
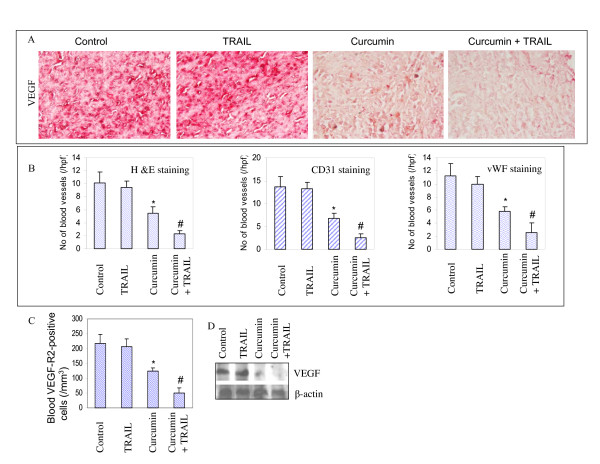
Whether regression in tumor growth by curcumin was due to inhibition of angiogenesis, we analyzed the markers of angiogenesis by immunohistochemistry in tumor samples. Examination of tumor tissues by immunohistochemistry showed that control mice had increased VEGF-positive endothelial cells compared to curcumin treated mice (Fig. 6A). TRAIL had no effect on VEGF staining. The combination of curcumin and TRAIL showed significantly less VEGF staining than that noted in tumors from mice treated with either agent alone.
Figure 6.
Effects of curcumin and/TRAIL on markers of angiogenesis. (A), Immunohistochemistry was performed to measure the expression of VEGF in tumor tissues derived from control and treated mice on week 6. (B), Left panel, tumor tissue sections derived from control and treated mice on week 6 were stained with H & E and the number of blood vessels in ten field at 400 × magnification were counted. Each column represents the mean ± SD. * or # = significantly different from control, P < 0.05. Middle panel, blood vessel quantification in tumors derived on week 6. Tumor sections from control and treated mice were stained with anti-CD31 antibody, and the number of CD31-positive blood vessels was counted. The results are shown as the mean ± SD. * or # = significantly different from control, P < 0.05. Right panel, tumor sections from control and treated mice obtained on week 6 were stained with anti-von Willebrand Factor (vWF) antibody to evaluate blood vessels. The results are shown as the mean ± SD. (C), VEGF receptor 2 (VEGF-R2)-positive circulating endothelial cells in mice on week 6. The blood cells from peripheral blood attached to the slide were stained with anti-VEGF-R2 antibody, and the number of positive cells was counted under a microscope. The results are shown as the mean ± SD. * or # = significantly different from control, P < 0.05. (D), Expression of VEGF and β-actin in tumor tissues derived on week 6 were measured by the Western blot analysis.
We next examined the effects of curcumin and/or TRAIL treatment on number of blood vessels in tumor tissues by utilizing three different approaches (Fig. 6B). Blood vessels were examined by staining the tumor tissues by H&E, anti-CD31 antibody, and anti-vWF antibody. TRAIL treatment had no effect on number of blood vessels. By comparison, treatment of mice with curcumin caused an inhibition in number of blood vessels. The combination of curcumin with TRAIL further inhibited the number of blood vessels.
Several laboratories, including ours, have demonstrated that numbers of circulating vascular endothelial growth factor receptor 2 (VEGF-R2)-positive endothelial cells correlate directly with increase in tumor angiogenesis and can serve as in vivo indicators of tumor angiogenesis [27,37,38]. As expected, control mice had increased circulating VEGF-R2-positive endothelial cells compared to curcumin treated mice (Fig. 6C). Curcumin plus TRAIL-treated group had more inhibitory effects on VEGFR2-positive cells than that of curcumin or TRAIL treated group. Thus, these data strongly demonstrate that curcumin can inhibit tumor growth by inhibiting angiogenesis, and may also sensitize TRAIL-resistant tumor cells in vivo.
We next confirmed the immunohistochemistry data of VEGF expression by examining the protein levels by the Western blot analysis (Fig. 6D). TRAIL has no significant effect on VEGF expression. By comparison, curcumin or curcumin plus TRAIL inhibited the expression of VEGF.
