Abstract
Transition from G2 to M phase, a cell cycle checkpoint, is regulated by the Cdc2-cyclin B1 complex. Here, we report that persistent infection with Borna disease virus (BDV), a noncytolytic RNA virus infecting the central nervous system, results in decelerated proliferation of infected host cells due to a delayed G2-to-M transition. Persistent BDV-infected rat fibroblast cells showed reduced proliferation compared to uninfected cells. In pull-down assays we observed an interaction of the viral nucleoprotein with the Cdc2-cyclin B1 complex. Transfection of the viral nucleoprotein but not of the phosphoprotein also results in decelerated proliferation. This phenomenon was found in BDV-susceptible primary rat fibroblast cells and also in primary mouse cells, which are not susceptible to BDV infection. This is the first evidence that the noncytolytic Borna disease virus can manipulate host cell functions via interaction of the viral nucleoprotein with mitotic entry regulators. BDV preferentially infects and persists in nondividing neurons. The present report could give an explanation for this selective choice of host cell by BDV.
Cell division of eukaryotic cells is a highly regulated process. One round of cell division requires accurate duplication of DNA during S phase of the cell cycle and proper segregation of duplicated chromosomes during mitosis. Progression through the cell cycle is mediated by the activation of members of a highly conserved family of protein kinases, the cyclin-dependent kinases (termed Cdk′s or Cdc′s) (22). Activation of a Cdk requires binding to a specific regulatory subunit, termed a cyclin. These Cdk-cyclin complexes function as universal cell cycle regulators, each controlling a specific transition to the next phase in the cell cycle.
The initiation of mitosis in vertebrate cells is triggered by the cyclin-dependent protein kinase Cdk1, also known as Cdc2. The activation of Cdc2 begins with the binding of cyclin B1, whose level gradually increases during S and G2 phases. The Cdc2-cyclin B1 complex remains in an inactive state before mitosis by phosphorylation of Cdc2 at Thr14 and Tyr15. At the end of G2, these residues are dephosphorylated by the phosphatase Cdc25C, and the active Cdc2-cyclin B1 complex is then competent to initiate the events of mitosis (19, 20, 30).
It is well known that many DNA viruses interact with the cell cycle machinery, since they are dependent on the DNA synthesis enzymes for viral replication (reviewed in reference 16). In contrast, little is known about the interference of RNA viruses with cell cycle checkpoints, where our knowledge is almost exclusively based on investigations of human immunodeficiency virus (reviewed in reference 5). In addition, it was recently reported that reovirus, a cytolytic, nonenveloped, double-stranded RNA virus, inhibits cellular proliferation by inducing G2 cell cycle arrest (25).
Borna disease virus (BDV), a noncytolytic single-stranded RNA virus, is the only known member of the Bornaviridae, in the order Mononegavirales. BDV is highly neurotropic and cell associated and leads to a persistent infection of the central nervous system. BDV induces Borna disease, a T-cell-mediated encephalomyelitis, in a wide variety of animals; furthermore, it is reported to be involved in human psychiatric disease (reviewed in references 12, 26, and 29). Little is known about BDV-host cell interactions, although it was shown recently that BDV infection interferes with the activation of the Raf/MEK/ERK signaling cascade and that blockade of this pathway results in reduced viral spread (24).
The 8.9-kb negative-strand BDV genome is replicated in the nucleus of the infected cell and codes for at least six different known viral proteins (reviewed in reference 13). The nucleoprotein, which is involved in nuclear transport processes, is present both in the cytoplasm and in the nucleus of the infected cell and forms complexes with the phosphoprotein and p10 (3, 33).
In the present report we demonstrate that the interaction of viral nucleoprotein with the Cdc2-cyclin B1 complex results in prolongation of the G2 phase. These findings are independent of the viral host cell specificity. Furthermore, these findings provide the first evidence that a noncytolytic RNA virus manipulates cell cycle progression in the host cell. We propose that this might enable the virus to establish a persistent infection.
MATERIALS AND METHODS
Virus and cells.
Fibroblast cells from Lewis rats (LEW cells) were used, as described earlier (23). Briefly, primary skin cells from 2-week-old Lewis rats were cultured for several passages in the absence of any transforming agent. After 15 passages the LEW cells were infected with BDV (designated hereafter BDV-LEW), using the Tübingen laboratory strain derivative of He/80. The infectivity rate was controlled by immunofluorescence and fluorescence-activated cell sorter (FACS) analysis. Around 90 to 95% of the cells harbored BDV-specific antigen. For the presented experiments, NL-LEW and BDV-LEW cells with comparable numbers of passages were used.
Plating efficiency.
BDV-LEW or uninfected LEW (NL-LEW) cells (5 × 102) were distributed uniformly into a 10-cm petri dish and cultured at 37°C (5% CO2) for 10 to 14 days. After 7 days of incubation, colony formation was analyzed. For Giemsa staining, cells were washed twice with phosphate-buffered saline (PBS), fixed with methanol-acetic acid for 10 min, washed twice with PBS, dried at room temperature, and stained.
Proliferation assay.
