Abstract
The ability of an environmental exposure to induce an epigenetic transgenerational adult onset disease phenotype is discussed in the current mini-review in the context of defining this phenomenon and the associated reproductive toxicology. A gestating female (F0 generation) exposure results in the F1 generation embryo and F2 generation germ-line being directly exposed, such that the F3 generation is the first not directly exposed to the environmental compound. In contrast, postnatal or adult exposure (F0 generation) results in the F1 generation germ-line being exposed, such that the F2 generation is the first to not be directly exposed to the environmental compound. The unequivocal transgenerational transmission of an adult onset disease phenotype through the germ-line requires assessment in the F3 generation for embryonic exposure, and F2 generation for postnatal exposure. This is in contrast to a number of F1 and F2 generation studies referred to as transgenerational. The reproductive toxicology associated with this transgenerational phenotype generally involves the reprogramming of the germ-line epigenome. The biological phenomenon involved in this reproductive toxicology deals with embryonic gonadal development and germ-line differentiation, or postnatally the gametogenesis process and germ cell development. The ability of an environmental compound (e.g. endocrine disruptor) to promote this reprogramming of the germ-line appears to be the causal factor in the epigenetic transgenerational phenotype.
Keywords: Epigenetics, Transgenerational, Endocrine Disruptors, Adult Onset Disease, Germ Cell, Sex Determination, Embryonic Exposure
INTRODUCTION
Previous studies have documented the ability of environmental compounds (e.g. endocrine disruptors), therapeutic treatments (e.g. diethylstilbestrol, DES) and physiological stress (e.g. caloric restriction) during embryogenesis and/or early postnatally to promote adult onset disease for multiple generations {1-8}. This is the basis for the proposal that adult onset disease may be in large part due to these embryonic and/or postnatal exposures {9, 10}. The types of environmental compounds and exposures involved range from plastics to agricultural pesticides, and are often endocrine disruptors {10-13}. Although the ability of these environmental factors to promote multi-generational/transgenerational adult onset disease has been shown, the mechanisms involved in the process remain to be elucidated. Multi-generational is defined here as an exposure that directly influences multiple generations, such as exposure of a pregnant female affects both the F0 mother and F1 embryo generations. Multi-generational involves direct exposure to the environmental factor. In contrast, transgenerational is defined here as transmission between generations, but not involving direct exposure. Therefore, transgenerational involves a germ line transmission between generations without direct exposure to the environmental factor. Recently, an endocrine disruptor (i.e. vinclozolin) exposure during embryonic gonadal sex determination was shown to induce an adult onset disease (i.e. male fertility and spermatogenic defect) for multiple generations (i.e. F1-F4) and involved epigenetic (i.e. DNA methylation) changes in several genes in the male germ-line {1, 11}. This transgenerational phenotype appears to involve altered DNA methylation and epigenetic programming of the male germ-line as the potential causal factor in the phenomenon {1, 14}. Subsequently it has been found that as these transgenerational animals age multiple adult onset disease are observed including tumor development, prostate disease, kidney disease and immune abnormalities {2, 11}. The ability of an environmental factor (e.g. endocrine disruptor) to promote an epigenetic change in the germ-line is postulated to be a mechanism involved in transgenerational adult onset disease {1, 2, 11, 14}. In addition to transgenerational germ line considerations, the exposure and epigenetic modification of any developing organ system may influence adult onset disease for the individual and tissue exposed. The focus of the current mini-review will be on the transgenerational phenomena and not direct exposures. Further investigation into how environmental toxicants may influence the epigenetic programming of the germ-line and subsequent developing organs will provide a better understanding of the mechanisms involved in transgenerational phenotypes, adult onset disease, and toxicology of the compounds.
EPIGENETIC TRANSGENERATIONAL PHENOTYPE
Adult onset disease can be induced through embryonic exposure {1, 2, 5}, and postnatal exposure during organ development {8, 15}. The physiological basis for adult onset disease is that altered programming of gene expression (i.e. transcriptome) during critical developmental periods will initiate a cascade of effects on cellular differentiation that do not manifest as abnormal physiologies and resulting disease until adulthood {1, 11}. Environmental toxicants (e.g. endocrine disruptors) and factors (e.g. nutritional status) can promote these adult onset disease states{1-3, 5, 8}. These diseases range from tumors {8, 16}, reproductive defects {1, 5, 15}, and metabolic defects (e.g. obesity and diabetes) {3, 9}. Most major disease states have been speculated to in part involve this type of mechanism {9, 10}. Although these environmental compounds and factors have been shown to influence adult onset disease, the molecular mechanisms involved are unclear.
