Abstract
Feline immunodeficiency virus (FIV) infection of domestic cats represents a valuable system through which to investigate criteria for antilentiviral vaccines in a natural host species. Here, we examined whether vaccination with a strain of FIV attenuated as a result of prolonged growth in vitro could protect against a fully virulent, highly heterologous intraclade challenge. The results indicated that the vaccine virus produced a low-grade infection with no detectable pathological effects and afforded a long-lasting sterilizing immunity if the challenge was delivered intraperitoneally as cell-free virus but not against a cell-associated intravaginal challenge. In the latter case, however, the replication and pathological consequences of the challenge virus were markedly suppressed. Together with similar results obtained in rhesus monkey models, these findings should give impulse to the development of attenuated FIV vaccines to be tested in controlled studies in field cats. Field studies may provide answers to some of the existing safety concerns surrounding attenuated AIDS vaccines in humans.
Despite numerous strategies involving animal models and clinical trials in human volunteers, effective vaccines against human immunodeficiency virus type 1 (HIV-1) remain a global health priority. Estimates indicate that HIV-1 causes >15,000 new infections every day. Furthermore, almost 90% of the new infections occur in resource-poor countries, mostly in women infected by sexual intercourse or in neonates infected during pregnancy or breast feeding (69), indicating that the major portal of entry for HIV-1 is mucosal. In these groups, where drug therapy is unaffordable and health care infrastructures are weak, the demand for an AIDS vaccine is therefore as great as ever.
Vaccines remain the method of choice for control of viral infections on the grounds of safety, efficacy, and cost. An ideal vaccine should confer long-lasting and broad-range protection, preferably after a single inoculation, and it should be inexpensive and easily administered (41, 54, 56, 64). Live attenuated virus vaccines fulfill these prerequisites, in that a single vaccine dose is normally sufficient to elicit a robust and long-lasting immune response in the host. Thus, it is conceivable that attenuated lentiviral vaccine may evoke broad protective immunity more effectively than inactivated virus vaccines or subunit vaccines. While live vaccines are currently considered to be impractical for clinical trials in humans because of safety concerns, studies conducted in animal models are providing useful information on the immune mechanisms of vaccinal protection. This approach has been successfully applied in the rhesus macaque models of simian and simian-human immunodeficiency viruses, although uncertainties still exist as to the relative importance of different immune effectors (1, 2, 15, 17, 22, 33-35, 40, 51, 65, 67, 74). However, the rhesus models do not lend themselves to testing vaccines under natural conditions of infection and therefore fall short of providing the ultimate proof that a given vaccinal approach is indeed working in the field.
Feline immunodeficiency virus (FIV) infection of domestic cats represents a valuable system through which to study the pathogenesis of immunodeficiency viruses and to evaluate antilentiviral vaccines in a natural host species. The course of infection, mechanisms of persistence, pathogenesis, and genetic diversity of FIV parallel those of HIV-1, allowing the definition of the protective immune mechanisms that may be important for the rational development of safe and effective HIV-1 vaccines. More than 10 years of FIV vaccination experiments have demonstrated that complete protection (sterilizing immunity) can be achieved only with certain vaccine formulations. Further, the vaccinal protection observed is limited to homologous or slightly heterologous virus challenges (for comprehensive reviews, see references 21 and 68). Taken together, these studies indicate that FIV vaccines perform better when virus challenge is administered systemically rather than mucosally (23, 24) and that protection conferred by inactivated vaccines requires multiple immunizing doses and is relatively short-lived (9, 47).
Long-term propagation in tissue culture has been widely used to develop live attenuated antiviral vaccines. This is the first study investigating whether vaccination with a strain of FIV rendered nonpathogenic as a result of prolonged growth in vitro could protect against a fully virulent, highly heterologous intraclade challenge. The results indicated that the vaccine afforded a long-lasting sterilizing immunity if the challenge was delivered intraperitoneally but not when it was delivered intravaginally. In the latter case, however, the replication and pathological consequences of the challenge virus were markedly suppressed.
MATERIALS AND METHODS
Animals, viruses, and experimental design.
Female specific-pathogen-free cats (Iffa Credo, L'Asbrege, France) were used for this study. Both controls and vaccinees were 9 months old and free from infectious FIV and feline leukemia virus and virus-specific antibodies at the commencement of the study. The animals were housed individually in our climate-controlled animal facility in accordance with European Community regulations. The vaccine virus was the Petaluma strain of FIV (Pet) produced by chronically infected FL4 cells (75; a generous gift of Janet K. Yamamoto, Gainesville, Fla.). In our laboratory, FL4 cells are routinely split 1:5 twice weekly. The viral stock used as a vaccine (FL-381) was cell-free supernatant harvested at cell passage 381 and was inoculated intravenously at 316 50% tissue culture infectious doses (TCID50) in 1 ml per cat. The challenge Glasgow-8 strain of FIV (GL8) and the interleukin-2-dependent feline T-cell line Q201 (72), in which this virus was propagated, were kindly provided by Margaret J. Hosie, Glasgow, United Kingdom. Intraperitoneal challenge consisted of 1 ml of cell-free supernatant and contained 30 TCID50. Intravaginal challenge was instead carried out with primary blasts that were ∼60% FIV positive by surface immunofluorescence. These were obtained by stimulating in vitro pooled peripheral blood mononuclear cells (PBMC) from independent uninfected cats with concanavalin A (infection with GL8 at a multiplicity of infection of 0.0015) and by harvesting 8 days later. The desired numbers of infected cells were deposited into the anterior vagina in 100-μl of sterile, pyrogen-free saline using smooth pipette tips in order to prevent tissue lacerations. No discharge from the vagina was observed after inoculation.
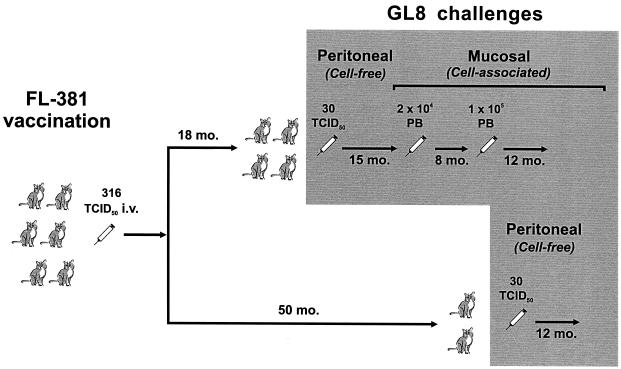
Six cats were inoculated with the vaccine virus, FL-381, and then monitored daily for clinical manifestations and at regular intervals for viral RNA in plasma, viral DNA and infectivity in the PBMC, virus-specific antibodies and lymphoproliferative responses, circulating CD4+-T-lymphocyte counts, and routine hematochemical test results. Eighteen months postvaccination (p.v.), four vaccinees and four uninfected age-matched control cats were challenged intraperitoneally with GL8 and then monitored for 15 months using assays capable of discriminating the challenge virus from the preexisting FL-381, as well as by the parameters described above. At the end of this period (33 months p.v.), because they were still GL8 free, the four vaccinees, together with another six control cats, were challenged intravaginally. Eight months later (41 months p.v.), five of the six control cats and all of the vaccines were still GL8 free and were therefore given a larger intravaginal challenge and monitored for another 1 year. Finally, at 50 months p.v., the two vaccinees that had been left unchallenged, together with a control cat that had resisted both intravaginal challenges, were challenged intraperitoneally exactly as described above and also monitored for 1 year (Fig. 1).
FIG. 1.
Outline of preinfection and challenge. Control cats used in the challenge experiments were omitted for simplicity. PB, infected primary blasts; i.v., intravenous.
Differential plasma viremia measurements.