Cell proliferation kit I (MTT) (Roche, Mannheim, Germany) was used to measure proliferation of BDV-infected and uninfected LEW cells according to the manufacturer's instructions. Between 103 and 104 cells were plated in a volume of 100 μl into each well of a 96-well plate. All assays were done in quadruplicate. After various incubation periods ranging from 24 to 72 h, cells were incubated with the yellow MTT solution (0.5 mg/ml) for approximately 4 h. After this incubation period, purple formazan salt crystals were formed. The solubilized formazan product was spectrometrically quantified using an enzyme-linked immunosorbent assay reader. To compare different growth rates of uninfected LEW versus BDV-LEW cells as a function of their starting growth characteristics, the formula described by Solyanik and colleagues was used (28).
GST pull-down assay. (i) Constructs.
Constructs for GST-p40, GST-p24, and GST-p16 were kind gifts from W. I. Lipkin, Irvine, Calif. (14, 15), and GST-p10 was a kind gift from J. R. Richt, Giessen, Germany (32). Construct pGEX-2T to produce glutathione S-transferase (GST) was purchased from Pharmacia Biotech. Propagation of the different plasmids was done with Escherichia coli strain Top 10F (Invitrogen). Growth, induction of protein synthesis, and preparation of cell extracts were done according to recommendations of the manufacturers. Fusion proteins were purified by the use of glutathione-Sepharose 4B (Pharmacia).
(ii) Preparation of cell lysates.
LEW cells were grown in a petri dish to confluency. Thereafter, the cell layer, approximately 2 × 106 cells, was washed twice with PBS before addition of 500 μl of Triton X-100 lysis buffer (TLB). The cell lysate was stored in 1-ml aliquots at −70°C for further use.
For pull down, 20 μg of GST fusion protein was incubated with 200 μl of LEW cell lysate at 4°C overnight. Next, 50 μl of glutathione-Sepharose 4B was added, and the samples were again incubated overnight at 4°C. Thereafter, samples were washed three times with PBS and centrifuged at 500 × g for 5 min. After the third washing step, PBS was removed and the pellet was incubated with 50 μl of electrophoresis buffer (13 μl of Roti-load, 37 μl of TLB), denatured for 5 min at 100°C. Thereafter, 25 μl was used directly for Western blot analysis using anti-Cdc2 (sc-54; Santa Cruz Biotechnology), anti-phospho-Cdc2 Tyr15 (Cell Signaling Technology), and anti-cyclin B1 (sc-245; Santa Cruz Biotechnology) antibodies.
(iii) Gel electrophoresis and Western blot analysis.
Gel electrophoresis and Western blot analysis were performed as described earlier (7) except that TBS Blotto A (sc-2333; Santa Cruz Biotechnology) was used as a blocking reagent. For Western blot analysis the following antibodies (all but two from Santa Cruz Biotechnology) were used: anti-Cdc2 (sc-54), anti-phospho-Cdc2 Tyr15 (Cell Signaling Technology), anti-cyclin B1 (sc-245), anti-Cdc25A (sc-7389), anti-pCdc25 Ser216 (Cell Signaling Technology), anti-Cdc25C (sc-327), anti-ERK2 (sc-1647), and anti PP2A (sc-6110). After incubation with species-specific peroxidase-labeled secondary antibody, chemiluminescence was performed using Luminol reagent (sc-2048; Santa Cruz Biotechnology). To confirm equal loading of the gel lanes, a Western blot analysis with anti-ERK2 antibody was used as a control. Furthermore, Western blot membranes were stained with Coomassie blue after the chemiluminescence reaction (data not shown).
Fluorimetric analysis.
BDV-LEW and uninfected LEW cells were cultured without serum for 24 h to induce a G1 arrest. After release of G1-arrested cells by addition of 5% serum to the medium, cells were harvested every 3 h for a total of 24 h and propidium iodide staining was performed to determine DNA content in the different cell cycle phases. For this, cells were washed, incubated with cold 70% ethanol overnight, and stained with 1 ml of propidium iodide (50 μg of propidium iodide/ml, 100 U of RNase A/ml, PBS) for 30 min.
Cells were used for fluorimetric analysis with FACSCalibur (Becton Dickinson). Flow-cytometric analysis for DNA content cannot distinguish between G2 and M. Therefore, the percentage of cells in the G1, S, and G2/M phases was determined at the different time points after release of G1 arrest. For calculation of the duration of the different cell cycle phases, the formula of Van Dilla and colleagues was used (27, 31).
Protein transfection.
Protein transfection was carried out by using the Chariot Transfection System (Active Motif). Briefly, 3 × 105 NL-LEW cells were plated into one well of a six-well plate (Greiner) 1 day prior to the experiment. Chariot transfection reagent was prepared according to the manual instructions. In contrast to the original protocol, for successful protein transfection of NL-LEW cells, twice the amount of transfection reagent was used. After incubation of BDV proteins with the transfection reagent for 30 min at room temperature, cells were washed and 200 μl of the transfection-protein mixture together with 400 μl of Iscore’s modified Dulbecco medium without serum was added to the cells and incubated for 1 h at 37°C. Thereafter, 1 ml of Iscove’s modified Dulbecco medium-10% fetal calf serum was added to the cell and incubated for further use at 37°C.