In vivo regulation of metastasis by curcumin and/or TRAIL
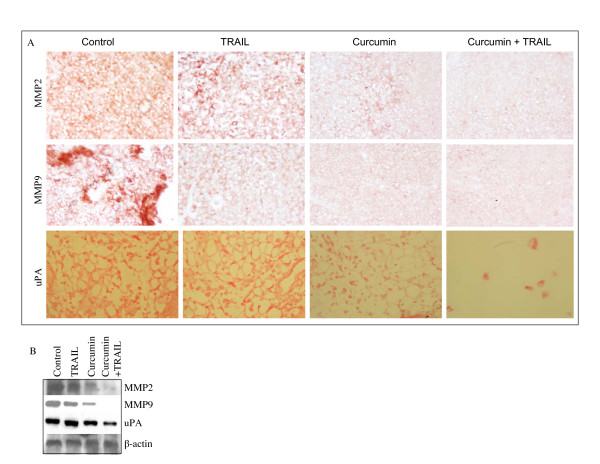
Elevated expression of matrix metalloproteinases (MMPs) and uPA are associated with increased metastatic potential in many tumor cells [39-42]. We therefore sought to examine the effects of curcumin on MMP-2, MMP-9, and uPA on tumor tissues derived from xenografted nude mice. Treatment of xenogrfated mice with curcumin resulted in inhibition of MMP-2, MMP-7, and uPA expression than those of control or TRAIL group (Fig. 7A). The combination of curcumin and TRAIL was more effective in inhibiting MMP-2, MMP-7, and uPA expression than single agent alone. We next confirmed the immunohistochemistry data by examining the expression of these proteins by the Western blot analysis (Fig. 7B). TRAIL has no significant effect on the expression of MMP-2, MMP-9 and uPA. By comparison, curcumin or curcumin plus TRAIL inhibited the expression of MMP-2, MMP-9 or uPA. These data suggest that curcumin can inhibit prostate cancer progression by inhibiting metastasis.
Figure 7.
Effects of curcumin and/TRAIL on markers of metastasis. (A), Immunohistochemistry was performed to measure the expression of MMPs (MMP-2 and MMP-9) and uPA in tumor tissues derived from control and treated mice on week 6. (B), Expression of MMP-2, MMP-9, uPA and β-actin in tumor tissues derived on week 6 were measured by the Western blot analysis.
Discussion
We have recently shown that curcumin induces apoptosis in TRAIL-sensitive PC-3 cells, and sensitizes TRAIL-resistant LNCaP cells in vitro through activation of multiple signaling pathways [35]. Curcumin-induced apoptosis engages mitochondria, which was evident by drop in mitochondrial membrane potential and activation of caspase-3 and caspase-9 in both prostate cancer PC-3 and LNCaP cells [35]. Curcumin induced expression of proapoptotic proteins (Bax, Bak, PUMA, Noxa and Bim), death receptors (TRAIL-R1/DR4 and TRAIL-R2/DR5), and inhibited expression of antiapoptotic proteins (Bcl-2 and Bcl-XL) and IAPs (XIAP and survivin) [35]. Since these proteins regulate cell-intrinsic and/or cell-extrinsic pathways of apoptosis, and they may be responsible for sensitization of TRAIL-resistant LNCaP cells. In the present study, we have demonstrated that curcumin inhibited the growth of LNCaP xenografts, metastasis and angiogenesis. Although the TRAIL was ineffective alone, the combination of curcumin and TRAIL had greater effect on tumor growth inhibition, metastasis and angiogenesis than curcumin.
In vitro curcumin downregulated the expression of Bcl-2, and Bcl-XL and upregulated the expression of p53, Bax, Bak, PUMA, Noxa, and Bim at mRNA and protein levels in prostate cancer cells [14]. We have also demonstrated that curcumin upregulated the expression, phosphorylation, and acetylation of p53 in androgen-dependent LNCaP cells [14]. The ability of curcumin to regulate gene transcription was also evident as it caused acetylation of histone H3 and H4 in LNCaP cells [14]. Furthermore, treatment of LNCaP cells with curcumin resulted in translocation of Bax and p53 to mitochondria, production of reactive oxygen species, drop in mitochondrial membrane potential, release of mitochondrial proteins (cytochrome c, Smac/DIABLO and Omi/HtrA2), and activation of caspase-3 leading to apoptosis [14]. Furthermore, deletion of Bax and Bak genes completely inhibited curcumin-induced cytochrome c and Smac/DIABLO release in mouse embryonic fibroblasts [13]. In the present study, tumor tissues derived from curcumin treated mice showed that curcumin inhibited the exprerssion of Bcl-2 and Bcl-XL, and induced the expression of Bax and Bak. The combinatioin of curcumin and TRAIL was more effective in regulating Bcl-2 family members than single agent alone. Our in vitro and in vivo studies demonstrate that curcumin can engage cell-intrinsic pathway of apoptosis by regulating the expression of Bcl-2 family of proteins.