The frequency and reproducibility of the majority of adult onset disease are such that genetic DNA sequence mutations are not likely involved. The frequency of a DNA sequence mutation, even with exposures such as radiation, are generally less than 0.01% and in a hot spot mutation in the 1-5% range {17, 18}. The reproducibility of the same mutation occurring is highly improbable {17, 18}. However, the reproducibility and frequency of most adult onset disease is significantly higher and reproducible {2, 9, 10}. For example, the frequency of the epigenetic transgenerational (i.e. F1-F4) adult onset diseases induced ranged from 20-90% for all progeny {1, 2}. Therefore, a mechanism not involving DNA sequence mutations or a genetic mechanism is suggested. Epigenetic regulation of the genome is a well established process and involves DNA methylation, histone modifications and chromosomal alterations {19, 20}. DNA methylation is the primary epigenetic mechanism involved in transgenerational heritable phenomena. A number of studies have demonstrated the ability of environmental compounds and factors to influence DNA methylation and epigenetics {1, 11, 13, 14, 20, 21}. Since the epigenome is a major regulator of the genome and gene expression (i.e. transcriptome), alterations in epigenetics (i.e. DNA methylation) is an important molecular component to consider in the actions of toxicant exposures. A number of environmental toxicants (e.g. endocrine disruptors) have been shown to induce epigenetic (i.e. DNA methylation) changes in a variety of different genes {1, 15, 21, 22}. Many DNA methylation changes are metastable {20} and not heritable, but imprinted genes maintain a DNA methylation pattern in a heritable manner {22-24}. Alterations in imprinted genes can promote disease states. Recently, the endocrine disruptor vinclozolin (i.e. antiandrogenic fungicide used in the fruit industry {25} has been shown after an embryonic exposure during sex determination to promote the induction of new imprinted-like genes that transmit an alteration in the epigenome transgenerationally {1, 14} and correlate with the development of adult onset disease {2}. Therefore, observations support the concept that the actions of environmental toxicants and factors on adult onset disease are in large part due to epigenetic phenomena {26-29}.
In regards to adult onset disease, the most sensitive developmental periods to environmental exposures are both the embryonic and early postnatal periods {10, 11, 27}. The reason for this is that various developmental processes are occurring that when altered permanently change subsequent organ development and function. For example, during gonadal sex determination and development the germ-line undergoes critical programming of its epigenome and transcriptome {30, 31} that then impacts the progeny from those germ cells. Alternatively, active organ development during late fetal and early postnatal periods also undergo critical programming of the epigenome and transcriptome associated with cellular differentiation and organogenesis. Recent studies have shown that both embryonic {1, 2, 14} and postnatal {15} exposures to environmental toxicants (e.g. endocrine disruptors) can modify the epigenome to promote adult onset disease. Only the modification of the germ-line can promote a transgenerational phenotype. Although the epigenome modification of the developing organ can be critical for the adult onset disease of the individual exposed, a germ-line reprogramming is required to transmit this phenotype trasngenerationally {1, 11}.
An embryonic exposure involves the F0 generation gestating female and the F1 generation developing embryo, Figure 1. A large number of studies have demonstrated that embryonic exposures to environmental factors can influence a disease or abnormal phenotypes in the F1 generation {4, 32-36}. Since the developing embyro is sensitive to environmental insults, the most common multigenerational phenotype reported is an F1 generation. The environmental factors include toxic substances {36}, endocrine disruptors {4, 10, 34} and physiological factors such as nutrition {32, 33, 35}. Since the F1 embryo is directly exposed to the environmental factor/toxicant this is not a transgenerational phenomenon.
Figure 1. Epigenetic transgenerational actions of endocrine disruptors through the male germ-line.