For the proliferation assay, cells were trypsinized and 5 × 103 cells were plated into wells of a 96-well plate (Greiner). For immunofluorescence, 104 cells were plated into a chamber of an eight-chamber slide (Nunc). Immunofluorescence was performed as described earlier (23) using the monoclonal antibody 38/17C1 for BDV detection.
RESULTS
Phenotypic changes of LEW cells after BDV infection.
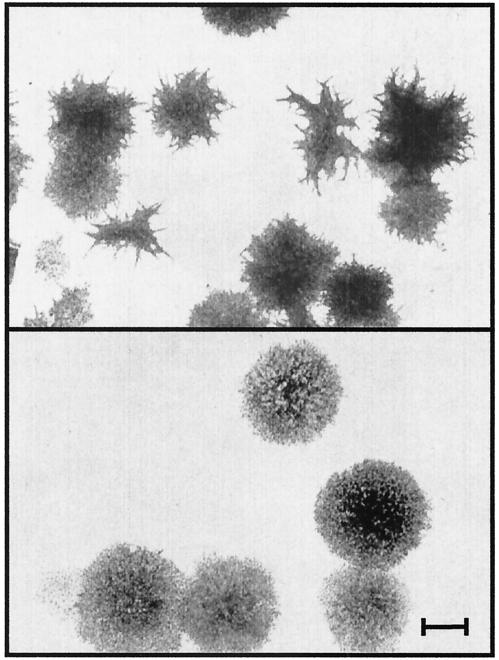
During culture of different BDV-infected and uninfected cell lines, we observed that growth of LEW cells which were persistently infected with BDV (BDV-LEW) was reduced compared to that of uninfected LEW cells. Experiments were performed to compare the plating efficacy and colony formation of BDV-infected LEW cells with those of their uninfected counterparts. The numbers of colonies formed by BDV-LEW or uninfected LEW cells were equal, but their morphologies significantly differed. Colonies formed by LEW were characterized by regular, round shapes. In contrast, colonies obtained from BDV-LEW cells showed extensions, resembling a more differentiated phenotype (Fig. 1). The proliferation rate of the cells was also measured, using an MTT method. For BDV-LEW cells the proliferation rate was reduced between 30 and 40% over a 3-day observation period compared to that of uninfected LEW cells (Fig. 2).
FIG. 1.
Morphological differences of uninfected LEW and BDV-LEW cells. Colony formation was assayed for persistently BDV-infected LEW cells (upper panel) and uninfected LEW cells (lower panel). Five hundred cells were plated on a 10-cm petri dish, and cells were allowed to grow for 9 to 12 days. Thereafter, cells were fixed with methanol-acetic acid and stained with Giemsa. The number, shape, and size of colonies were determined. Scale bar, 1 mm.
FIG. 2.
Proliferation rates of uninfected LEW and BDV-LEW cells. BDV-LEW cells (▪) and the uninfected parental cells (□) were cultured in 96-well plates for 24, 48, or 72 h before an MTT proliferation assay was performed as described in Materials and Methods. The proliferation rate of uninfected LEW cells at each time point was arbitrarily set to 100%, and the relative proliferation rate of BDV-LEW cells at the same time is given as a percentage thereof.
Prolonged G2/M cell cycle phases in BDV-infected LEW.
Since BDV-LEW cells differed significantly from parental cells in their proliferative potential, a detailed analysis of the cell cycle phases by analysis of DNA content was performed. Upon serum starvation for 24 h, both cell types were mainly arrested in G1, and only a few cells were in the G2 or M phase (Fig. 3A). Six hours after release of G1 arrest, LEW cells showed a decreased number of cells in G1 phase corresponding to an increased number of cells in G2 or M phase (19%). In contrast, the DNA profile of BDV-LEW cells 6 h after G1 release did not obviously differ from that of the starting serum-starved cells. At 15 h, most LEW cells were in S and G2 or M, while the proportion of BDV-LEW cells in the G1 phase was even higher. Twenty-four hours after release from G1 arrest, almost all uninfected LEW cells were found in the G1 phase. In G2/M phase only 15% were found, which is almost the same percentage as at the initiation of the experiment (14%). For BDV-LEW cells, 21% of the cells were still in the G2 or M phase, compared to 11% at the beginning of the experiment.
FIG. 3.
Cell cycle analysis of uninfected LEW versus BDV LEW cells. (A) For flow-cytometric analysis for DNA content, cells were cultured without serum for 24 h. Thereafter, serum was added to the culture medium, and the cells were harvested at indicated time points, labeled with propidium iodide, and analyzed using FACSCalibur (Becton Dickinson). Percentages of cells in G2/M phase are shown. (B) Percentage of cells in G1, S, and G2/M phases were determined at different time points after release from G1 arrest, and therefrom the durations of the different cell cycle phases were calculated.