We and others have recently shown that curcumin caused a growth arrest at G1/S stage in several cancers including prostate [43-45]. The G1/S phase arrest by curcumin was associated with the induction of p21/WAF1, p27/KIP1, and p16, and inhibition of cyclin D1, cyclin E, Cdk4 and cdk 6 [45]. The ability of curcumin to induce cdk inhibitors p21/CIP1 and p27/KIP1 and inhibit cyclin D1 expression was also confirmed in our xenograft experiment. In a recent study, we have demonstrated that inhibition of p21/CIP1 inhibited curcumin-induced cell cycle arrest and apoptosis [46]. We and others have also demonstrated that curcumin induces the degradation of cyclin E expression through ubiquitin-dependent pathway in several cancer cell lines [44,45]. Interestingly, deregulated expression of cyclin E correlated with chromosome instability [47], malignant trasformation [48], tumor progression [49], and patient survival [50]. Overall, our data suggest that curcumin induces growth arrest at G1/S stage of cell cycle.
Entry of malignant cells into the vasculature (i.e. intravasation) requires proteolytic remodeling of the extracellular matrix so that tumor cells may pass through the local stroma and penetrate the vessel wall. The circulatory system then provides a means of transporting tumor cells to distant sites where they extravasate and establish metastatic lesions. Matrix metalloproteinase (MMP) is up-regulated in many tumor types and has been implicated in tumor progression and metastasis. MMP is critical for pericellular degradation of the extracellular matrix, thereby promoting tumor cell invasion and dissemination. To grow efficiently in vivo, tumor cells induce angiogenesis in both primary solid tumors and metastatic foci. Our results showed that curcumin significantly inhibited the growth of TRAIL-resistant LNCaP xenografts and sensitized these xenografts to undergo apoptosis by TRAIL. Tumor tissues derived from curcumin treated mice showed that curcumin inhibibited proliferation (PCNA and Ki67 staining), induced apoptosis (TUNEL staining), metastasis (uPA, MMP-2 and MMP-9 staining), and angiogenesis (CD31 and VEGF staining). Curcumin also inhibited VEGFR2-positive circulating endothelial cells. Treatment of LNCaP xenografted mice with TRAIL alone had no effect on tumor growth, apoptosis, metastasis and angiogenesis. Our recent in vitro studies demonstrated that curcumin inhibits capillary tube formation and endothelial cell migration, and the inhibitory effects of curcumin were enhanced in the presence of ERK MAP kinase inhibitor [35]. These data suggest that curcumin can inhibit tumor growth by inhibiting apoptosis, metastasis and angiogenesis.
TRAIL induces apoptosis in cancer cells which express TRAIL-R1/DR4 and TRAIL-R2/DR5. We have shown that the upregulation of death receptors by chemotherapeutic drugs, irradiation and chemopreventive agents enhance or sensitize cancer cells to TRAIL treatment [28,30,35,51-58]. Specifically, TRAIL-resistant LNCaP cells can be sensitized by chemotherapeutic drugs and irradiation in vitro and in vivo through upregulation of death receptors DR4 and/or DR5 [27,28]. Similarly, our in vitro study has demonstrated the upregulation of DR4 and DR5 in PC-3 and LNCaP cells by curcumin [35]. Interestigly, curcumin sensitized TRAIL-resistant LNCaP xenografts by inhibiting tumor cell proliferation and inducing apoptosis which were correlated with induction of death receptors DR4 and DR5. Death receptor (DR4 and/or DR5) regulation has been shown to be under the control of transcription factor NFκB, SP1 and p53 [59-64]. Inducible silencing of KILLER/DR5 in vivo promoted bioluminescent colon tumor xenograft growth and confers resistance to chemotherapeutic agent 5-fluorouracil [65]. These finding suggest that upregulation of death receptors DR4 and DR5 by curcumin may be one of the mechanisms by which curcumin enhances the therapeutic potnetial of TRAIL.