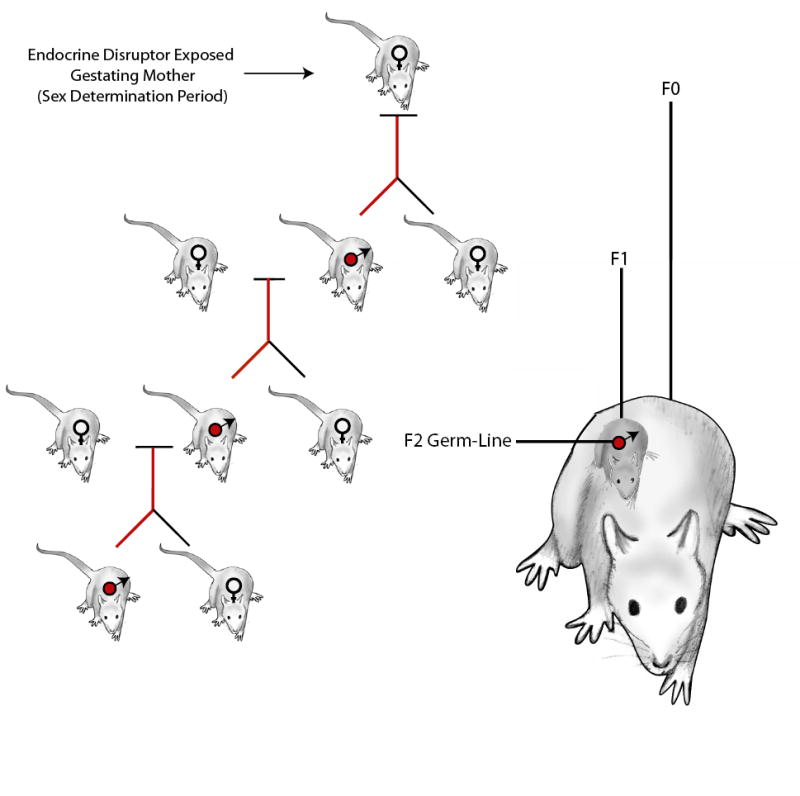
Schematic of an endocrine disruptor induced transgenerational phenomena and direct exposure of the F0 mother, F1 embryo, and F2 germ-line. The F3 generation is the first without direct exposure.
Exposure of an F0 gestating female also exposes the F1 generation embryo, including the F2 generation germ-line present, Figure 1. During embryonic gonadal development the germ-line is present that will be involved in generating the F2 generation progeny. Therefore, alterations in the F2 generation germ cell due to direct embryonic exposure can influence the F2 generation progeny due to a direct toxicant exposure. When an F0 generation gestating mother is exposed the F1 generation embryo and F2 generation germ-line are directly exposed. Several observations have shown that an embryonic exposure (i.e. F0 generation mother) can promote an F2 generation phenotype {5, 6, 37-42}. Since the germ-line generating this F2 generation was directly exposed, is this a transgenerational phenotype? If the phenotype was due to an abnormality generated in the germ cell due to direct toxicant exposure, then no. If the phenotype was due to a permanent reprogramming of the germ-line (e.g. epigenome), then yes. However, definitive conclusions that the F2 phenotype was transgenerational requires the next F3 generation to be produced to determine the transgenerational nature of the phenotype. Although many of the F2 generation phenotypes described may be due to a transgenerational mechanism {5, 41}, further analysis involving the F3 generation is required to eliminate the variable of direct F2 generation germ-line exposure.
After an F0 generation gestating female exposure the F3 generation is the first that has not had a direct exposure, Figure 1. This will be the first that can be unequivocally concluded to be a transgenerational phenomenon. Several studies have shown the effects of environmental toxicants on the F3 generation {1, 2, 7}. The mechanism involved requires a germ-line transmission and permanent reprogramming of the germ-line. A recent study has suggested this is likely at the level of altering the epigenome of the germ cell {1, 14}. Since a class of imprinted genes exist that can transfer their DNA methylation pattern transgenerationally, the epigenetic transgenerational phenomenon will likely involve an alteration in the imprinted-like pattern of the epigenome {1, 11, 14}. For embryonic exposure to environmental factors or toxicants the F3 generation is needed to identify a transgenerational phenotype.