Next, the duration of the different cell cycle phases for BDV-LEW versus uninfected LEW cells was calculated according to the algorithm described by Van Dilla and colleagues (31). For BDV-LEW cells the duration of G1 was 10.5 ± 2.3 h, compared to 10.6 ± 2.5 h for uninfected cells (Fig. 3B). The average duration of the S phase of BDV-LEW cells (4.6 ± 3.0 h) was also not different from that measured for the uninfected cells (5.2 ± 2.6 h). Since cell microfluorimetric analysis for DNA content does not allow one to distinguish the G2 and M phases, the combined durations of the G2 plus M phases of both cell lines were compared. Here, we observed a clear-cut 2.4-h prolongation in BDV-LEW cells (7.2 ± 1.4 h) compared to the duration for uninfected LEW cells (4.8 ± 1.2 h). This 50% prolongation in G2/M is remarkable, since in general the lengths of the cell cycle are determined by the duration of G1. It accounts for the overall length for a complete cell cycle in BDV-LEW cells to 22.2 ± 0.7 h or 10% longer than that seen for the uninfected LEW cells (20.6 ± 0.6 h). These experiments were performed four times in independent analyses.
BDV nucleoprotein p40 binds to Cdc2.
Since the duration of the G2 and M phases of BDV-LEW cells was remarkably prolonged, we examined whether viral proteins might be responsible for this effect. To investigate a possible interaction of BDV proteins with cell cycle-regulating kinases, phosphatases, or cyclins, pull-down assays using different recombinant BDV proteins tagged with GST and glutathione beads were performed. After incubation of GST-tagged BDV proteins with cell lysates of uninfected LEW cells, glutathione-bound protein complexes were eluted by excess glutathione and further analyzed by Western blotting. We focused on proteins that control progression through the G2 to M phase and thus might be candidates to coprecipitate with viral proteins. When an antibody directed against Cdc2 was used, this protein could be detected in the p40 and (to a lesser extent) the p24 precipitations but not when pull-down assays were performed with p16 or p10 or with GST alone (Fig. 4A). Moreover, Cdc2 could also be detected by the use of a phospho-specific antibody directed against its Tyr15 phosphorylation site, suggesting that the inactive form of Cdc2 interacts with the viral nucleoprotein. In addition, cyclin B1 was also detectable in these samples, further substantiating that BDV nucleoprotein physically interacts with the Cdc2-cyclin B1 complex. To further characterize nucleoprotein-Cdc2 binding, truncated forms of p40 fused to GST were used for precipitation. While the truncated amino-terminal fragment GST-p40 13-171 still precipitated Cdc2, no interaction was found with the carboxy-terminal fragment GST-p40 67-370 (data not shown). When antibodies directed against other cell cycle regulators, such as the Cdc25 phosphatase or the retinoblastoma suppressor protein (Rb), were used for Western blot analysis, no coprecipitated protein could be detected (data not shown). Interaction of the viral nucleoprotein with the Cdc2-cyclin B1 complex in infected cells was also found for two other cell lines tested (Lewis rat astrocytes [F10] and neuronal guinea pig cells [subclone of CRL 1405]; data not shown).
FIG. 4.
BDV p40 protein interacts with the Cdc2-cyclin B1 complex. (A) Coprecipitations using glutathione beads after adding GST-p40 (lane 1), GST-p24 (lane 2), GST-p16 (lane 3), GST-p10 (lane 4), and GST (lane 5) were performed with 200-μl lysates of uninfected LEW cells. Lane 6 represents a Western blot with 10 μl of whole NL-LEW lysate as a control. After precipitation and elution of all precipitated proteins with sodium dodecyl sulfate loading buffer (Roti-load, Roth, Germany), a Western blot analysis was performed using anti-phospho-Cdc2-specific antibody (panel a), a Cdc2-specific antibody (panel b), or a cyclin B1-specific antibody (panel c). Equal amounts of the different GST fusion proteins were used, as demonstrated by a Coomassie blue-stained gel (panel d). (B) MTT assay of protein-transfected LEW cells with GST-p40, GST-p40 13-171, GST-p40 67-370, and GST of two individual experiments. Each bar represents the mean value of 16 to 24 individual wells. Variation of the single values was less than 10%. Cell growth was measured after a 2-day (▪) and 3-day (□) culture period. (C) MTT assay of protein-transfected primary mouse fibroblast cells (B.10S) with GST-p40, GST-p40 13-171, and GST. Cell growth was measured after a 2-day (▪) and 3-day (□) culture period. The bars represent the mean value of seven individual wells. Variation of individual wells after a 3-day culture period was 3.6% for GST-p40, 6.4% for GST, 6.7% GST-p40 13-171, and 7.2% for untransfected cells. (D) MTT assay of protein-transfected NL-LEW cells with GST-p24, GST, and untransfected LEW cells. Cell growth was measured after 2-day (▪) and 3-day (□) culture periods. Each bar represents the mean value of 24 individual wells. Variation of individual wells after a 3-day culture period was 3.5% for GST-p24, 10.3% for GST, and 9.7% for untransfected cells.
Protein transfection of the BDV nucleoprotein results in reduced proliferation rates for rat fibroblast cells.