The NFκB family of transcription factors has been shown to be constitutively activated in various human malignancies, including a number of solid tumors, leukemias, and lymphomas [66]. NFκB is shown to contribute to development and/or progression of malignancy by regulating the expression of genes involved in cell growth, differentiation, apoptosis, angiogenesis and metastasis [66]. Prostate cancer cells have been reported to have constitutive NFκB activity due to increased activity of the IκB kinase complex [67]. Furthermore, an inverse correlation between androgen receptor (AR) status and NFκB activity was observed in prostate cancer cell lines [68]. In prostate cancer cells, NFκB may promote cell growth and proliferation by regulating expression of genes such as c-myc, cyclin D1, and IL-6 [66,69], and inhibit apoptosis through activation of expression of anti-apoptotic genes, such as Bcl-2 and Bcl-XL. NFκB-mediated expression of genes, involved in angiogenesis (IL-8, VEGF), invasion and metastasis (MMP-9, uPA, uPA receptor), may further contribute to the progression of prostate cancer. Constitutive NFκB activity has also been demonstrated in primary prostate cancer tissue samples and suggested to have prognostic importance for a subset of primary tumors. In the present study, curcumin inhibited the activation of NFκB and its gene products such as VEGF, Bcl-2, Bcl-XL, uPA, cyclin D1, MMP-2, MMP-9, COX-2 and IL-8 in LNCaP xenografted tumors. These findings suggest that NFκB may play a role in human prostate cancer development, and/or progression, and curcumin can inhibit these processes through regulation of NFκB-regulated gene products.
Conclusion
Our in vivo experiments have demonstrated that curcumin sensitizes TRAIL-resistant LNCaP cells through multiple mechanisms. It induces death receptors, upregulates proapoptotic members of Bcl-2 family (Bax and Bak), inhibits antiapoptotic Bcl-2 proteins (Bcl-2 and Bcl-XL) and markers of cell proliferation (PCNA and Ki67), and induces expression of cell cycle inhibitors p21/CIP1, and p27/KIP1. Furthermore, curcumin can also inhibit the activation of NFκB and its gene products (e.g. VEGF, Bcl-2, Bcl-XL, uPA, cyclin D1, MMP-2, MMP-9, COX-2 and IL-8) in vitro [35], and in vivo (current study), which play significant roles in invasion, metastasis and angiogenesis. All these events will significantly contribute to the antiproliferative and antitumor activities of curcumin. Clinical trials on curcumin have demonstrated that (1) curcuma extract can be administered safely to patients at doses of up to 2.2 g daily, equivalent to 180 mg of curcumin; and (2) curcumin has low oral bioavailability in humans and may undergo intestinal metabolism [70]. Our studies posses strong clinical potential because curcumin either alone or in combination with TRAIL can be used to prevent and/or treat prostate cancer.
Methods
Reagents
Antibodies against CD31, VEGF, VEGFR2, Bcl-2, Bcl-XL, Bax, Bak, TRAIL-R1/DR4, TRAIL-R2/DR5 and β-actin were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Antibodies against p21, p27, phospho-p65-NFκB, Cox-2, IL-8, cyclin D1, uPA, MMP-2, and MMP-9 were purchased from Cell Signaling Technology, Inc. (Danvers, MA). Enhanced chemiluminescence (ECL) Western blot detection reagents were from Amersham Life Sciences Inc. (Arlington Heights, IL). Terminal Deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL) assay kit was purchased from EMD Biosciences/Calbiochem (San Diego, CA). TRAIL was purified as described elsewhere [71]. Curcumin was purchased from LKT Laboratories, Inc. (St. Paul, MN).
Western blot analysis
Western blot analysis was performed as we described earlier [13]. Protein bands were visualized on X-ray film using an enhanced chemiluminescence system.
Xenograft assays in nude mice
Athymic nude mice (Balb c nu/nu, 4–6 weeks old) were purchased from the National Cancer Institute (Frederick, MD). LNCaP cells (2 × 106cells as a 50% suspension in matrigel, Becton Dickinson, Bedford, MA) in a final volume of 0.1 ml were injected subcutaneously at right flank of Balb c nude mice. When the average tumor volume reached about 100 mm3, mice were randomized into four groups of 10 mice/group, and the following treatment protocol was implemented: Group 1, vehicle control (0.1 ml normal saline containing 0.5 % DMSO) administered by oral injection, three times/week (Monday, Wednesday and Friday) beginning the day of tumor cell implantation through out the duration of experiment; Group 2, TRAIL (15 mg/kg) administered i.v. on day 1, 7, 14, and 21; Group 3, curcumin (30 mg/kg, in 0.1 ml normal saline containing 0.5 % DMSO) administered by oral injection, three times/week (Monday, Wednesday and Friday) beginning the day of tumor cell implantation through out the duration of experiment; Group 4, curcumin and TRAIL, curcumin administered through oral injection, and TRAIL administered i.v. Mice were housed under pathogen-free conditions and maintained on a 12 h light/12 h dark cycle, with food and water supplied ad libitum. Tumor volume was calculated using the equation: (volume = length × width × depth × 0.5236 mm3). The in vivo experiment was performed under IACUC's approved protocol.