In contrast to an embryonic exposure, a postnatal or adult exposure to an environmental factor or toxicant has different implications for transgenerational phenomena. The postnatal or adult individual is the F0 generation exposed. Therefore, the F1 generation germ-line is directly exposed. The germ cells that will generate the F1 generation are directly exposed to the toxicant or factor. Several observations have suggested F1 generation phenotypes after an environmental toxicant/factor {43-48}. Since the F1 generation germ-line is directly exposed the F1 phenotype observed cannot be unequivocally defined as a transgenerational phenomenon. Therefore, the F2 generation needs to be produced to determine a potential transgenerational phenotype. The mechanism involved in such a transgenerational phenotype would require an alteration of the process of gametogenesis to reprogram the germ-line. Epigenetic programming of the germ-line during gametogenesis has been reported {49-52}, but alterations that could generate an epigenetic transgenerational phenotype have not been reported.
SUMMARY
The discussion above can now be used to define an epigenetic transgenerational phenotype. The term multigenerational can be used to help clarify the phenomena. An embryonic exposure involves the multigenerational exposure of the F0 generation gestating female, F1 generation embryo and F2 generation germ-line, Figure 1. This multigenerational exposure indicates that the phenotypes of the F0-F2 generations may be due to direct exposure to the environmental toxicant/factor and cannot be characterized as a transgenerational phenomena. For an embryonic exposure this requires minimally an F3 generation to be investigated as this is the first generation not directly exposed. In contrast, a postnatal or adult exposure involves the multigenerational exposure of the F0 generation adult and the F1 generation germ line. This multigenerational exposure indicates the phenotypes of the F0 and F1 generations may be due to direct exposure to the environmental toxicant/factor and not be concluded to be a transgenerational phenomenon. For a postnatal/adult exposure this requires minimally the F2 generation to be investigated.
The mechanism involved in these transgenerational phenotypes is postulated to be primarily epigenetic {1, 2, 11, 14}. Due to the infrequency and random nature of the DNA mutations {17, 18}, epigenetics is the most viable mechanism for the germ-line to transmit an environmentally influenced heritable phenotype. The germ-line is required for the epigenetic transgenerational phenotype. The embryonic exposure during gonadal sex determination can alter the epigenetic programming of the germ-line {1, 14}. During embryonic development the primordial germ cells migrate down the genital ridge and undergo de-methylation (i.e. erasure) of the genomic DNA, such that at the onset of sex determination they are in the gonad and are de-methylated. During sex determination the germ cells re-methylate in a sex specific manner {30, 53}. Exposure of the germ cells during this period to environmental toxicants (e.g. endocrine disruptors) {1, 2} has the ability to reprogram the germ-line. If imprinted-like genes are involved to permanently alter the epigenome {14}, an epigenetic transgenerational phenotype develops {2}. If postnatal/adult exposures to environmental toxicants occur then the process of gametogenesis can be affected (e.g. spermatogenesis) that then can potentially reprogram the germ-line. Although epigenetic effects on gametogenesis have been observed {49-52, 54}, the transgenerational nature of this phenomenon remains to be determined. Therefore, the reproductive toxicology involved in an epigenetic transgenerational phenotype is the development of the germ-line during embryonic and adult gonadal development.