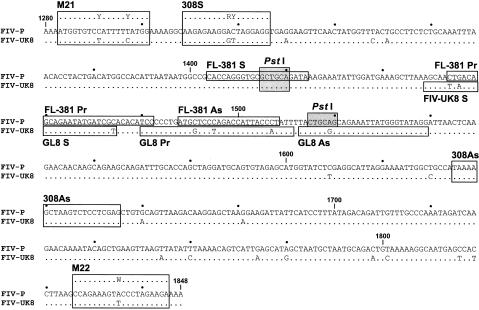
The numbers of FL-381 and GL8 RNA genomes in plasma were initially calculated by determining the percentage of each strain in samples by gag nested reverse transcriptase (RT)-PCR followed by a fluorescence-based restriction fragment length polymorphism (RFLP) analysis which exploits restriction site differences in PCR products (see below). The percentages were then used to calculate the numbers of copies of FL-381 and GL8 RNA genomes from the total number of FIV genomes as determined by gag RT-competitive PCR (59). From January 1999, corresponding to the first intravaginal challenge, this approach was replaced by strain-specific RT-TaqMan-PCR (RT-TM-PCR) assays, which employed strain-specific primers and probes designed by exploiting the mismatches found in the alignment of the gag nucleotide sequence (Fig. 2). To evaluate assay specificity, the p24 regions of both strains were cloned into pGEM plasmids (Promega, Milan, Italy), 10-fold diluted, and amplified in parallel with both strain-specific oligonucleotide sets. The numbers of input copies were correctly estimated using the homologous oligonucleotide sets: FL-381 was underestimated by a factor of 106 with the GL8-specific oligonucleotides, and GL8 was underestimated by a factor of 104 with the FL-381-specific oligonucleotides (data not shown). Plasma samples were processed as described elsewhere (60). Briefly, RNA genomes were extracted from plasma by using the QIAamp Viral RNA kit (Qiagen, Milan, Italy), reverse transcribed with the antisense primer FL-381 AS (900 nM) or GL8 AS (300 nM), and then amplified with the sense primer FL-381 S or GL8 S (300 nM) and the 6-carboxyfluorescein (FAM)/6-carboxytetramethylrhodamine (TAMRA) probe FL-381 PR or GL8 PR (200 nM). Amplification was carried out with Universal PCR Master Mix (Applied Biosystems, Monza, Italy) in a 50-μl reaction volume and the default amplification protocol (50 cycles) on the ABI Prism 7700 Sequence Detection System instrument (Applied Biosystems). Samples and controls (negative and positive) were tested in duplicate and in triplicate, respectively, and a few no-template controls were also interspersed randomly in each amplification plate. Serial 10-fold dilutions (101 to 107) of FL-381 and GL8 gag p24 RNA transcripts were used to produce standard curves. Intra- and interassay precision and reproducibility were assessed as described previously (26, 42, 60). The sensitivities of both assays were 200 copies per ml of plasma, as evaluated by extracting and amplifying FIV-negative plasma spiked with serial 10-fold dilutions of RNA transcripts.
FIG. 2.
Nucleotide alignment of the FIV p24 gag region detected by nested and TM-PCR. Homologous regions are indicated by dots. The shaded boxes represent the PstI sites, one present in both FL-381 and GL8 and the other in FL-381 only, that were used for discriminating between the two strains following reisolation in tissue culture. The positions of outer (M21 and M22) and inner (308S and 308AS) degenerated nested PCR primers are indicated along the alignment. The localizations of FL-381- and GL8-specific TM-PCR primers and probes are also shown.
For a better comparison and since RNA loads determined by RT-TM-PCR were on average 1 log unit higher than those determined by RT-competitive PCR (M. Pistello, personal observations), all plasma samples were reanalyzed in retrospect, and only the quantitative data obtained by RT-TM-PCR assay are reported here.
Differential provirus detection and quantitation.
The strain-specific provirus load was initially calculated from the total proviral load and the percentage of each strain in samples, as determined by competitive PCR and the combined nested-PCR-RFLP analysis in the gag region, respectively (59). From early 1999, this method was replaced by TM-PCR, and previous samples were reanalyzed by the latter method. TM-PCR was carried out as described for RT-TM-PCR, except that the reaction volume was 25 μl and genomic DNA was extracted from the buffy coat using the QIAamp DNA Blood Mini Kit (Qiagen). Standard curves were produced with 10-fold dilutions of FL-381 and GL8 gag p24 plasmids. The assay sensitivity was 100 plasmid copies in a background of 1 μg of genomic DNA for both viruses.
Virus isolation and infectious-PBMC enumeration.
FIV was isolated from the PBMC by cocultivating 106 Ficoll-Hypaque-separated PBMC with feline T-lymphoid MBM cells and testing the supernatant fluids for RT once per week. Cultures regarded as negative showed no evidence of RT in any sample collected during the 5-week culture period. Numbers of infectious units in the PBMC were determined by limiting dilution (48).
Differentiation of FL-381 and GL8 in positive cultures.
Discrimination of FL-381 and GL8 in the RT-positive cultures was carried out by RFLP analysis of gag PCR products. Genomic DNA extracted from the positive cocultures was amplified by nested PCR with degenerate primers to permit amplification of both FL-381 and GL8. Primer sets M21-M22 and 308S-308AS were used in the first and second amplification rounds, respectively. The amplicons were then digested with the enzyme PstI (New England Biolabs, Beverly, Mass.), selected on the basis of the presence of two and one PstI restriction sites in the gag p24 region of FL-381 and GL8, respectively (Fig. 2). Briefly, 15 μl of PCR products was diluted to 50 μl in PstI restriction buffer and digested with the enzyme at 37°C for 2 h. The restriction patterns were finally analyzed in 2% agarose gels. The assay had a lower sensitivity of 500 copies for both the FL-381 and GL8 genomes and was capable of detecting either genome mixed 1/100 with the other one.
Serology.
Plasma samples were tested for antibodies against whole FIV antigen by enzyme-linked immunosorbent assay (ELISA) (48). Neutralizing antibodies (NA) were measured against 10 TCID50 of Pet or GL8 by using MBM cells as an indicator and 50% inhibition of RT production as a readout and were expressed exactly as described previously (18).
Lymphoproliferation assay.
Ficoll-purified PBMC were incubated for 4 days with 0.1 μg of gradient-purified, sonicated Pet grown in FL-4 cells and then pulsed with [3H]thymidine for 18 h (48). The stimulation index was calculated as the ratio of radioactivity incorporated by PBMC in the presence of antigen to that incorporated in the absence of antigen. Only stimulation indices of ≥2 were considered indicative of FIV-specific lymphoproliferation.
Lymphocyte subset counts.
EDTA anticoagulated blood was processed for flow cytometry analysis, and counts of CD4+ and CD8+ T lymphocytes were obtained (48).
RESULTS
Low-grade infection established by the vaccine virus.