Immunohistochemistry
Immunohistochemistry was performed as described earlier (28, 29). In brief, tumor tissues were collected on week 6, excised and fixed with 10% formalin, embedded in paraffin and sectioned. Tissue sections were stained with primary antibodies against Bax, Bak, Bcl-2, Bcl-XL, DR4, DR5, Ki-67, PCNA, p21/WAF1/CIP1, p27/Kip1, IL-8, Cox-2, phospho-p65-NFkB, CD31, VEGF, VEGFR2, MMP-2, MMP-9 and uPA or TUNEL reaction mixture. For immunohistochemistry, sections were fixed in cold 100% acetone for 3 min, air-dried, and incubated with various primary antibodies at room temperature for 4 h. Subsequently, slides were washed three times in PBS and incubated with secondary antibody at room temperature for 1 h. Finally, alkaline phosphatase or hydrogen peroxide polymer-AEC chromagen substrate kits were used as per manufacturer' instructions (Lab Vision Corporation). After washing with PBS, Vectashield (Vector Laboratories) mounting medium was applied and sections were coverslipped and imaged.
Electrophoretic mobility shift assay
EMSA was performed as we described elsewhere [59].
Statistical analysis
The mean and SD were calculated for each experimental group. Differences between groups were analyzed by one or two way ANOVA. The non-parametric Mann-Whitney U test was performed to assess the difference of tumor volume between control and treatment group. To assess the difference between two groups under multiple conditions, one-way ANOWA followed by Bonferoni's multiple comparison tests were performed using PRISM statistical analysis software (GrafPad Software, Inc., San Diego, CA). Significant differences among groups were calculated at P < 0.05.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
SS, SG, and QC have performed the experiments and drafted the manuscript. RS has directed the project and edited the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
The project was funded by the Department of Defense US Army and the National Institutes of Health. We thank all the lab members for critically reading the manuscript.
Contributor Information
Sharmila Shankar, Email: sharmila.shankar@uthct.edu.
Suthakar Ganapathy, Email: suthakar.ganapathy@uthct.edu.
Qinghe Chen, Email: Qinghe.chen@uthct.edu.
Rakesh K Srivastava, Email: rakesh.srivastava@uthct.edu.
References
- Hahn WC, Weinberg RA. Modelling the molecular circuitry of cancer. Nat Rev Cancer. 2002;2:331–341. doi: 10.1038/nrc795. [DOI] [PubMed] [Google Scholar]
- Nowak MA, Komarova NL, Sengupta A, Jallepalli PV, Shih IeM, Vogelstein B, Lengauer C. The role of chromosomal instability in tumor initiation. Proc Natl Acad Sci USA. 2002;99:16226–16231. doi: 10.1073/pnas.202617399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artandi SE, DePinho RA. Mice without telomerase: what can they teach us about human cancer? Nat Med. 2000;6:852–855. doi: 10.1038/78595. [DOI] [PubMed] [Google Scholar]
- Blasco MA. Telomerase beyond telomeres. Nat Rev Cancer. 2002;2:627–633. doi: 10.1038/nrc862. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med. 2003;3:643–651. doi: 10.2174/1566524033479465. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- Plummer SM, Hill KA, Festing MF, Steward WP, Gescher AJ, Sharma RA. Clinical development of leukocyte cyclooxygenase 2 activity as a systemic biomarker for cancer chemopreventive agents. Cancer Epidemiol Biomarkers Prev. 2001;10:1295–1299. [PubMed] [Google Scholar]
- Singh S, Khar A. Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med Chem. 2006;6:259–270. doi: 10.2174/187152006776930918. [DOI] [PubMed] [Google Scholar]
- Bae MK, Kim SH, Jeong JW, Lee YM, Kim HS, Kim SR, Yun I, Bae SK, Kim KW. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol Rep. 2006;15:1557–1562. [PubMed] [Google Scholar]
- Yoysungnoen P, Wirachwong P, Bhattarakosol P, Niimi H, Patumraj S. Effects of curcumin on tumor angiogenesis and biomarkers, COX-2 and VEGF, in hepatocellular carcinoma cell-implanted nude mice. Clin Hemorheol Microcirc. 2006;34:109–115. [PubMed] [Google Scholar]