Environmental exposures/factors that influence embryonic or early postnatal development have been shown to be associated with adult onset disease {9, 10}. The ability of an environmental toxicant/factor to influence the germ-line and/or organogenesis early in development can cause a cascade of molecular events that manifest in the adult as disease. One major mechanism postulated to be involved in such an event is the ability of an environmental factor to influence the epigenome. Since very few environmental factors or toxicants can directly influence DNA mutations, alterations in the epigenome (e.g. DNA methylation) provides a critical mechanism for these adult onset disease states. Therefore, an environmental exposure that can promote an epigenetic transgenerational phenotype would have a significant impact on disease etiology and toxicology. The ability of a toxicant to not only influence the individual exposed but all subsequent progeny, needs to be considered in future toxicology studies. The recent studies demonstrating the ability of endocrine disruptors, such as vinclozolin, to induce a transgenerational disease phenotype {1, 2} need to be qualified in regards to conclusions on the toxicology of these compounds. The doses used were above that expected in the environment, such that studies are now needed to compare environmental versus effective doses. In addition, whether the endocrine disruptor activity or other metabolites may be causal also needs to be assessed. Although caution is needed regarding toxicology conclusions, the phenomena identified of a transgenerational disease phenotype and relationship with epigenetic modifications does provide a novel etiology to consider for disease. The role of epigenetic transgenerational phenotypes in adult onset disease needs to be seriously considered {1, 2, 11, 20, 26, 28, 29, 55}. Further investigation of the mechanisms involved in these epigenetic transgenerational phenotypes will likely provide significant insights into future diagnosis and therapy for disease, and develop a better understanding of the reproductive toxicology of many environmental toxicants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–9. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anway MD, Leathers C, Skinner MK. Endocrine disruptor vinclozolin induced epigenetic transgenerational adult-onset disease. Endocrinology. 2006;147:5515–23. doi: 10.1210/en.2006-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cesani MF, Orden B, Zucchi M, Mune MC, Oyhenart EE, Pucciarelli HM. Effect of undernutrition on the cranial growth of the rat. An intergenerational study. Cells Tissues Organs. 2003;174:129–35. doi: 10.1159/000071153. [DOI] [PubMed] [Google Scholar]
- 4.Klip H, Verloop J, van Gool JD, Koster ME, Burger CW, van Leeuwen FE. Hypospadias in sons of women exposed to diethylstilbestrol in utero: a cohort study. Lancet. 2002;359:1102–7. doi: 10.1016/S0140-6736(02)08152-7. [DOI] [PubMed] [Google Scholar]
- 5.Newbold RR, Padilla-Banks E, Jefferson WN. Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology. 2006;147:S11–7. doi: 10.1210/en.2005-1164. [DOI] [PubMed] [Google Scholar]
- 6.Popova NV. Transgenerational effect of orthoaminoasotoluol in mice. Cancer Lett. 1989;46:203–6. doi: 10.1016/0304-3835(89)90131-6. [DOI] [PubMed] [Google Scholar]
- 7.Turusov VS, Nikonova TV, Parfenov Yu D. Increased multiplicity of lung adenomas in five generations of mice treated with benz(a)pyrene when pregnant. Cancer Lett. 1990;55:227–31. doi: 10.1016/0304-3835(90)90123-f. [DOI] [PubMed] [Google Scholar]
- 8.Yamasaki H, Loktionov A, Tomatis L. Perinatal and multigenerational effect of carcinogens: possible contribution to determination of cancer susceptibility. Environ Health Perspect. 1992;98:39–43. doi: 10.1289/ehp.929839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gluckman PD, Hanson MA. Developmental origins of disease paradigm: a mechanistic and evolutionary perspective. Pediatr Res. 2004;56:311–7. doi: 10.1203/01.PDR.0000135998.08025.FB. [DOI] [PubMed] [Google Scholar]
- 10.Heindel JJ. Role of exposure to environmental chemicals in the developmental basis of reproductive disease and dysfunction. Semin Reprod Med. 2006;24:168–77. doi: 10.1055/s-2006-944423. [DOI] [PubMed] [Google Scholar]
- 11.Anway MD, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology. 2006;147:S43–9. doi: 10.1210/en.2005-1058. [DOI] [PubMed] [Google Scholar]
- 12.Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–84. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crews D, McLachlan JA. Epigenetics, evolution, endocrine disruption, health, and disease. Endocrinology. 2006;147:S4–10. doi: 10.1210/en.2005-1122. [DOI] [PubMed] [Google Scholar]