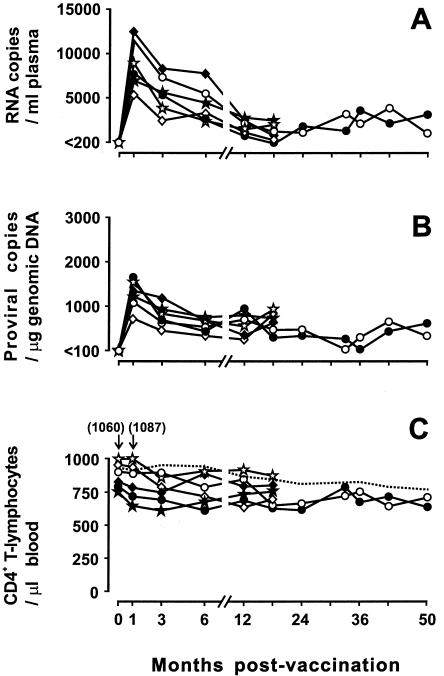
The vaccine virus, FL-381, was administered intravenously to six cats at a dose of 316 TCID50 per animal. As depicted in Fig. 1, the infection established by the vaccine virus in the absence of challenges was monitored for either 18 (cats 521, 560, 575, and 576) or 50 (cats 580 and 590) months. All vaccinees readily became infected, as revealed by the occurrence of peak viremia levels at 1 month p.v., but developed infections that remained extremely mild. In fact, the steady-state plasma viremia and proviral viremia loads observed from 12 months p.v. onward (Fig. 3A and B) only rarely exceeded 4,000 RNA copies per ml and 1,000 DNA copies per μg of genomic DNA, respectively, and attempts to reisolate the virus in culture occasionally yielded negative results (data not shown). Anti-FIV antibodies, determined by both ELISA and a sensitive NA assay against the homologous virus, became detectable in all animals early after vaccination and remained at high titers throughout the observation period, while FIV-specific lymphoproliferative responses were positive only sporadically (data not shown). Most importantly, all vaccinees remained clinically healthy throughout the variably long prechallenge periods, as demonstrated by periodic clinic examinations and routine hematochemical analyses, and circulating CD4+-T-lymphocyte counts remained essentially stable or underwent transient modest reductions (Fig. 3C). Thus, collectively, these data showed that the long-term tissue culture virus used as a vaccine had established productive infections that remained low grade and exerted no detectable clinical consequences for >4 years, exhibiting a possibly greater degree of attenuation than when the same virus had been used to inoculate cats at a lower in vitro passage (7, 31, 59). The virological and immunological statuses of the vaccinees at the times of challenge are detailed in Table 1. Of note, at these times, NA to the vaccine virus were relatively high, as expected for a tissue culture-adapted strain of FIV, while NA to the challenge virus were either very low or absent, as expected for a heterologous primary isolate of FIV (18).
FIG. 3.
Dynamics of vaccine virus FL-381 infection in vaccinated cats. (A) Plasma viremia as determined by FL-381-specific RT-TM-PCR. (B) Provirus load as determined by FL-381-specific TM-PCR. (C) Circulating CD4+ T-lymphocytes as determined by flow cytometry. Solid lines, individual vaccinated cats; dotted line, mean of six age-matched uninfected cats; ⋄, cat 521; ♦, cat 560; ⋆, cat 575; ★, cat 576; •, cat 580; ○, cat 590.
TABLE 1.
Virological and immunological status of vaccinees at times of challenge
| Mo. p.v. | Cat no. | Copies of viral RNA in plasmaa | Proviral DNA load in PBMCb | No. of infectious units in PBMCc | Amt of ELISA antibodyd | Amt of NAe
|
No. of CD4+ T lymphocytesf
|
||
|---|---|---|---|---|---|---|---|---|---|
| Anti-Pet | Anti-GL8 | Per μl of blood | % | ||||||
| 18 | 521 | <200 | 340 | <1 | 8,000 | >512 | 11 | 693 | 31.3 |
| 560 | 980 | 890 | 32 | 8,000 | 512 | <8 | 519 | 34.6 | |
| 575 | 2100 | 950 | 10 | 16,000 | >512 | <8 | 816 | 37.1 | |
| 576 | 2300 | 750 | 10 | 4,000 | >512 | <8 | 811 | 37.1 | |
| 33 | 521 | <200 | 128 | 10 | 32,000 | 181 | <8 | 591 | 32.9 |
| 560 | 2560 | 189 | 103 | 64,000 | 181 | ND | 750 | 37.5 | |
| 575 | 1870 | 104 | 102 | 64,000 | >512 | 8 | 821 | 37.3 | |
| 576 | 3570 | 353 | 102 | 32,000 | >512 | 11 | 684 | 31.1 | |
| 41 | 521 | <200 | 199 | 10 | 32,000 | >512 | <8 | 627 | 31.7 |
| 560 | <200 | 638 | 103 | 8,000 | 45 | <8 | 599 | 30.6 | |
| 575 | 2130 | 910 | 102 | 16,000 | 512 | <8 | 650 | 32.5 | |
| 576 | 1540 | 633 | 102 | 16,000 | 18 | <8 | 572 | 30.9 | |
| 50 | 580 | 1270 | 880 | 1 | 16,000 | ND | <8 | 618 | 30.2 |
| 590 | 1908 | 539 | 10 | 32,000 | ND | <8 | 597 | 33.6 | |
Copies per milliliter, as determined by RT-TM-PCR.
Load in 1 μg of genomic DNA, as determined by TM-PCR.
Number of infectious cells per 106 cells, as determined by quantitative isolation in MBM cells.
Reciprocal of the highest serum dilution that scored positive against whole Pet.
Reciprocal of the serum dilution that gave 50% inhibition of RT production relative to virus exposed to equal dilutions of normal cat serum, as calculated by the method of Reed and Muench (63). ND, not done.
Normal values: 739 ± 225/μl of blood and 35% ± 5%.
Complete protection from intraperitoneal challenge.
Vaccinees 521, 560, 575, and 576, together with four control naive cats (644, 652, 668, and 3063), were challenged intraperitoneally with 30 TCID50 of cell-free GL8 and then monitored systematically for 15 months.
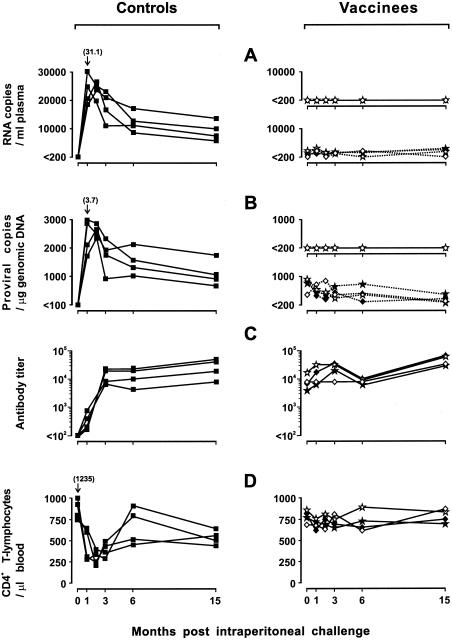
As expected for a virulent virus such as GL8 (30, 31), in spite of the smaller dose inoculated, replication of the challenge virus in naive cats was more florid than that observed with the vaccine virus. Plasma viremia peaked at levels that ranged between 18,240 and 31,100 copies per ml at 1 month postchallenge (p.c.) and then decreased to stabilize at ∼10,000 to 15,000 copies per ml at 6 months. Proviral loads in the PBMC followed a roughly parallel kinetics, with values that at 1 month p.c. ranged from 1,770 to 3,716 copies per μg of genomic DNA and subsequently declined rapidly to a mean steady-state level of ∼1,500 copies. Quantitative reisolation from the PBMC performed at selected times also showed high infectious-unit numbers (Table 2). Anti-FIV antibodies were already present by 1 month p.c. in all cats and increased steadily in titer thereafter (Fig. 4C). On the other hand, flow cytometry revealed a rapid loss of circulating CD4+ T lymphocytes, which were more than halved in number by 2 months p.c. and then rebounded at 6 months but never returned to prechallenge values (Fig. 4D).
TABLE 2.