- Lin YG, Kunnumakkara AB, Nair A, Merritt WM, Han LY, Armaiz-Pena GN, Kamat AA, Spannuth WA, Gershenson DM, Lutgendorf SK, et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res. 2007;13:3423–3430. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- Shankar S, Srivastava RK. Bax and Bak genes are essential for maximum apoptotic response by curcumin, a polyphenolic compound and cancer chemopreventive agent derived from turmeric, Curcuma longa. Carcinogenesis. 2007;28:1277–1286. doi: 10.1093/carcin/bgm024. [DOI] [PubMed] [Google Scholar]
- Shankar S, Srivastava RK. Involvement of Bcl-2 family members, phosphatidylinositol 3'-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int J Oncol. 2007;30:905–918. [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/S0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Lin A, Karin M. NF-kappaB in cancer: a marked target. Semin Cancer Biol. 2003;13:107–114. doi: 10.1016/S1044-579X(02)00128-1. [DOI] [PubMed] [Google Scholar]
- Divya CS, Pillai MR. Antitumor action of curcumin in human papillomavirus associated cells involves downregulation of viral oncogenes, prevention of NFkB and AP-1 translocation, and modulation of apoptosis. Mol Carcinog. 2006;45:320–332. doi: 10.1002/mc.20170. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- Kamat AM, Sethi G, Aggarwal BB. Curcumin potentiates the apoptotic effects of chemotherapeutic agents and cytokines through down-regulation of nuclear factor-kappaB and nuclear factor-kappaB-regulated gene products in IFN-alpha-sensitive and IFN-alpha-resistant human bladder cancer cells. Mol Cancer Ther. 2007;6:1022–1030. doi: 10.1158/1535-7163.MCT-06-0545. [DOI] [PubMed] [Google Scholar]
- Chirnomas D, Taniguchi T, de la Vega M, Vaidya AP, Vasserman M, Hartman AR, Kennedy R, Foster R, Mahoney J, Seiden MV, D'Andrea AD. Chemosensitization to cisplatin by inhibitors of the Fanconi anemia/BRCA pathway. Mol Cancer Ther. 2006;5:952–961. doi: 10.1158/1535-7163.MCT-05-0493. [DOI] [PubMed] [Google Scholar]
- HemaIswarya S, Doble M. Potential synergism of natural products in the treatment of cancer. Phytother Res. 2006;20:239–249. doi: 10.1002/ptr.1841. [DOI] [PubMed] [Google Scholar]
- Du B, Jiang L, Xia Q, Zhong L. Synergistic inhibitory effects of curcumin and 5-fluorouracil on the growth of the human colon cancer cell line HT-29. Chemotherapy. 2006;52:23–28. doi: 10.1159/000090238. [DOI] [PubMed] [Google Scholar]
- Wahl H, Tan L, Griffith K, Choi M, Liu JR. Curcumin enhances Apo2L/TRAIL-induced apoptosis in chemoresistant ovarian cancer cells. Gynecol Oncol. 2007;105:104–112. doi: 10.1016/j.ygyno.2006.10.050. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–260. doi: 10.1016/S0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- Shankar S, Chen X, Srivastava RK. Effects of sequential treatments with chemotherapeutic drugs followed by TRAIL on prostate cancer in vitro and in vivo. Prostate. 2005;62:165–186. doi: 10.1002/pros.20126. [DOI] [PubMed] [Google Scholar]
- Shankar S, Singh TR, Srivastava RK. Ionizing radiation enhances the therapeutic potential of TRAIL in prostate cancer in vitro and in vivo: Intracellular mechanisms. Prostate. 2004;61:35–49. doi: 10.1002/pros.20069. [DOI] [PubMed] [Google Scholar]
- Shankar S, Srivastava RK. Enhancement of therapeutic potential of TRAIL by cancer chemotherapy and irradiation: mechanisms and clinical implications. Drug Resist Updat. 2004;7:139–156. doi: 10.1016/j.drup.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Srivastava RK. TRAIL/Apo-2L: mechanisms and clinical applications in cancer. Neoplasia. 2001;3:535–546. doi: 10.1038/sj.neo.7900203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Thakkar H, Tyan F, Gim S, Robinson H, Lee C, Pandey SK, Nwokorie C, Onwudiwe N, Srivastava RK. Constitutively active Akt is an important regulator of TRAIL sensitivity in prostate cancer. Oncogene. 2001;20:6073–6083. doi: 10.1038/sj.onc.1204736. [DOI] [PubMed] [Google Scholar]
