Abstract
Disuse uncouples bone formation from resorption, leading to increased porosity, decreased bone geometrical properties, and decreased bone mineral content which compromises bone mechanical properties and increases fracture risk. However, black bear bone properties are not adversely affected by aging despite annual periods of disuse (i.e., hibernation), which suggests that bears either prevent bone loss during disuse or lose bone and subsequently recover it at a faster rate than other animals. Here we show decreased cortical bone turnover during hibernation with balanced formation and resorption in grizzly bear femurs. Hibernating grizzly bear femurs were less porous and more mineralized, and did not demonstrate any changes in cortical bone geometry or whole bone mechanical properties compared to active grizzly bear femurs. The activation frequency of intracortical remodeling was 75% lower during hibernation than during periods of physical activity, but the normalized mineral apposition rate was unchanged. These data indicate bone turnover decreases during hibernation, but osteons continue to refill at normal rates. There were no changes in regional variation of porosity, geometry, or remodeling indices in femurs from hibernating bears, indicating that hibernation did not preferentially affect one region of the cortex. Thus, grizzly bears prevent bone loss during disuse by decreasing bone turnover and maintaining balanced formation and resorption, which preserves bone structure and strength. These results support the idea that bears possess a biological mechanism to prevent disuse osteoporosis.
Keywords: cortical bone, bone remodeling, bone mechanics, grizzly bear, disuse osteoporosis
Introduction
The removal of routine bone stresses (e.g., from immobilization, inactivity, or reduced gravity) has deleterious consequences on bone integrity. Reduced skeletal loading causes net bone loss by unbalancing bone formation and bone resorption [1, 2]. Rat hindlimb immobilization, human spaceflight, and human bedrest can all cause increased bone resorption and decreased bone formation [2-4], whereas forelimb immobilization in dogs caused an unbalanced increase in bone resorption over bone formation [5]. Long-term human bedrest decreased bone mineral density (up to -9.2%) in skeletal weight-bearing locations [6], and decreased the mineral apposition rate in the iliac crest by 34% [7]. Similarly, two weeks of immobilization from casting decreased (-28%) the osteonal mineral apposition rate in the femoral diaphysis of monkeys, and caused a trend of fewer osteons undergoing formation (relative to the total number of osteons) during immobilization [8]. Following 8 weeks of disuse, there was a 1.4 fold increase in the density of remodeling sites in turkey ulnae, and the ratio of formation foci to resorption foci decreased by 68% [9]. It is clear from these studies that disuse uncouples bone formation from resorption.
Disuse-induced changes in cortical bone remodeling lead to detrimental alterations in the microstructural, geometrical, and mechanical properties of bones. At the microstructural level, four weeks of immobilization in turkey ulnae led to a 5-fold increase in porous area compared to controls [10], and both spinal cord injury and spaceflight decreased femoral bone mineral content in humans [11, 12]. Geometrically, hindlimb suspension and spinal cord injury decreased the cross-sectional moment of inertia of the femur [12, 13], and twelve months of canine forelimb immobilization decreased the polar moment of inertia of the second metacarpal by 70% [5]. These disuse-induced changes in bone decrease the mechanical properties of whole bones. For example, immobilizing canine forelimbs for 16 weeks decreased (-12 to -29%) cortical bone mechanical properties [14], and 12 months of canine forelimb immobilization decreased the ultimate bending load of the second metacarpal by 80% [5]. Spaceflights lasting 4-6 months also decreased femoral indices of bone strength [11]. Considered together, these disuse-induced changes in bones (i.e., increased porosity, decreased mineralization, cross-sectional properties, and mechanical properties) explain the increased rates of bone fracture in chronic disuse conditions such as spinal cord injury and stroke [15, 16].
When lost bone can be completely recovered following disuse, the required remobilization time is 2-3 times longer than the length of the disuse period because the rate of bone loss during inactivity is 2-3 times greater than the rate of bone recovery during remobilization [14, 17, 18]. However, bears in northern climates experience annual periods of disuse (hibernation) and activity that are approximately equal in length (6 months), yet black bear (Ursus americanus) cortical bone does not suffer any detrimental mechanical or structural consequences with age despite these relatively short recovery periods [19-22]. Black bear trabecular bone architecture and bone volume also appear to be preserved during hibernation [23-25]. These findings suggest that bears have evolved a unique biological mechanism to mitigate disuse-induced bone loss. Bears may prevent bone loss during hibernation by maintaining balanced bone remodeling [24, 26].