Infectious units and genotype of FIV reisolated from PBMC of vaccinated and control cats after challenges
| Group | Cat no. | No. of infectious units per 106 PBMC and genotype of the virus at (mo p.c.):
|
|||||
|---|---|---|---|---|---|---|---|
| 1 | 3 | 6 | 8 | 12 | 15 | ||
| Intraperitoneal challenge at 18 mo p.v. | |||||||
| Controls | 644 | 104a | 103 | 103 | NDc | ND | 103a |
| 3063 | 104a | 103 | 102 | ND | ND | 103a | |
| 652 | 103a | 103 | 102 | ND | ND | 103a | |
| 668 | 103a | 103 | 102 | ND | ND | 103a | |
| Vaccinees | 521 | <1 | <1b | 1b | ND | ND | 1b |
| 560 | 10b | 10b | 10b | ND | ND | 10b | |
| 575 | 10b | 1b | 102b | ND | ND | 103b | |
| 576 | 1 | 103b | 102b | ND | ND | 10b | |
| Mucosal challenge at 33 mo p.v. | |||||||
| Controls | 3056 | 10a | 104 | 103 | 103a | ||
| 3076 | <1 | <1 | <1 | <1 | |||
| 2633 | <1 | <1 | <1 | <1 | |||
| 2617 | <1 | <1 | <1 | <1 | |||
| 2625 | <1 | <1 | <1 | <1 | |||
| 138 | <1 | <1 | <1 | <1 | |||
| Vaccinees | 521 | 1b | 1b | <1 | 10 | ||
| 560 | 102b | 10b | 10b | 103b | |||
| 575 | 102b | 102b | 102b | 102b | |||
| 576 | 102b | 102b | 10b | 102b | |||
| Mucosal challenge at 41 mo p.v. | |||||||
| Controls | 3076 | <1 | <1 | <1 | ND | <1 | |
| 2633 | 102a | 10 | 103 | ND | 103a | ||
| 2617 | 102a | 103 | 103 | ND | 102a | ||
| 2625 | 1a | 105 | 103 | ND | 102a | ||
| 138 | <1 | <1 | <1 | ND | |||
| Vaccinees | 521 | 1a,b | 1a,b | <1 | ND | <1 | |
| 560 | 102a,b | 10a | 10a | ND | 1b | ||
| 575 | 102b | 104b | 102b | ND | 102b | ||
| 576 | 10a,b | 102a,b | 102a,b | ND | 10a,b | ||
| Intraperitoneal challenge at 50 mo p.v. | |||||||
| Controls | 138 | 103a | 103 | 103 | ND | 102a | |
| Vaccinees | 580 | 1b | 102b | 102b | ND | 10b | |
| 590 | 10b | 10b | 10b | ND | 10b | ||
Challenge genotype.
Vaccine genotype.
ND, not done.
FIG. 4.
Outcome of intraperitoneal GL8 challenge performed 18 months p.v. in four vaccinated cats and four naive controls. (A) Plasma viremia as determined by GL8 (continuous lines)- and FL-381 (broken lines)-specific RT-TM-PCR. (B) Proviral loads as determined by GL8 (continuous lines)- and FL-381 (broken lines)-specific RT-TM-PCR. (C) ELISA antibody titers to whole FIV antigen. The titers are expressed as reciprocals of the highest serum dilution that gave optical density readings at least threefold higher than the values obtained with 10 control FIV-negative serum samples. (D) Circulating CD4+ T lymphocytes as determined by flow cytometry. See the legend to Fig. 2 for symbols for vaccinees.
In contrast, there was no indication in the vaccinees that the challenge virus was replicating or present, as determined by repeatedly negative GL8-specific RT-TM-PCR and TM-PCR assays (Fig. 4A and B), RFLP genotyping of the virus reisolated from the PBMC (Table 2), and stable CD4+-T-lymphocyte counts (Fig. 4D). Also, preexisting levels of the vaccine virus in plasma (Fig. 4A), the vaccine provirus in PBMC (Fig. 4B), and antiviral antibodies (Fig. 4C) did not change relative to prechallenge values or showed insignificant fluctuations over time. Except for cat 521, from which virus could be retrieved only from the third month p.c. onward, challenged vaccinees yielded cultures positive for FL-381 alone at all times tested, and the numbers of infectious units detected were generally lower than those found in their unvaccinated counterparts (Table 2). Altogether, these results indicated that vaccinated cats were totally protected against an intraperitoneal GL8 challenge that had readily infected all of the unvaccinated controls.
Partial protection from mucosal challenge.
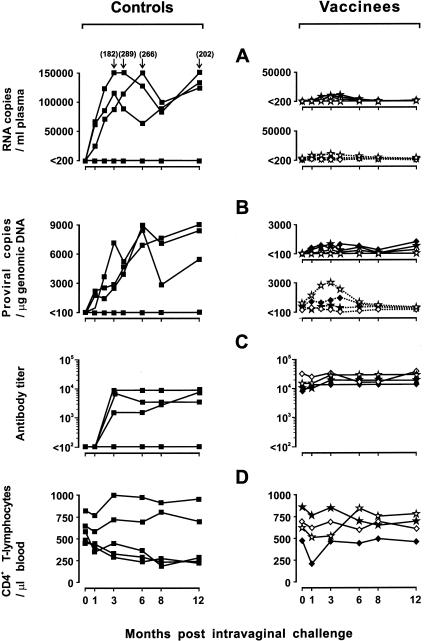
Fifteen months after the challenge described above (33 months p.v.), the vaccinees were tested for the ability to resist a mucosal challenge. GL8 was given intravaginally in the form of cell-associated virus, since previous results had shown that cell-free FIV was poorly effective at establishing infection by this route (12, 13, 48). The inoculum consisted of 2 × 104 primary blasts that had been infected 8 days earlier with GL8 and were 60% virus positive as determined by surface immunofluorescence. However, this inoculum proved weak, since only one of six matched naive control cats became clearly infected after challenge while one had low numbers of viral genomes detectable in plasma and PBMC only at 1 and 2 months p.c. Since little new information was gleaned from this first mucosal challenge (Table 2), at the end of an 8-month observation period, we rechallenged the four vaccinees and the five controls that had not become permanently infected following the first mucosal challenge using a larger intravaginal inoculum of 105 GL8-infected primary blasts. This challenge was more effective, since three control cats became clearly infected, with plasma viremias and provirus curves that were slightly delayed relative to those observed after intraperitoneal challenge but reached much higher levels, and the other parameters of infection (quantitative virus isolation, anti-FIV antibody development, and decline of CD4+-T-lymphocyte counts) also clearly indicated that the cats were infected (Fig. 5 and Table 2). Three of the four vaccinees also did not resist challenge, since they had GL8 RNA in plasma and GL8 DNA in PBMC from the first sampling performed at 1 month p.c. RFLP genotyping of the viruses reisolated from these animals confirmed that superinfection had occurred, since all positive cultures were found to contain both the vaccine virus and GL8. However, the GL8 replication curves observed in the vaccinees were much lower than in the unvaccinated cats, demonstrating a remarkable containment of the challenge virus (Fig. 5 and Table 2). Furthermore, antiviral antibody titers did not increase p.c. (Fig. 5C), and CD4+-T-lymphocyte counts remained essentially unchanged throughout the observation period, except in cat 560, which showed a transient, albeit sharp, decline at 1 month p.c. (Fig. 5D). Notably, the vaccine virus loads also were unchanged in these animals, except for a marginal and transient increase observed in cat 575 in the early months p.c. Collectively, these findings showed that the attenuated vaccine had failed to afford complete protection against mucosal challenge with GL8 but had nevertheless strongly restrained both its replication and its negative consequences for circulating CD4+-T-lymphocyte counts.
FIG. 5.
Outcome of intravaginal GL8 challenge performed 41 months p.v. in the four vaccinated cats shown in Fig. 4 and four naive controls. See the legend to Fig. 4 for details.
Complete protection from intraperitoneal challenge is long-lived.
In the experiments described above, the vaccinees proved to be fully protected against intraperitoneal challenge given 18 months p.v. but only partially protected against mucosal challenge given at 41 months p.v. To appraise whether this difference was due to the different mode of challenge delivery or the longer time elapsed after vaccination, at 50 months p.v. the two vaccinees, 580 and 590, that had never been challenged before were inoculated intraperitoneally with cell-free GL8, exactly as in the intraperitoneal-challenge experiment described above. The unvaccinated cat 138, which had not become infected in the previous experiments, was also similarly challenged and used as a control. While the last cat readily became infected, with indices of infection similar to the ones observed after the previous intraperitoneal challenge, the two vaccinees showed no changes in their preexisting virological and immunological situations and proved constantly GL8 negative over 12 months of observation (data not shown and Table 2). Thus, the ability of the vaccinees to completely resist an intraperitoneal challenge with GL8 had not waned with time p.v.