To analyze whether binding of p40 to the Cdc2-cyclin B1 complex has functional consequences for cell cycle progression similar to that observed in BDV-infected cells, protein transfection of GST-p40 and of two truncated forms of the nucleoprotein (GST-p40 13-171 and GST-p40 67-370) was performed. After a 3-day observation period, trypan blue staining of the cells revealed no increase in cell death of transfected cells over that of untransfected control cells. Transfection efficiencies of LEW cells were controlled by immunofluorescence (data not shown). If transfection efficiency was greater than 60%, growth performance was measured using the MTT proliferation assay. During a 3-day observation period, LEW cells transfected either with GST-p40 or with GST-p40 13-171 but not cells transfected with GST-p40 67-370 showed a reduced proliferation of about 40 to 60% compared to GST-transfected LEW or NL-LEW cells (Fig. 4B). The same growth-inhibitory effects of p40 or p40 13-171 were also observed in primary mouse fibroblast cells, which are not susceptible to BDV infection (Fig. 4C). In addition, LEW cells were transfected with GST-p24. No significant reduction of proliferation was observed when GST-p24-transfected LEW cells were compared to GST-transfected cells or untransfected LEW cells (Fig. 4D). These results indicate that the inhibitory proliferative effect is due to the nucleoprotein alone and that host cell specificity is not required.
Synthesis, phosphorylation profiles, and gene expression of different cell cycle regulators.
To further examine the influence of BDV infection on cell cycle events, the synthesis or activation status of various proteins that regulate the G2-to-M transition of the cell cycle was analyzed in BDV-infected LEW cells and uninfected control LEW cells by Western blotting using protein-specific or activation state-specific antibodies.
A stronger phosphorylation of Cdc2 at Tyr15 was detected in virus-infected LEW cells (Fig. 5) up to 12 h after release from serum starvation and G1 mitotic arrest, indicating that more Cdc2 is kept in an inactive phosphorylated state after infection. The synthesis of Cdc2 protein was not altered in general. In contrast, the level of cyclin B1, which is bound to Cdc2 in G2 phase, is upregulated in BDV-LEW cells during the first 12 h after release from cell cycle arrest. Dephosphorylation of Cdc2 is regulated by the phosphatase Cdc25C. For Cdc25, as shown in Fig. 5, neither its synthesis (anti-Cdc25C) nor its activity (anti-phospho-Cdc25C) appears to be altered. Another phosphatase regulating G2-to-M-phase transition is protein phosphatase 2A (PP2A), which dephosphorylates Cdc25C. When PP2A levels were analyzed, overall no difference was found between BDV-LEW cells and uninfected LEW cells. The PP2A activity state was also not altered, as determined in a phosphatase assay (data not shown). In contrast, an up-regulation of Cdc25A, a regulator of the G1-to-S-phase transition, was detected in BDV-LEW cells, persisting up to 15 h postrelease into the cell cycle. In addition, Western blot analysis and RNase protection assays of other regulators of the G1 or S phase, such as cyclin D1, cyclin D3, and p21, did not reveal any alterations in their expression or protein synthesis (data not shown).
FIG. 5.
Effect of persistent BDV infection on synthesis and activity of G2/M phase regulators. BDV-infected LEW cells and uninfected control LEW cells were cultured for 24 h without serum to induce an accumulation of cells in the G1 phase. Thereafter, cells were released from the cell cycle arrest by stimulation with 5% fetal calf serum. At various time points the cells were lysed to examine the synthesis level or activation status of various proteins that regulate the G2 phase of the cell cycle by using protein- or activation state-specific antibodies.
These Western blot analyses indicated that besides the interaction of BDV p40 with the Cdc2-cyclin B1 complex, which appears to prevent activation and dephosphorylation of Cdc2, BDV infection also causes a transient misexpression of the genes coding for G2 or M phase regulators.
DISCUSSION
In this study we have identified the BDV nucleoprotein as a regulator of cell cycle progression that most likely acts through an interaction with the Cdc2-cyclin B1 phase in late G2. This feature of BDV p40 is observed both in cells that are permissive and in those that are nonpermissive for BDV infection and appears to be the basis of growth retardation in persistently infected LEW cells. In order to be able to investigate a possible mechanism in the context of viral persistency, we have chosen to use primary cells, since transformation of cells often interferes with cell cycle regulation. Primary rat fibroblasts can be infected primarily and can also be maintained as persistently infected cell lines. Cells of neuronal origin are the primary targets for BDV. Nevertheless, primary neuronal cultures are very delicate to establish and to maintain for several passages.
Comparison of BDV-infected rat fibroblast cells (BDV-LEW) with uninfected LEW cells revealed phenotypical changes. Similar observations were made after BDV infection of PC12 cells, where neuronal differentiation was blocked and the extracellular regulated kinase was activated (9). Extracellular regulated kinase activation was also found after BDV infection of LEW cells; nevertheless, from the present data, we cannot propose whether this leads to the observed morphological changes (24).