It is not known if bears prevent disuse-associated cortical bone loss or if they experience similar losses, but make faster recoveries than other animals. To answer this question, we examined cortical bone geometry, whole bone mechanical properties, ash fraction, microstructure, and remodeling dynamics of grizzly bear (Ursus arctos horribilis) femurs from bears killed during periods of hibernation and physical activity. We hypothesized that hibernating bears do not lose cortical bone strength because bone remodeling remains balanced, which preserves bone geometry and mineral content.
Materials and methods
All handling and treatment procedures were approved by the Washington State University Institutional Animal Care and Use Committee. Eight age-matched grizzly bears were used for the study; four bears were 1 year old (3 male, 1 female), and four bears were 17-20 years old (2 male, 2 female) (Table 1). Exact pairings by age and sex were not available for all bears, but deviations were anticipated to have minimal impact on data interpretation because body masses between the paired animals were comparable. One 1 year old active female bear was age-matched with a 1 year old hibernating male bear; gender-related differences in body mass are small in grizzly bears less than 2-4 years of age [27-30]. Additionally, one 20 year old female active bear was matched with a 17 year old hibernating female bear; female grizzly bears do not demonstrate annual increases in body weight after 13 years of age [27]. The bears were housed at the Washington State University Bear Research, Education, and Conservation Facility (Pullman, WA). At the time of death, four had hibernated for 16-18 weeks, and the other four had been active following hibernation for at least 14 weeks. The bears were administered an IV solution of calcein at 5 mg/kg body mass twice prior to death; injections were given 9 to 11 days apart, and 5 to 8 days passed after the second label was administered before animals were sacrificed (i.e., labeling schedules ranged from 1-9-1:5 to 1-11-1:8). The bears were euthanized by an injection of pentobarbital (10 mls/100 lbs body weight). After sacrifice, the hind legs were removed from each bear, cleaned of soft tissue, and frozen at -20° C. One randomly chosen femur from each bear was used for histology, and the contralateral femur was used for mechanical testing, geometrical measurements, and ashing (Figure 1). Only the left femur was available from one of the 1 year old hibernating bears, so this femur was histologically prepared after mechanical testing.
Table 1.
Bears in the same row were paired for all statistical analyses comparing active and hibernating bears.
| Active bear age / sex | Hibernating bear age / sex |
|---|---|
| 1 year / M | 1 year / M |
| 1 year / F | 1 year / M |
| 18 years / M | 18 years / M |
| 20 years / F | 17 years / F |
Figure 1.

One femur was used for mechanical testing (A) and processed post-fracture to determine cross-sectional properties (B) and ash fraction (C). Mechanically tested femurs fractured at midshaft below the point of load application. The contralateral femur was used for histological analyses of static and dynamic remodeling indices and quantification of intracortical porosity (D).
Geometrical and Mechanical Properties
Femurs were loaded to failure in three-point bending with the anterior side in tension on an Instru-met / Sintech 20 KIP computer controlled system (Instru-met, Union, NJ) using MTS Testworks software (MTS, Eden Prairie, MN) (Figure 1A). The span between the lower supports was adjusted to metaphyseal bony landmarks in order to assure the same relative testing conditions for bones of different sizes [31]. After fracture, the bones were reconstructed with cyanoacrylate and a 15 mm cortical bone segment centered about the fracture was removed from the diaphysis (Figure 1B). This segment was embedded in methyl methacrylate and sectioned on a diamond saw to expose the face at midshaft. Images of the cross-section were captured with a digital camera (SPOT Insight QE, Diagnostic Instruments, Sterling Heights, MI). Periosteal area (Ps.Ar), cortical area (Ct.Ar), endosteal area (Es.Ar), and endosteal area fraction (Es.Ar/Ps.Ar) were quantified with image analysis software (Scion Image, Frederick, MD). Cortical thickness (Ct.Th) was measured in 1 mm intervals for each anatomical quadrant and for the entire cross-section (Bioquant Osteo, Nashville, TN). The mediolateral, anteroposterior, and maximum moments of inertia (IML, IAP, and Imax, respectively), were quantified with a custom macro as described previously [21]. Section modulus (SM) was calculated as the mediolateral moment of inertia divided by one-half of the outer (i.e., periosteal surface) anteroposterior diameter. Elastic modulus (E), ultimate stress (σU), energy to failure (Uf), and modulus of toughness (u) were calculated from the load-displacement data and cross-sectional properties with beam bending theory [31].