DISCUSSION
This study set out to investigate the residual pathogenicity for its natural host species of a long-term in vitro-grown FIV and the ability of the virus to protect against a virulent and highly heterologous virus of the same clade administered systemically or mucosally. The vaccine virus, FL-381, was Pet (clade A) produced by chronically infected FL4 cells that had undergone 381 passages, corresponding to >3 years, in culture. FL4 cells were established 12 years ago by Yamamoto and her group (75) and have since been distributed to many laboratories. Long-term propagation in vitro is the classic method for producing attenuated live virus vaccines, and although newer approaches show promise (8, 17, 29, 38, 44, 53, 65), it is still widely used for developing contemporary vaccines (5, 46). The method also proved successful at attenuating FIV, as demonstrated by the present study and previous findings (6, 59). Notably, the virus produced by FL4 cells was found to revert to broad neutralization resistance (7) and to reacquire a partially virulent phenotype following prolonged readaptation in vivo and subsequent reinoculation into naive cats (31). Here, however, the low-grade infections established by FL-381 were characterized by a complete absence of detectable pathological effects for at least 4 years, despite the reasonably well-preserved replication capacity of the virus. The latter may have been an important feature in determining the considerable protective activity demonstrated in cats by FL-381. Findings showing that simian immunodeficiency virus mutants with markedly reduced replicative efficiencies are poorly protective have in fact indicated that a substantial preservation of replication capacity is essential for attenuated antilentiviral vaccines to be potently effective (19, 20, 35, 45).
The challenge virus, GL8, was from clade A, like the vaccine virus, but diverged from it by 10% at the amino acid level in Env, thus showing an extent of divergence among the widest so far detected between FIV isolates of the same clade (4). Importantly, this challenge virus had been passed only a limited number of times in vitro and had proved extremely difficult or impossible to protect against in previous experiments (30). The importance of testing vaccinal protection with virulent lentiviruses has been discussed previously (52, 73). Indeed, when inoculated into naive cats, GL8 also proved highly virulent in our hands, as revealed by the florid replication curves and by the rapid decline in circulating CD4+ T lymphocytes observed. In our experience, a reduction in CD4+ T cells is the most sensitive index for assessing the severity of FIV infection in cats, particularly in short-term studies (47, 59).
The vaccinees were first challenged 18 months p.v., when FL-381 replication appeared to be steady state. At this time, FL-381 loads in plasma and PBMC were generally low, although only occasionally below the detection thresholds, and circulating CD4+ T lymphocytes were within the normal range. The first challenge was administered intraperitoneally to investigate how vaccinated cats dealt with virus delivered systemically, and the result was complete protection. In fact, throughout the 15-month follow-up, challenged cats were free from GL8, as determined by all the exhaustive tests carried out. These included sensitive assays for GL8 RNA and DNA detection and strain discrimination of reisolated virus. Consistent with these findings, challenged vaccinees also showed no changes in preexisting total viremia levels and lymphocyte subset counts that might be suggestive of a superinfection with virulent FIV. Notably, in a further experiment, cats challenged intraperitoneally 50 months p.v. also proved to be completely protected, thus showing that the resistance against systemic challenge afforded by the attenuated vaccine was not only robust but also long-lived.
In nature, FIV transmission mainly occurs through bite wounds, although other routes, such as mucosal transmission, are also possible (58). However, sexual transmission is of the utmost importance for HIV, and thus, vaginal or rectal challenges are commonly used to test vaccinal protection in FIV (23), as well as in other animal models (16, 40, 50, 71). Therefore, we examined whether the vaccinees that had resisted intraperitoneal challenge could also resist an intravaginal challenge. Because cell-free FIV had previously proved poorly effective at initiating infection when delivered by this route (12, 48), in this case the challenge consisted of in vitro-infected primary blasts. Infected cells are believed to represent an important element in the transmission of lentiviruses through mucosal surfaces (25, 28, 66), and in a previous study, cats immunized with fixed FIV-infected cells and challenged systemically proved to be protected for a longer period against infected cells than against cell-free virus (47). Consistent with previous findings with a clade B strain of FIV (48), we encountered some difficulty in infecting cats by the vaginal route: a first inoculum of 2 × 104 infected cells produced overt infections in only one of six unvaccinated control cats, and a subsequent inoculum of 105 cells failed to infect two of five such cats. However, all control cats that became infected by these mucosal inocula exhibited infections that were slightly delayed yet more robust relative to infections following intraperitoneal inoculation of cell-free virus. This was revealed by persistently very high viral loads and profound falls in circulating CD4+ T lymphocytes and was consistent with previous reports showing that the route of exposure, as well as cell association of the inoculum, significantly affects the kinetics of early lentiviral infections (13, 14, 32). Furthermore, vaccination did not consistently confer sterilizing immunity against this type of challenge, since GL8 was readily detected in three of four vaccinees starting from the first sampling performed at 1 month p.c. This could reflect the different form of challenge, the unequal virus dosage inherent therein, or the fact that the challenge virus expanded locally before spreading systemically (14, 57), thus possibly eluding vaccinal immunity more effectively than following intraperitoneal challenge. However, the vaccinees exerted substantial control of the challenge virus even when delivered intravaginally, as evidenced by much lower GL8 loads than in the unvaccinated controls and by substantially stable circulating CD4+-T-lymphocyte counts. Although one vaccinee showed a decline in CD4+ T lymphocytes at 1 month p.c., this effect was, in fact, extremely transient.
Notably, the challenge virus was found to persist in intravaginally challenged vaccinated cats for at least 1 year. This is consistent with findings in macaques showing that the protection achieved by a live attenuated vaccine did not permit the eradication of the challenge lentivirus once it had become established in the host (39). Interestingly, the beneficial effects of the attenuated vaccine tested here occurred in spite of the fact that the relatively high titers of NA detected in vaccinated cats were specific for the vaccine virus but were essentially ineffective against the heterologous challenge virus. This finding suggests that NA may have played a role in containing the vaccine virus but were unimportant in the control of challenge virus. In a recent exhaustive study by Abel et al. (1), protection against pathogenic simian immunodeficiency virus observed in simian-human immunodeficiency virus-infected macaques was attributed to a combination of cytotoxic T lymphocytes and alpha interferon responses. In the present study, analysis of the cell-mediated immune reactivity of vaccinees was limited to testing the lymphoproliferative responses to whole FIV antigen, which were found to be very modest throughout the observation period. Identification of the mechanisms responsible for the protection afforded by the attenuated FIV vaccine tested here will therefore need a much finer dissection of the numerous innate and adaptive defense mechanisms that might be involved.
Recently, superinfections have generated alarm due to increased numbers of mixed infections with two or more virus strains—even of different clades—in HIV-1 patients (3, 36, 62) and consequent increased disease severity (10, 43). Moreover, the occurrence of superinfections has cast doubts on the feasibility of vaccinating against AIDS, especially with live vaccines (27, 37). By showing that cats infected with an attenuated FIV were completely and durably protected against infection by a virulent and highly heterologous virus of the same clade administered systemically and against disease when the same challenge virus was given mucosally, the present results suggest that severe superinfections are a significant issue only when the preexisting virus is fully virulent and capable of compromising the host's immune system. In contrast, viruses that are still capable of significant replication but unable to impact heavily on immune system functions appear to be prophylactically valuable and capable of either preventing intraclade superinfections or minimizing their pathological consequences, depending on the route of exposure. It is also noteworthy that in previous cat infection studies, the same strain of FIV cultured for fewer passages in vitro than described here was also found to exert significant long-term beneficial effects against superinfection by fully virulent FIV of a different clade (59). Thus, together with similar results obtained in monkey models (1, 2, 15, 17, 22, 33-35, 40, 51, 55, 61, 65, 67, 74), these findings should provide impulse to the development of attenuated FIV vaccines. Should the FIV strain used to vaccinate in the present study also prove safe and protective in neonatal and juvenile specific-pathogen-free kittens (70), it could, for example, be tested in controlled studies in field cats, similar to the one already carried out with a fixed infected-cell vaccine (49). In addition to permitting an assessment of safety and efficacy in natural settings, the results may provide answers to some of the existing safety concerns surrounding attenuated AIDS vaccines in humans (11, 26), as well as grounds for establishing the molecular bases of the attenuation obtained by prolonged in vitro propagation.