- 14.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–62. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66:5624–32. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng RY, Hockman T, Crawford E, Anderson LM, Shiao YH. Epigenetic and gene expression changes related to transgenerational carcinogenesis. Mol Carcinog. 2004;40:1–11. doi: 10.1002/mc.20022. [DOI] [PubMed] [Google Scholar]
- 17.Gray J. News and Information. Journal of Radiological Protection. 2005;25:499–502. [Google Scholar]
- 18.Dubrova YE. Radiation-induced transgenerational instability. Oncogene. 2003;22:7087–93. doi: 10.1038/sj.onc.1206993. [DOI] [PubMed] [Google Scholar]
- 19.Callinan PA, Feinberg AP. The emerging science of epigenomics. Hum Mol Genet. 2006;15(Spec No 1):R95–101. doi: 10.1093/hmg/ddl095. [DOI] [PubMed] [Google Scholar]
- 20.Peaston AE, Whitelaw E. Epigenetics and phenotypic variation in mammals. Mamm Genome. 2006;17:365–74. doi: 10.1007/s00335-005-0180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rakyan VK, Chong S, Champ ME, Cuthbert PC, Morgan HD, Luu KV, Whitelaw E. Transgenerational inheritance of epigenetic states at the murine Axin(Fu) allele occurs after maternal and paternal transmission. Proc Natl Acad Sci U S A. 2003;100:2538–43. doi: 10.1073/pnas.0436776100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jirtle RL, Sander M, Barrett JC. Genomic imprinting and environmental disease susceptibility. Environ Health Perspect. 2000;108:271–8. doi: 10.1289/ehp.00108271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy SK, Jirtle RL. Imprinted genes as potential genetic and epigenetic toxicologic targets. Environ Health Perspect. 2000;108(Suppl 1):5–11. doi: 10.1289/ehp.00108s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy SK, Jirtle RL. Imprinting evolution and the price of silence. Bioessays. 2003;25:577–88. doi: 10.1002/bies.10277. [DOI] [PubMed] [Google Scholar]
- 25.Kelce WR, Gray LE, Wilson EM. Antiandrogens as environmental endocrine disruptors. Reprod Fertil Dev. 1998;10:105–11. doi: 10.1071/r98051. [DOI] [PubMed] [Google Scholar]
- 26.Di Croce L, Buschbeck M, Gutierrez A, Joval I, Morey L, Villa R, Minucci S. Altered epigenetic signals in human disease. Cancer Biol Ther. 2004;3:831–7. doi: 10.4161/cbt.3.9.1103. [DOI] [PubMed] [Google Scholar]
- 27.Heindel JJ, McAllister KA, Worth LJ, Tyson FL. Epigenetics. Durham, NC: 2005. Environmental Epigenomics, Imprinting and Disease Susceptibility. [DOI] [PubMed] [Google Scholar]
- 28.Jiang YH, Bressler J, Beaudet AL. Epigenetics and human disease. Annu Rev Genomics Hum Genet. 2004;5:479–510. doi: 10.1146/annurev.genom.5.061903.180014. [DOI] [PubMed] [Google Scholar]
- 29.Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–610. doi: 10.1038/nrg1655. [DOI] [PubMed] [Google Scholar]
- 30.Morgan HD, Santos F, Green K, Dean W, Reik W. Epigenetic reprogramming in mammals. Hum Mol Genet. 2005;14(Spec No 1):R47–58. doi: 10.1093/hmg/ddi114. [DOI] [PubMed] [Google Scholar]
- 31.Small CL, Shima JE, Uzumcu M, Skinner MK, Griswold MD. Profiling gene expression during the differentiation and development of the murine embryonic gonad. Biol Reprod. 2005;72:492–501. doi: 10.1095/biolreprod.104.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carter MJ, Lardies MA, Nespolo RF, Bozinovic F. Heritability of progeny size in a terrestrial isopod: transgenerational environmental effects on a life history trait. Heredity. 2004;93:455–9. doi: 10.1038/sj.hdy.6800523. [DOI] [PubMed] [Google Scholar]
- 33.Golding J. The Avon Longitudinal Study of Parents and Children (ALSPAC)--study design and collaborative opportunities. Eur J Endocrinol. 2004;151(Suppl 3):U119–23. doi: 10.1530/eje.0.151u119. [DOI] [PubMed] [Google Scholar]
- 34.Kang IJ, Yokota H, Oshima Y, Tsuruda Y, Oe T, Imada N, Tadokoro H, Honjo T. Effects of bisphenol a on the reproduction of Japanese medaka (Oryzias latipes) Environ Toxicol Chem. 2002;21:2394–400. [PubMed] [Google Scholar]
- 35.Portha B. Programmed disorders of beta-cell development and function as one cause for type 2 diabetes? The GK rat paradigm. Diabetes Metab Res Rev. 2005;21:495–504. doi: 10.1002/dmrr.566. [DOI] [PubMed] [Google Scholar]
- 36.Tsui MT, Wang WX. Maternal transfer efficiency and transgenerational toxicity of methylmercury in Daphnia magna. Environ Toxicol Chem. 2004;23:1504–11. doi: 10.1897/03-310. [DOI] [PubMed] [Google Scholar]