The duration of a complete cell cycle in BDV-infected LEW cells was prolonged due to a delay in G2-to-M-phase transition. The lengths of all phases of the cycle are variable to some extent, but by far the greatest variation occurs in the duration of G1 in most of the commonly studied cells types. Here, we show that the duration of G1 is equal in infected and uninfected cells, but the duration of G2/M shows a 50% increase in BDV-infected cells over that in uninfected cells. When the duration of the complete cell cycle was calculated with a formula (31), roughly 10% (2 h) prolongation was found for BDV-LEW cells to complete one cell cycle. In contrast, proliferation assays revealed that proliferation is reduced by 40% over a 3-day observation period. Increased cell death was not found in BDV-infected cells. The retarded growth of LEW cells and primary mouse fibroblasts was also observed upon transfection of the viral nucleoprotein. Consistent with this observation, an interaction of the viral nucleoprotein with the inactive Cdc2-cyclin B1 complex was demonstrated. This binding may transiently interfere with the activation of Cdc2 in late G2, which is most likely the basis for growth retardation in BDV-infected cells.
Replication of DNA viruses requires the cellular DNA synthesis machinery of the host cell, so it is not surprising that these viruses interact with cell cycle regulators (e.g., Cdc2) and alter cellular functions to increase cellular activities related to cell cycle progression. However, alteration in Cdc2 activity not only might be caused by a virus to induce cell cycle progression but also may be required for phosphorylation of a viral protein (1, 34, 35).
As for RNA viruses, in a human immunodeficiency virus (HIV) model system, first investigations indicated that the viral Vpr protein inhibits Cdc2 activity and consequently leads to a G2-phase cell cycle arrest (10). More recent publications suggest that the inhibition of Cdc2 activity by the HIV Vpr protein is due to its direct physical interaction with PP2A (11, 18). PP2A inhibits Cdc25C activity by dephosphorylation, whereas Cdc25C phosphatase dephosphorylates Thr14/Tyr15 of Cdc2, which leads to activation of Cdc2. In contrast to Cdc2 activation, Wee1 kinase activity is required to phosphorylate and inactivate Cdc2 (reviewed in reference 30). Measles virus induces unresponsiveness of peripheral blood lymphocytes to mitogenic stimulation by deregulation of the expression of CDK4, CDK6, cyclin D3, and cyclin E, which are essential for the G1/S-phase transition (6).
Most recently, another RNA virus was identified as interacting with the host cell cycle. Infection of cells with reovirus, a cytolytic double-stranded RNA virus, also leads to G2-phase cell cycle arrest. The mechanism is not yet fully understood, but an interaction of reovirus with Wee1 and Cdc25 is proposed (25).
In light of these reports, the detection of a complex formed by the BDV nucleoprotein and Cdc2-cyclin B1, which appears to be responsible for a decreased proliferation rate of BDV-infected LEW cells, represents a novel mechanism by which a virus interferes with G2-to-M progression. As a consequence, Cdc2 seems to remain at least transiently in a phosphorylated and inactivated state. The fact that Cdc2 was coprecipitated with the truncated amino-terminal fragment p40 13-171 but not with the carboxy-terminal fragment p40 67-370, which directly correlates with the effects of the two protein fragments on cell proliferation, indicates that the binding domain of the BDV nucleoprotein for functional interaction with Cdc2 must be located between amino acids 13 and 67. Also, a very weak but reproducible interaction of the viral phosphoprotein (p24) with unphosphorylated Cdc2 was found. It is puzzling that p24 interacts only with Cdc2 but not with the Cdc2-cyclin B1 complex, and currently it is unknown whether this has any functional relevance. Furthermore, another explanation might be that the signals for phospho-cdc2 and cyclin B1 are undetectable, if p24 pulls down the same percentage of cdc2 versus phospho-cdc2 as p40. Additional direct or indirect interactions of viral proteins to affect the activity or synthesis of cell cycle proteins are also possible. Western blot analyses revealed higher levels of cyclin B1 and Cdc25A in BDV-LEW cells, and this may also be directly caused by the virus. Another likely explanation might be that during long-term culture of the persistently infected cells, populations have been selected which somehow counteract the cell cycle-inhibitory effect of BDV p40. Greater production of cyclin B1 as the Cdc2 binding partner during G2 phase as well as higher levels of the phosphatase Cdc25A might represent such a regulatory counteraction. Cdc25A is a main regulator of the G1-to-S transition, but involvement in S-to-G2 regulation has also been discussed (4). An overproduction of Cdc25A and cyclin B1 might also explain why we do not observe a full G2 cell cycle arrest but rather a delay in cell cycle progression in BDV-LEW cells. If this is the correct explanation, such a counteraction is quite selective, since for Cdc25C no difference in protein synthesis or phosphatase activity was found. There were also no detectable effects on the upstream phosphatase PP2A, indicating a mechanism different from that shown with HIV Vpr.