Acknowledgments
This work was supported by the Ministero della Salute-Istituto Superiore di Sanità, “Programma per l'AIDS,” and the Ministero dell'Istruzione, dell'Università e della Ricerca, Programma Cofinanziamento 2001.
REFERENCES
- 1.Abel, K., L. Compton, T. Rourke, D. Montefiori, D. Lu, K. Rothaeusler, L. Fritts, K. Bost, and C. J. Miller. 2003. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T lymphocytes and alpha interferon responses. J. Virol. 77:3099-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almond, N., and J. Stott. 1999. Live attenuated SIV—a model of a vaccine for AIDS. Immunol. Lett. 66:167-170. [DOI] [PubMed] [Google Scholar]
- 3.Altfeld, M., T. M. Allen, X. G. Yu, M. N. Johnston, D. Agrawal, B. T. Korber, D. C. Montefiori, D. H. O'Connor, B. T. Davis, P. K. Lee, E. L. Maier, J. Harlow, P. J. Goulder, C. Brander, E. S. Rosenberg, and B. D. Walker. 2002. HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature 420:434-439. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann, M. H., D. L. Sodora, M. Matthiason-Dubard, G. H. Learn, A. G. Rodrigo, P. Mazzetti, E. A. Hoover, and J. I. Mullins. 1997. Genetic diversity of feline immunodeficiency virus: dual infection, recombination and distinct evolutionary rates between envelope sequence clades. J. Virol. 71:4241-4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badgett, M. R., A. Auer, L. E. Carmichael, C. R. Parrish, and J. J. Bull. 2002. Evolutionary dynamics of viral attenuation. J. Virol. 76:10524-10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barlough, J. E., T. W. North, C. L. Oxford, K. M. Remington, S. Dandekar, M. N. Ellis, and N. C. Pedersen. 1993. Feline immunodeficiency virus infection of cats as a model to test the effect of certain selection pressures on the infectivity and virulence of resultant lentivirus variants. Antivir. Res. 22:259-272. [DOI] [PubMed] [Google Scholar]
- 7.Bendinelli, M., M. Pistello, D. Del Mauro, G. Cammarota, F. Maggi, A. Leonildi, S. Giannecchini, C. Bergamini, and D. Matteucci. 2001. During readaptation in vivo, a tissue culture-adapted strain of feline immunodeficiency virus reverts to broad neutralization resistance at different times in individual hosts but through changes at the same position of the surface glycoprotein. J. Virol. 75:4584-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berkhout, B., K. Verhoef, G. Marzio, B. Klaver, M. Vink, X. Zhou, and A. T. Das. 2002. Conditional virus replication as an approach to a safe live attenuated human immunodeficiency virus vaccine. J. Neurovirol. 8:134-137. [DOI] [PubMed] [Google Scholar]
- 9.Bishop, S. A., C. R. Stokes, T. J. Gruffydd-Jones, C. V. Whiting, J. E. Humphries, R. Osborne, M. Papanastasopoulou, and D. A. Harbour. 1996. Vaccination with fixed feline immunodeficiency virus (FIV) infected cells: protection, breakthrough and specificity of response. Vaccine 14:1243-1250. [DOI] [PubMed] [Google Scholar]
- 10.Blackard, J. T., D. E. Cohen, and K. H. Mayer. 2002. Human immunodeficiency virus superinfection and recombination: current state of knowledge and potential clinical consequences. Clin. Infect. Dis. 34:1108-1114. [DOI] [PubMed] [Google Scholar]
- 11.Blower, S. M., K. Koelle, D. E. Kirschner, and J. Mills. 2001. Live attenuated HIV vaccines: predicting the tradeoff between efficacy and safety. Proc. Natl. Acad. Sci. USA 98:3618-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burkhard, M. J., L. A. Obert, L. L. O'Neil, L. J. Diehl, and E. A. Hoover. 1997. Mucosal transmission of cell-associated and cell-free feline immunodeficiency virus. AIDS Res. Hum. Retrovir. 13:347-355. [DOI] [PubMed] [Google Scholar]
- 13.Burkhard, M. J., C. K. Mathiason, K. O'Halloran, and E. A. Hoover. 2002. Kinetics of early FIV infection in cats exposed via the vaginal versus intravenous route. AIDS Res. Hum. Retrovir. 18:217-226. [DOI] [PubMed] [Google Scholar]
- 14.Butterworth, J. L., R. V. English, H. L. Jordan, and M. B. Tompkins. 2001. Distribution of immune cells in the female reproductive tract in uninfected and FIV infected cats. Vet. Immunol. Immunopathol. 83:37-51. [DOI] [PubMed] [Google Scholar]
- 15.Connor, R., D. Montefiori, J. Binley, J. Moore, S. Bonhoeffer, A. Gettie, E. Fenamore, K. Sheridan, D. Ho, P. Dailey, and P. Marx. 1998. Temporal analyses of virus replication, immune responses, and efficacy in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J. Virol. 72:7501-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cranage, M. P., A. M. Whatmore, S. A. Sharpe, N. Cook, N. Polyanskaya, S. Leech, J. D. Smith, E. W. Rud, M. J. Dennis, and G. A. Hall. 1997. Macaques infected with live attenuated SIVmac are protected against superinfection via the rectal mucosa. Virology 229:143-154. [DOI] [PubMed] [Google Scholar]
- 17.Daniel, M. D., F. Kirchhoff, S. C. Czajak, P. K. Sehgal, and R. C. Desrosiers. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 258:1938-1941. [DOI] [PubMed] [Google Scholar]
- 18.Del Mauro, D., D. Matteucci, S. Giannecchini, F. Maggi, M. Pistello, and M. Bendinelli. 1998. Autologous and heterologous neutralization analyses of primary feline immunodeficiency virus isolates. J. Virol. 72:2199-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denesvre, C., R. Le Grand, F. Boissin-Cans, L. Chakrabarti, B. Hurtrel, B. Vaslin, D. Dormont, and P. Sonigo. 1995. Highly attenuated SIVmac142 is immunogenic but does not protect against SIVmac251 challenge. AIDS Res. Hum. Retrovir. 11:1397-1406. [DOI] [PubMed] [Google Scholar]
- 20.Desrosiers, R. C., J. D. Lifson, J. S. Gibbs, S. C. Czajak, A. Y. Howe, L. O. Arthur, and R. P. Johnson. 1998. Identification of highly attenuated mutants of simian immunodeficiency virus. J. Virol. 72:1431-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elder, J. H., G. A. Dean, E. A. Hoover, J. A. Hoxie, M. H. Malim, L. Mathes, J. C. Neil, T. W. North, E. E. Sparger, M. B. Tompkins, W. A. Tompkins, J. Yamamoto, N. Yuhki, N. C. Pedersen, and R. H. Miller. 1998. Lessons from the cat: feline immunodeficiency virus as a tool to develop intervention strategies against human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 14:797-801. [DOI] [PubMed] [Google Scholar]
- 22.Enose, Y., M. Ui, A. Miyake, H. Suzuki, H. Uesaka, T. Kuwata, J. Kunisawa, H. Kiyono, H. Takahashi, T. Miura, and M. Hayami. 2002. Protection by intranasal immunization of a nef-deleted, nonpathogenic SHIV against intravaginal challenge with a heterologous pathogenic SHIV. Virology 298:306-316. [DOI] [PubMed] [Google Scholar]
- 23.Finerty, S., C. R. Stokes, T. J. Gruffydd-Jones, T. J. Hillman, N. A. Reeves, C. V. Whiting, W. M. Schaaper, K. Dalsgaard, and D. A. Harbour. 2000. Mucosal immunization with experimental feline immunodeficiency virus (FIV) vaccines induces both antibody and T cell responses but does not protect against rectal FIV challenge. Vaccine 18:3254-3265. [DOI] [PubMed] [Google Scholar]