- Deeb DD, Jiang H, Gao X, Divine G, Dulchavsky SA, Gautam SC. Chemosensitization of hormone-refractory prostate cancer cells by curcumin to TRAIL-induced apoptosis. J Exp Ther Oncol. 2005;5:81–91. [PubMed] [Google Scholar]
- Jung EM, Park JW, Choi KS, Park JW, Lee HI, Lee KS, Kwon TK. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis through CHOP-independent DR5 upregulation. Carcinogenesis. 2006. [DOI] [PubMed]
- Shankar S, Chen Q, Sarva K, Siddiqui I, Srivastava RK. Curcumin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells: molecular mechanisms of apoptosis, migration and angiogenesis. J Mol Signal. 2007;2:10. doi: 10.1186/1750-2187-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwarf DM, Karin M. The NF-kappa B activation pathway: a paradigm in information transfer from membrane to nucleus. Sci STKE. 1999;1999:RE1. doi: 10.1126/stke.1999.5.re1. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis and apoptosis. Semin Cancer Biol. 2003;13:159–167. doi: 10.1016/S1044-579X(02)00133-5. [DOI] [PubMed] [Google Scholar]
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- Suh J, Rabson AB. NF-kappaB activation in human prostate cancer: important mediator or epiphenomenon? J Cell Biochem. 2004;91:100–117. doi: 10.1002/jcb.10729. [DOI] [PubMed] [Google Scholar]
- Madsen MA, Deryugina EI, Niessen S, Cravatt BF, Quigley JP. Activity-based protein profiling implicates urokinase activation as a key step in human fibrosarcoma intravasation. J Biol Chem. 2006;281:15997–16005. doi: 10.1074/jbc.M601223200. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- Shenouda NS, Zhou C, Browning JD, Ansell PJ, Sakla MS, Lubahn DB, Macdonald RS. Phytoestrogens in common herbs regulate prostate cancer cell growth in vitro. Nutr Cancer. 2004;49:200–208. doi: 10.1207/s15327914nc4902_12. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Banerjee S, Bharadwaj U, Sung B, Shishodia S, Sethi G. Curcumin induces the degradation of cyclin E expression through ubiquitin-dependent pathway and up-regulates cyclin-dependent kinase inhibitors p21 and p27 in multiple human tumor cell lines. Biochem Pharmacol. 2007;73:1024–1032. doi: 10.1016/j.bcp.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Srivastava RK, Chen Q, Siddiqui I, Shankar S. Mechanisms of cell cycle regulation by curcumin in prostate cancer. Front Biosci. 2007.
- Srivastava RK, Chen Q, Siddiqui I, Sarva K, Shankar S. Linkage of curcumin-induced cell cycle arrest and apoptosis by cyclin-dependent kinase inhibitor p21/WAF1/CIP1. Cell Cycle. 2007 doi: 10.4161/cc.6.23.4951. [DOI] [PubMed] [Google Scholar]
- Spruck CH, Won KA, Reed SI. Deregulated cyclin E induces chromosome instability. Nature. 1999;401:297–300. doi: 10.1038/45836. [DOI] [PubMed] [Google Scholar]
- Haas K, Johannes C, Geisen C, Schmidt T, Karsunky H, Blass-Kampmann S, Obe G, Moroy T. Malignant transformation by cyclin E and Ha-Ras correlates with lower sensitivity towards induction of cell death but requires functional Myc and CDK4. Oncogene. 1997;15:2615–2623. doi: 10.1038/sj.onc.1201434. [DOI] [PubMed] [Google Scholar]
- Rosen DG, Yang G, Deavers MT, Malpica A, Kavanagh JJ, Mills GB, Liu J. Cyclin E expression is correlated with tumor progression and predicts a poor prognosis in patients with ovarian carcinoma. Cancer. 2006;106:1925–1932. doi: 10.1002/cncr.21767. [DOI] [PubMed] [Google Scholar]
- Keyomarsi K, Tucker SL, Buchholz TA, Callister M, Ding Y, Hortobagyi GN, Bedrosian I, Knickerbocker C, Toyofuku W, Lowe M, et al. Cyclin E and survival in patients with breast cancer. N Engl J Med. 2002;347:1566–1575. doi: 10.1056/NEJMoa021153. [DOI] [PubMed] [Google Scholar]
- Singh TR, Shankar S, Chen X, Asim M, Srivastava RK. Synergistic interactions of chemotherapeutic drugs and tumor necrosis factor-related apoptosis-inducing ligand/Apo-2 ligand on apoptosis and on regression of breast carcinoma in vivo. Cancer Res. 2003;63:5390–5400. [PubMed] [Google Scholar]
- Singh TR, Shankar S, Srivastava RK. HDAC inhibitors enhance the apoptosis-inducing potential of TRAIL in breast carcinoma. Oncogene. 2005;24:4609–4623. doi: 10.1038/sj.onc.1208585. [DOI] [PubMed] [Google Scholar]