At this point the question why BDV infection induces a delay in cell cycle progression must be raised. For HIV it could be shown that Vpr-mediated manipulation of the host cell cycle leads to increased virus production (8). Evidence that this might also be the case for BDV comes from treatment of BDV-infected cells with N-butyrate, which leads to an increase in the number of viral particles (17, 21). N-butyrate treatment results in an alteration of various cellular functions in proliferating cells, including inhibition of cyclin D1 and c-myc transcription. Furthermore, N-butyrate induces p21/CIP-1 expression, which leads to cell cycle arrest (2). Concerning the possible benefit for the virus, we hypothesize that interaction of BDV nucleoprotein with the Cdc2-cyclin B1 complex and subsequent delay in G2 progression are conducive to establishment of a persistent virus infection. In this regard the degree of cell cycle inhibition might also be critical. A complete cell cycle arrest, as observed upon HIV or reovirus infection, will kill the infected cell; consequently, a persistent infection cannot be established, in contrast to the case with BDV-infected LEW cells.
In summary, our findings identify for the first time a protein of an RNA virus that directly interacts with the Cdc2-cyclin B1 complex. They reveal a specific virus-host interaction between the BDV nucleoprotein and the Cdc2-cyclin B1 complex of the host cell. As a consequence, Cdc2 appears to be kept in an inactive state, resulting in a delay in G2 phase progression. The effects appear to be transient, allowing the infected cell to further replicate on a low level and thereby ensuring a persistent infection. These findings not only provide novel insights in BDV host-cell interactions but also may be relevant for cell cycle manipulation of rapidly proliferating cells, such as cancer cells, by the use of specific viral proteins. In the central nervous system the vast majority of neurons represent terminally differentiated nondividing cells. The present finding that Borna disease virus is capable of modulating mitotic entry to a certain extent indicates that the virus prefers to replicate in nondividing cells. This might explain why neurons are the preferential target cells of BDV and could explain why BDV infection outside the central nervous system is a rather rare event.
Acknowledgments
This work was supported in part by Deutsche Forschungsgemeinschaft grant Pl 256/1-2.
We thank Mandy Laukner for technical assistance, R. Cassada for critical reading of the manuscript, and H. J. Rziha for helpful discussion and critical reading of the manuscript.
REFERENCES
- 1.Advani, S. J., R. R. Weichselbaum, and B. Roizman. 2000. The role of cdc2 in the expression of herpes simplex virus genes. Proc. Natl. Acad. Sci. USA 97:10996-11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archer, S., S. Meng, J. Wu, J. Johnson, R. Tang, and R. Hodin. 1998. Butyrate inhibits colon carcinoma cell growth through two distinct pathways. Surgery 124:248-253. [PubMed] [Google Scholar]
- 3.Berg, M., C. Ehrenborg, J. Blomberg, R. Pipkorn, and A. L. Berg. 1998. Two domains of the Borna disease virus p40 protein are required for interaction with the p23 protein. J. Gen. Virol. 79:2957-2963. [DOI] [PubMed] [Google Scholar]
- 4.Bernardi, R., D. A. Liebermann, and B. Hoffman. 2000. Cdc25A stability is controlled by the ubiquitin-proteasome pathway during cell cycle progression and terminal differentiation. Oncogene 19:2447-2454. [DOI] [PubMed] [Google Scholar]
- 5.Elder, R. T., Z. Benko, and Y. Zhao. HIV-1 VPR modulates cell cycle G2/M transition through an alternative cellular mechanism other than the classic mitotic checkpoints. Front. Biosci. 7:349-357. [DOI] [PubMed]
- 6.Engelking, O., L. M. Fedorov, R. Lilischkis, V. ter Meulen, and S. Schneider-Schaulies. 1999. Measles virus induced immunosuppression in vitro is associated with deregulation of G1 cell cycle control proteins. J. Gen. Virol. 80:1599-1608. [DOI] [PubMed] [Google Scholar]
- 7.Furrer, E., T. Bilzer, L. Stitz, and O. Planz. 2001. Neutralizing antibodies in persistent Borna disease virus infection: prophylactic effect of gp94-specific monoclonal antibodies in preventing encephalitis. J. Virol. 75:943-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goh, W. C., M. E. Rogel, C. M. Kinsey, S. F. Michael, P. N. Fultz, M. A. Nowak, B. H. Hahn, and M. Emerman. 1998. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat. Med. 4:65-71. [DOI] [PubMed] [Google Scholar]
- 9.Hans, A., S. Syan, C. Crosso, P. Sassone-Corsi, M. Brahic, and D. Gonzalez-Dunia. 2001. Borna disease virus persistent infection activates mitogen activated protein kinase and blocks neuronal differentiation of PC12 cells. J. Biol. Chem. 276:7258-7265. [DOI] [PubMed] [Google Scholar]