- 24.Finerty, S., C. R. Stokes, T. J. Gruffydd-Jones, T. J. Hillman, F. J. Barr, and D. A. Harbour. 2001. Targeted lymph node immunization can protect cats from a mucosal challenge with feline immunodeficiency virus. Vaccine 20:49-58. [DOI] [PubMed] [Google Scholar]
- 25.Fultz, P. N., Q. Wei, and L. Yue. 1999. Rectal transmission of human immunodeficiency virus type 1 to chimpanzees. J. Infect. Dis. 179:418-421. [DOI] [PubMed] [Google Scholar]
- 26.Goto, Y., Y. Nishimura, K. Baba, T. Mizuno, Y. Endo, K. Masuda, K. Ohno, and H. Tsujimoto. 2002. Association of plasma viral RNA load with prognosis in cats naturally infected with feline immunodeficiency virus. J. Virol. 76:10079-10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goulder, P. J. R., and B. D. Walker. 2002. HIV-1 superinfection—a word of caution. N. Engl. J. Med. 347:756-758. [DOI] [PubMed] [Google Scholar]
- 28.Gupta, P., K. B. Collins, D. Ratner, S. Watkins, G. J. Naus, D. V. Landers, and B. K. Patterson. 2002. Memory CD4(+) T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J. Virol. 76:9868-9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosie, M. J., J. N. Flynn, M. A. Rigby, C. Cannon, T. Dunsford, N. A. Mackay, D. Argyle, B. J. Willett, T. Miyazawa, D. E. Onions, O. Jarrett, and J. C. Neil. 1998. DNA vaccination affords significant protection against feline immunodeficiency virus infection without inducing detectable antiviral antibodies. J. Virol. 72:7310-7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosie, M. J., T. Dunsford, D. Klein, B. J. Willett, C. Cannon, R. Osborne, J. MacDonald, N. Spibey, N. Mackay, O. Jarrett, and J. C. Neil. 2000. Vaccination with inactivated virus but not viral DNA reduces virus load following challenge with a heterologous and virulent isolate of feline immunodeficiency virus. J. Virol. 74:9403-9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosie, M. J., B. J. Willett, D. Klein, T. H. Dunsford, C. Cannon, M. Shimojima, J. C. Neil, and O. Jarrett. 2002. Evolution of replication efficiency following infection with a molecularly cloned feline immunodeficiency virus of low virulence. J. Virol. 76:6062-6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ignatius, R., K. Tenner-Racz, D. Messmer, A. Gettie, J. Blanchard, A. Luckay, C. Russo, S. Smith, P. A. Marx, R. M. Steinman, P. Racz, and M. Pope. 2002. Increased macrophage infection upon subcutaneous inoculation of rhesus macaques with simian immunodeficiency virus-loaded dendritic cells or T cells but not with cell-free virus. J. Virol. 76:9787-9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson, R. P. 2002. Mechanisms of protection against simian immunodeficiency virus infection. Vaccine 20:1985-1987. [DOI] [PubMed] [Google Scholar]
- 34.Johnson, R. P., and R. C. Desrosiers. 1998. Protective immunity induced by live attenuated simian immunodeficiency virus. Curr. Opin. Immunol. 10:436-443. [DOI] [PubMed] [Google Scholar]
- 35.Johnson, R. P., J. D. Lifson, S. C. Czajak, K. S. Cole, K. H. Manson, R. Glickman, J. Yang, D. C. Montefiori, R. Montelaro, M. S. Wyand, and R. C. Desrosiers. 1999. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relationship of degree of protection with level of attenuation. J. Virol. 73:4952-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jost, S., M. C. Bernard, L. Kaiser, S. Yerly, B. Hirschel, A. Samri, B. Autran, L. E. Goh, and L. Perrin. 2002. A patient with HIV-1 superinfection. N. Engl. J. Med. 347:731-736. [DOI] [PubMed] [Google Scholar]
- 37.Kahn, P. 2002. Superinfection: what does it mean for vaccines? IAVI Rep. 4:3-4. [Google Scholar]
- 38.Kent, S. J., C. J. Dale, S. Preiss, J. Mills, D. Campagna, and D. F. J. Purcell. 2001. Vaccination with attenuated simian immunodeficiency virus by DNA inoculation. J. Virol. 75:11930-11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khatissian, E., V. Monceaux, M.-C. Cumont, M.-P. Kieny, A.-M. Aubertin, and B. Hurtrel. 2001. Persistence of pathogenic challenge virus in macaques protected by simian immunodeficiency virus SIVmacΔnef. J. Virol. 75:1507-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar, A., S. Mukherjee, J. Shen, S. Buch, Z. Li, I. Adany, Z. Liu, W. Zhuge, M. Piatak, Jr., J. Lifson, H. McClure, and O. Narayan. 2002. Immunization of macaques with live simian human immunodeficiency virus (SHIV) vaccines conferred protection against AIDS induced by homologous and heterologous SHIVs and simian immunodeficiency virus. Virology 301:189-205. [DOI] [PubMed] [Google Scholar]
- 41.Letvin, N. J., D. H. Barouch, and D. C. Montefiori. 2002. Prospects for vaccine protection against HIV-1 infection and AIDS. Annu. Rev. Immunol. 20:73-99. [DOI] [PubMed] [Google Scholar]
- 42.Leutenegger, C. M., D. Klein, R. Hofman-Lehmann, C. Mislin, U. Hummel, J. Boni, F. Boretti, W. H. Guenzburg, and H. Lutz. 1999. Rapid feline immunodeficiency virus provirus quantitation by polymerase chain reaction using the TaqMan fluorogenic real-time detection system. J. Virol. Methods 78:105-116. [DOI] [PubMed] [Google Scholar]
- 43.Liu, S.-L., J. E. Mittler, D. C. Nickle, T. M. Mulvania, D. Shriner, A. G. Rodrigo, B. Kosloff, X. He, L. Corey, and J. I. Mullins. 2002. Selection for human immunodeficiency virus type 1 recombinants in a patient with rapid progression to AIDS. J. Virol. 76:10674-10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lockridge, K. M., M. Chien, G. A. Dean, K. Stefano Cole, R. C. Montelaro, P. A. Luciw, and E. E. Sparger. 2000. Protective immunity against feline immunodeficiency virus induced by inoculation with vif-deleted proviral DNA. Virology 273:67-79. [DOI] [PubMed] [Google Scholar]
- 45.Lohman, B. L., M. B. McChesney, C. J. Miller, E. McGowan, S. M. Joye, K. K. Van Rompay, E. Reay, L. Antipa, N. C. Pedersen, and M. L. Marthas. 1994. A partially attenuated simian immunodeficiency virus induces host immunity that correlates with resistance to pathogenic virus challenge. J. Virol. 68:7021-7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macadam, A. J., G. Ferguson, D. M. Stone, J. Meredith, J. W. Almond, and P. D. Minor. 2001. Live-attenuated strains of improved genetic stability. Dev. Biol. 105:179-187. [PubMed] [Google Scholar]
- 47.Matteucci, D., M. Pistello, P. Mazzetti, S. Giannecchini, D. Del Mauro, I. Lonetti, L. Zaccaro, C. Pollera, S. Specter, and M. Bendinelli. 1997. Studies of AIDS vaccination using an ex vivo feline immunodeficiency virus model: protection conferred by a fixed-cell vaccine against cell-free and cell-associated challenge differs in duration and is not easily boosted. J. Virol. 71:8368-8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matteucci, D., M. Pistello, P. Mazzetti, S. Giannecchini, P. Isola, A. Merico, A. Rizzuto, and M. Bendinelli. 2000. AIDS vaccination studies using feline immunodeficiency virus as a model: immunisation with inactivated whole virus suppresses viral replication following intravaginal challenge with infected cats. Vaccine 18:119-130. [DOI] [PubMed] [Google Scholar]