- Shankar S, Chen Q, Siddiqui I, Sarva K, Srivastava RK. Sensitization of TRAIL-resistant LNCaP cells by resveratrol (3, 4', 5 tri-hydroxystilbene): molecular mechanisms and therapeutic potential. J Mol Signal. 2007;2:7. doi: 10.1186/1750-2187-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar S, Ganapathy S, Hingorani SR, Srivastava RK. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front Biosci. 2008;13:440–452. doi: 10.2741/2691. [DOI] [PubMed] [Google Scholar]
- Shankar S, Siddiqui I, Srivastava RK. Molecular mechanisms of resveratrol (3,4,5-trihydroxy-trans-stilbene) and its interaction with TNF-related apoptosis inducing ligand (TRAIL) in androgen-insensitive prostate cancer cells. Mol Cell Biochem. 2007 doi: 10.1007/s11010-007-9510-x. [DOI] [PubMed] [Google Scholar]
- Shankar S, Singh G, Srivastava RK. Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front Biosci. 2007;12:4839–4854. doi: 10.2741/2432. [DOI] [PubMed] [Google Scholar]
- Shankar S, Singh TR, Chen X, Thakkar H, Firnin J, Srivastava RK. The sequential treatment with ionizing radiation followed by TRAIL/Apo-2L reduces tumor growth and induces apoptosis of breast tumor xenografts in nude mice. Int J Oncol. 2004;24:1133–1140. [PubMed] [Google Scholar]
- Shankar S, Singh TR, Fandy TE, Luetrakul T, Ross DD, Srivastava RK. Interactive effects of histone deacetylase inhibitors and TRAIL on apoptosis in human leukemia cells: Involvement of both death receptor and mitochondrial pathways. Int J Mol Med. 2005;16:1125–1138. [PubMed] [Google Scholar]
- Chen X, Kandasamy K, Srivastava RK. Differential roles of RelA (p65) and c-Rel subunits of nuclear factor kappa B in tumor necrosis factor-related apoptosis-inducing ligand signaling. Cancer Res. 2003;63:1059–1066. [PubMed] [Google Scholar]
- Takimoto R, El-Deiry WS. Wild-type p53 transactivates the KILLER/DR5 gene through an intronic sequence-specific DNA-binding site. Oncogene. 2000;19:1735–1743. doi: 10.1038/sj.onc.1203489. [DOI] [PubMed] [Google Scholar]
- Wu GS, Burns TF, McDonald ER, 3rd, Meng RD, Kao G, Muschel R, Yen T, el-Deiry WS. Induction of the TRAIL receptor KILLER/DR5 in p53-dependent apoptosis but not growth arrest. Oncogene. 1999;18:6411–6418. doi: 10.1038/sj.onc.1203025. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Maeda A, Tani N, Sakai T. Promoter structure and transcription initiation sites of the human death receptor 5/TRAIL-R2 gene. FEBS Lett. 2001;507:381–385. doi: 10.1016/S0014-5793(01)02947-7. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Sakai T. Promoter of TRAIL-R2 gene. Vitam Horm. 2004;67:35–49. doi: 10.1016/S0083-6729(04)67003-8. [DOI] [PubMed] [Google Scholar]
- Liu X, Yue P, Khuri FR, Sun SY. p53 upregulates death receptor 4 expression through an intronic p53 binding site. Cancer Res. 2004;64:5078–5083. doi: 10.1158/0008-5472.CAN-04-1195. [DOI] [PubMed] [Google Scholar]
- Wang S, El-Deiry WS. Inducible silencing of KILLER/DR5 in vivo promotes bioluminescent colon tumor xenograft growth and confers resistance to chemotherapeutic agent 5-fluorouracil. Cancer Res. 2004;64:6666–6672. doi: 10.1158/0008-5472.CAN-04-1734. [DOI] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- Peant B, Diallo JS, Lessard L, Delvoye N, Le Page C, Saad F, Mes-Masson AM. Regulation of IkappaB kinase epsilon expression by the androgen receptor and the nuclear factor-kappaB transcription factor in prostate cancer. Mol Cancer Res. 2007;5:87–94. doi: 10.1158/1541-7786.MCR-06-0144. [DOI] [PubMed] [Google Scholar]
- Karin M. NF-kappaB and cancer: mechanisms and targets. Mol Carcinog. 2006;45:355–361. doi: 10.1002/mc.20217. [DOI] [PubMed] [Google Scholar]
- Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, Pirmohamed M, Marnett LJ, Gescher AJ, Steward WP. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]
- Kim EJ, Suliman A, Lam A, Srivastava RK. Failure of Bcl-2 to block mitochondrial dysfunction during TRAIL-induced apoptosis. Tumor necrosis-related apoptosis-inducing ligand. Int J Oncol. 2001;18:187–194. [PubMed] [Google Scholar]