- 37.Anderson CM, Lopez F, Zimmer A, Benoit JN. Placental insufficiency leads to developmental hypertension and mesenteric artery dysfunction in two generations of Sprague-Dawley rat offspring. Biol Reprod. 2006;74:538–44. doi: 10.1095/biolreprod.105.045807. [DOI] [PubMed] [Google Scholar]
- 38.Csaba G, Inczefi-Gonda A. Transgenerational effect of a single neonatal benzpyrene treatment on the glucocorticoid receptor of the rat thymus. Hum Exp Toxicol. 1998;17:88–92. doi: 10.1177/096032719801700203. [DOI] [PubMed] [Google Scholar]
- 39.Csaba G, Karabelyos C. Transgenerational effect of a single neonatal benzpyrene treatment (imprinting) on the sexual behavior of adult female rats. Hum Exp Toxicol. 1997;16:553–6. doi: 10.1177/096032719701601001. [DOI] [PubMed] [Google Scholar]
- 40.Ottinger MA, Wu JM, Hazelton JL, Abdelnabi MA, Thompson N, Quinn ML, Jr, Donoghue D, Schenck F, Ruscio M, Beavers J, Jaber M. Assessing the consequences of the pesticide methoxychlor: neuroendocrine and behavioral measures as indicators of biological impact of an estrogenic environmental chemical. Brain Res Bull. 2005;65:199–209. doi: 10.1016/j.brainresbull.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjostrom M, Golding J. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet. 2006;14:159–66. doi: 10.1038/sj.ejhg.5201538. [DOI] [PubMed] [Google Scholar]
- 42.Zambrano E, Martinez-Samayoa PM, Bautista CJ, Deas M, Guillen L, Rodriguez-Gonzalez GL, Guzman C, Larrea F, Nathanielsz PW. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol. 2005;566:225–36. doi: 10.1113/jphysiol.2005.086462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Byrnes EM. Transgenerational consequences of adolescent morphine exposure in female rats: effects on anxiety-like behaviors and morphine sensitization in adult offspring. Psychopharmacology (Berl) 2005;182:537–44. doi: 10.1007/s00213-005-0122-4. [DOI] [PubMed] [Google Scholar]
- 44.Guest RM. National study inthe UK of occupational exposure to radiofrequency fields. J Radiol Prot. 1998;18:301–303. [Google Scholar]
- 45.Kaati G, Bygren LO, Edvinsson S. Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. Eur J Hum Genet. 2002;10:682–8. doi: 10.1038/sj.ejhg.5200859. [DOI] [PubMed] [Google Scholar]
- 46.Moret Y. “Trans-generational immune priming”: specific enhancement of the antimicrobial immune response in the mealworm beetle, Tenebrio molitor. Proc Biol Sci. 2006;273:1399–405. doi: 10.1098/rspb.2006.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nomura T. Transgenerational carcinogenesis: induction and transmission of genetic alterations and mechanisms of carcinogenesis. Mutat Res. 2003;544:425–32. doi: 10.1016/j.mrrev.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Reusens B, Remacle C. Programming of the endocrine pancreas by the early nutritional environment. Int J Biochem Cell Biol. 2006;38:913–22. doi: 10.1016/j.biocel.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 49.Allegrucci C, Thurston A, Lucas E, Young L. Epigenetics and the germline. Reproduction. 2005;129:137–49. doi: 10.1530/rep.1.00360. [DOI] [PubMed] [Google Scholar]
- 50.Durcova-Hills G, Hajkova P, Sullivan S, Barton S, Surani MA, McLaren A. Influence of sex chromosome constitution on the genomic imprinting of germ cells. Proc Natl Acad Sci U S A. 2006;103:11184–8. doi: 10.1073/pnas.0602621103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCarrey JR, Geyer CB, Yoshioka H. Epigenetic regulation of testis-specific gene expression. Ann N Y Acad Sci. 2005;1061:226–42. doi: 10.1196/annals.1336.025. [DOI] [PubMed] [Google Scholar]
- 52.Trasler JM. Origin and roles of genomic methylation patterns in male germ cells. Semin Cell Dev Biol. 1998;9:467–74. doi: 10.1006/scdb.1998.0225. [DOI] [PubMed] [Google Scholar]
- 53.McLaren A. Primordial germ cells in the mouse. Dev Biol. 2003;262:1–15. doi: 10.1016/s0012-1606(03)00214-8. [DOI] [PubMed] [Google Scholar]
- 54.Flanagan JM, Popendikyte V, Pozdniakovaite N, Sobolev M, Assadzadeh A, Schumacher A, Zangeneh M, Lau L, Virtanen C, Wang SC, Petronis A. Intra- and interindividual epigenetic variation in human germ cells. Am J Hum Genet. 2006;79:67–84. doi: 10.1086/504729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–6. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]