- 49.Matteucci, D., A. Poli, P. Mazzetti, S. Sozzi, F. Bonci, P. Isola, L. Zaccaro, S. Giannecchini, M. Calandrella, M. Pistello, S. Specter, and M. Bendinelli. 2000. Immunogenicity of an anti-clade B feline immunodeficiency fixed-cell virus vaccine in field cats. J. Virol. 74:10911-10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller, C. J., M. B. McChesney, X. Lü, P. J. Dailey, C. Chutkowski, D. Lu, P. Brosio, B. Roberts, and Y. Lu. 1997. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J. Virol. 71:1911-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mills, J., R. Desrosiers, E. Rud, and N. Almond. 2000. Live attenuated HIV vaccines: a proposal for further research and development. AIDS Res. Hum. Retrovir. 16:1453-1461. [DOI] [PubMed] [Google Scholar]
- 52.Mooij, P., W. M. J. M. Bogers, H. Oostermeijer, W. Koornstra, P. J. F. Ten Haaft, B. E. Verstrepen, G. Van Der Auwera, and J. L. Heeney. 2000. Evidence for viral virulence as a predominant factor limiting human immunodeficiency virus vaccine efficacy. J. Virol. 74:4017-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mori, K., Y. Yasutomi, S. Ohgimoto, T. Nakasone, S. Takamura, T. Shioda, and Y. Nagai. 2001. Quintuple deglycosylation mutant of simian immunodeficiency virus SIVmac239 in rhesus macaques: robust primary replication, tightly contained chronic infection, and elicitation of potent immunity against the parental wild-type strain. J. Virol. 75:4023-4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nabel, G. J. 2002. HIV vaccine strategies. Vaccine 20:1945-1947. [DOI] [PubMed] [Google Scholar]
- 55.Nilsson, C., B. Makitalo, R. Thorstensson, S. Norley, D. Binninger-Schinzel, M. Cranage, E. Rud, G. Biberfeld, and P. Putkonen. 1998. Live attenuated simian immunodeficiency virus (SIV)mac in macaques can induce protection against mucosal infection with SIVsm. AIDS 12:2261-2270. [DOI] [PubMed] [Google Scholar]
- 56.Norley, S., B. Beer, D. Binninger-Schinzel, T. Vogel, O. Hohn, E. Seibold, D. Radke, C. Cosma, and R. Kurth. 2002. Vaccine development using the simian immunodeficiency virus model for AIDS. Intervirology 45:267-274. [DOI] [PubMed] [Google Scholar]
- 57.Obert, L. A., and E. A. Hoover. 2002. Early pathogenesis of transmucosal feline immunodeficiency virus infection. J. Virol. 76:6311-6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pedersen, N. C., J. K. Yamamoto, T. Ishida, and H. Hansen. 1989. Feline immunodeficiency virus infection. Vet. Immunol. Immunopathol. 21:111-129. [DOI] [PubMed] [Google Scholar]
- 59.Pistello, M., D. Matteucci, G. Cammarota, P. Mazzetti, S. Giannecchini, D. Del Mauro, S. Macchi, L. Zaccaro, and M. Bendinelli. 1999. Kinetics of preinfecting and challenge virus replication during a three-year interclade feline immunodeficiency virus superinfection experiment in cats. J. Virol. 73:1518-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pistello, M., M. Moscardini, P. Mazzetti, S. Macchi, F. Bonci, P. Isola, G. Freer, D. Matteucci, S. Specter, and M. Bendinelli. 2002. Development of feline immunodeficiency virus ORF-A (tat) mutants: in vitro and in vivo characterization. Virology 298:84-95. [DOI] [PubMed] [Google Scholar]
- 61.Quesada-Rolander, M., B. Makitalo, R. Thorstensson, Y. J. Zhang, E. Castanos-Velez, G. Biberfeld, and P. Putkonen. 1996. Protection against mucosal SIVsm challenge in macaques infected with a chimeric SIV that expresses HIV type 1 envelope. AIDS Res. Hum. Retrovir. 12:993-999. [DOI] [PubMed] [Google Scholar]
- 62.Ramos, A., D. J. Hu, L. Nguyen, K.-O. Phan, S. Vanichseni, N. Promadej, K. Choopanya, M. Callahan, N. L. Young, J. McNicholl, T. D. Mastro, T. M. Folks, and S. Subbarao. 2002. Intersubtype human immunodeficiency virus type 1 superinfection following seroconversion to primary infection in two injection drug users. J. Virol. 76:7444-7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reed, L. J., and H. A. Muench. 1938. A simple method for estimating fifty percent end points. Am. J. Hyg. 27:493-497. [Google Scholar]
- 64.Robinson, H. L. 2002. New hope for an AIDS vaccine. Nat. Rev. Immunol. 2:239-250. [DOI] [PubMed] [Google Scholar]
- 65.Shacklett, B. L., K. E. S. Shaw, L. A. Adamson, D. T. Wilkens, C. A. Cox, D. C. Montefiori, M. B. Gardner, P. Sonigo, and P. A. Luciw. 2002. Live, attenuated simian immunodeficiency virus SIVmac-M4, with point mutations in the env transmembrane protein intracytoplasmic domain, provides partial protection from mucosal challenge with pathogenic SIVmac251. J. Virol. 76:11363-11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sodora, D. L., A. Gettie, C. J. Miller, and P. A. Marx. 1998. Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res. Hum. Retrovir. 14:S119-S123. [PubMed] [Google Scholar]
- 67.Stebbings, R. J., N. M. Almond, E. J. Stott, N. Berry, A. M. Wade-Evans, R. Hull, J. Lines, P. Silvera, R. Sangster, T. Corcoran, J. Rose, and K. B. Walker. 2002. Mechanisms of protection induced by attenuated simian immunodeficiency virus. Virology 296:338-353. [DOI] [PubMed] [Google Scholar]
- 68.Uhl, E. W., T. G. Heaton-Jones, R. Pu, and J. K. Yamamoto. 2002. FIV vaccine development and its importance to veterinary and human medicine: a review. FIV vaccine 2002 update and review. Vet. Immunol. Immunopathol. 90:113-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.United Nations Program on HIV/AIDS-World Health Organization. 2002. AIDS epidemic update. December 2001. United Nations Program on HIV/AIDS-World Health Organization, Geneva, Switzerland.
- 70.Van Rompay, K. K. A., J. L. Greenier, K. Stefano Cole, P. Earl, B. Moss, J. D. Steckbeck, B. Pahar, T. Rourke, R. C. Montelaro, D. R. Canfield, R. P. Tarara, C. Miller, M. B. McChesney, and M. L. Marthas. 2003. Immunization of newborn rhesus macaques with simian immunodeficiency virus (SIV) vaccines prolongs survival after oral challenge with virulent SIVmac251. J. Virol. 77:179-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Warren, J. 2002. Preclinical AIDS vaccine research: survey of SIV, SHIV, and HIV challenge studies in vaccinated nonhuman primates. J. Med. Primatol. 31:237-256. [DOI] [PubMed] [Google Scholar]
- 72.Willett, B. J., M. J. Hosie, T. H. Dunsford, J. C. Neil, and O. Jarrett. 1991. Productive infection of T-helper lymphocytes with feline immunodeficiency virus is accompanied by reduced expression of CD4. AIDS 5:1469-1475. [DOI] [PubMed] [Google Scholar]
- 73.Willett, B. J., J. N. Flynn, and M. J. Hosie. 1997. FIV infection of the domestic cat: an animal model for AIDS. Immunol. Today 18:182-189. [DOI] [PubMed] [Google Scholar]
- 74.Wyand, M. S., K. Manson, D. C. Montefiori, J. D. Lifson, R. P. Johnson, and R. C. Desrosiers. 1999. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J. Virol. 73:8356-8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamamoto, J. K., C. D. Ackley, H. Zochlinski, H. Louie, E. Pembroke, M. Torten, H. Hansen, R. Munn, and T. Okuda. 1991. Development of interleukin-2 independent feline lymphoid cell lines chronically infected with feline immunodeficiency virus: importance for diagnostic reagents and vaccines. Intervirology 32:361-375. [DOI] [PubMed] [Google Scholar]