- 10.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hrimech, M., X. J. Yao, P. E. Branton, and E. A. Cohen. 2000. Human immunodeficiency virus type 1 Vpr-mediated G(2) cell cycle arrest: Vpr interferes with cell cycle signaling cascades by interacting with the B subunit of serine/threonine protein phosphatase 2A. EMBO J. 19:3956-3967. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Ikuta, K., M. S. Ibrahim, T. Kobayashi, and K. Tomonaga. 2002. Borna disease virus and infection in humans. Front. Biosci. 7:470-495. [DOI] [PubMed] [Google Scholar]
- 13.Jordan, I., and W. I. Lipkin. 2001. Borna disease virus. Rev. Med. Virol. 11:37-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kliche, S., T. Briese, A. H. Henschen, L. Stitz, and W. I. Lipkin. 1994. Characterization of a Borna disease virus glycoprotein, gp18. J. Virol. 68:6918-6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kliche, S., L. Stitz, H. Mangalam, L. Shi, T. Binz, H. Niemann, T. Briese, and W. I. Lipkin. 1996. Characterization of the Borna disease virus phosphoprotein, p23. J. Virol. 70:8133-8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knipe, D. M. 1996. Virus-host cell interactions, p. 273-299. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 17.Kohno, T., T. Goto, T. Takasaki, C. Morita, T. Nakaya, K. Ikuta, I. Kurane, K. Sano, and M. Nakai. 1999. Fine structure and morphogenesis of Borna disease virus. J. Virol. 73:760-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masuda, M., Y. Nagai, N. Oshima, K. Tanaka, H. Murakami, H. Igarashi, and H. Okayama. 2000. Genetic studies with the fission yeast Schizosaccharomyces pombe suggest involvement of wee1, ppa2, and rad24 in induction of cell cycle arrest by human immunodeficiency virus type 1 Vpr. J. Virol. 74:2636-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan, D. O. 1997. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 13:261-291. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson, I., and I. Hoffmann. 2000. Cell cycle regulation by the Cdc25 phosphatase family. Prog. Cell Cycle Res. 4:107-114. [DOI] [PubMed] [Google Scholar]
- 21.Pauli, G., and H. Ludwig. 1985. Increase of virus yields and releases of Borna disease virus from persistently infected cells. Virus Res. 2:29-33. [DOI] [PubMed] [Google Scholar]
- 22.Pines, J.1995. Cyclins and cyclin-dependent kinases: a biochemical view. Biochem. J. 308:697-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Planz, O., T. Bilzer, M. Sobbe, and L. Stitz.1993. Lysis of major histocompatibility complex class I-bearing cells in Borna disease virus-induced degenerative encephalopathy. J. Exp. Med. 178:163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Planz, O., S. Pleschka, and S. Ludwig. 2001. Mek-specific inhibitor u0126 blocks spread of Borna disease virus in cultured cells. J. Virol. 75:4871-4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poggioli, G. J., T. S. Dermody, and K. L. Tyler. 2001. Reovirus-induced σ1s-dependent G2/M phase cell cycle arrest is associated with inhibition of p34cdc2. J. Virol. 75:7429-7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rott, R., and H. Becht. 1995. Natural and experimental Borna disease in animals. Curr. Top. Microbiol. Immunol. 190:17-30. [DOI] [PubMed] [Google Scholar]
- 27.Smith, J. A., and L. Martin. 1973. Do cells cycle? Proc. Natl. Acad. Sci. USA 70:1263-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solyanik, G. I., N. M. Berezetskaya, R. I. Bulkiewicz, and G. I. Kulik.1995. Different growth patterns of a cancer cell population as a function of its starting growth characteristics: analysis by mathematical modelling. Cell Prolif. 28:263-278. [DOI] [PubMed] [Google Scholar]
- 29.Stitz, L., and R. Rott. 1999. Borna disease virus (Bornaviridae), p. 167-173. In A. Granoff and R. G. Webster (ed.), Encyclopedia of virology. Academic Press, New York, N.Y.
- 30.Takizawa, C. G., and D. O. Morgan. 2000. Control of mitosis by changes in the subcellular location of cyclin-B1, Cdk1 and Cdc25C. Curr. Opin. Cell Biol. 12:658-665. [DOI] [PubMed] [Google Scholar]
- 31.Van Dilla, M. A., T. T. Trujillo, P. F. Mullaney, and J. R. Coulter. 1969. Cell microfluorometry: a method for rapid fluorescence measurement. Science 163:1213-1214. [DOI] [PubMed] [Google Scholar]
- 32.Wehner, T., A. Ruppert, C. Herden, K. Frese, H. Becht, and J. A. Richt. 1997. Detection of a novel Borna disease virus-encoded 10 kDa protein in infected cells and tissues. J. Gen. Virol. 78:2459-2466. [DOI] [PubMed] [Google Scholar]
- 33.Wolff, T., R. Pfleger, T. Wehner, J. Reinhardt, and J. A. Richt. 2000. A short leucine-rich sequence in the Borna disease virus p10 protein mediates association with the viral phospho- and nucleoproteins. J. Gen. Virol. 81:939-947. [DOI] [PubMed] [Google Scholar]
- 34.Ye, M., K. M. Duus, J. Peng, D. H. Price, and C. Grose. 1999. Varicella-zoster virus Fc receptor component gI is phosphorylated on its endodomain by a cyclin-dependent kinase. J. Virol. 73:1320-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zafrullah, M., M. H. Ozdener, S. K. Panda, and S. Jameel. 1997. The ORF3 protein of hepatitis E virus is a phosphoprotein that associates with the cytoskeleton. J. Virol. 71:9045-9053. [DOI] [PMC free article] [PubMed] [Google Scholar]