Ash Fraction
Following the bending test, a 10 mm segment of the diaphysis located 7.5 mm proximal to the fracture (Figure 1C) was removed, cleaned of marrow, and used for ashing. The bone sections were placed in a 100 °C furnace for 24 hours, weighed (dry mass), then placed in a 600 °C furnace for 48 hours and weighed again (ash mass). The ash fraction was calculated as the ash mass divided by the dry mass.
Static and Dynamic Histomorphometry
A 30 mm long segment of the diaphysis was removed 20 mm distal to the midshaft of the femur for static and dynamic histomorphometry (Figure 1D). Each bone segment was fixed in 70% ethanol and embedded in methyl methacrylate. Thin sections (approximately 30 μm thick for dynamic sections and 70 μm thick for static sections) were cut with a diamond saw. All histomorphometric measurements were performed using a digital camera (SPOT Insight QE, Diagnostic Instruments, Sterling Heights, MI), microscope (Leitz LABORLUX S, Leica Microsystems, Inc., Bannockburn, IL), and image analysis software (Bioquant Osteo, Nashville, TN). Measurements were made by anatomical quadrant as well as for the entire cross-section in order to assess regional variation in each property.
For porosity quantification, thin sections were stained in four increasing ethanol concentrations of 1% basic fuchsin stain (70-100% ethanol) for 30 seconds each, rinsed in 100% ethanol for 30 seconds, and mounted on a glass slide. This staining protocol provided good contrast between the bone and porous areas. Intracortical porosity (Por), which included all porous spaces except osteocyte lacunae and canaliculi, was quantified at 40x total magnification. For quantification of remodeling cavities, thin sections were stained in Villanueva osteochrome bone stain for 48 hours, differentiated with 0.01% acetic acid in 95% methanol, cleared in xylene, and mounted on glass slides. Porous cavities were judged to be refilling if the osteoid seam thickness was ≥ 3 μm [32]. Resorption cavities were identified as porous spaces with a scalloped border without a surrounding cement line. Both refilling and resorption cavities were quantified at 100x magnification, and refilling and resorption cavity densities (Rf.Ca.Dn and Rp.Ca.Dn) were calculated as the respective number of cavities divided by cortical area. Completed secondary osteons were identified by a cement line with a non-remodeling central cavity. Haversian canal radius (H.Rd) was measured at 200x total magnification for a maximum of 50 randomly selected completed osteons from each quadrant.
The thin sections for dynamic histomorphometry were mounted unstained on glass slides. Calcein labels were viewed with an excitation wavelength of 480 ± 15 nm and an emission wavelength of 535 ± 20 nm (31001 FITC filter block, Chroma Technology Corp., Rockingham, VT) with the same microscope and digital camera used for the static slides. The total number of labeled osteons (single, double, or partially double-labeled) was quantified at 250x total magnification, and labeled osteon density (L.On.Dn) was calculated by dividing the number of labeled osteons by cortical area. Next, a maximum of 50 double labeled osteons were randomly selected from each quadrant for further morphological analysis. Inter-label width (Ir.L.Wi) was measured at 400x total magnification in 5 μm intervals between the label midpoints of these double-labeled osteons, and the osteonal mineral apposition rate (On.MAR) was calculated. The radii of the outer calcein label (Ro), inner calcein label (Ri), and cement line (Cm.Rd) were quantified for each double labeled osteon by measuring the perimeter of each feature and calculating the radius by assuming circularity (Figure 2). The decay constant α (which characterizes the non-linear refilling rate of osteons), was calculated for each osteon using the outer and inner label radii [33]. The percentage of the osteon left to be refilled (%W.Wi.UF) was calculated using the outer label, cement line, and Haversian canal radii using Equation 1 [33]:
Figure 2.

The distance between calcein labels (Ir.L.Wi) was quantified for double labeled osteons and was used to calculate the osteonal mineral apposition rate (On.MAR) by dividing Ir.L.Wi by the time between calcein injections. The percentage of the osteon left to be refilled (%W.Wi.UF) was calculated using the outer calcein label (Ro), cement line (Cm.Rd), and Haversian canal radii. The osteonal mineral apposition rate was normalized (On.MARN) by %W.Wi.UF to account for the non-linear refilling rate of osteons.
| (1) |
Biological changes in osteonal mineral apposition rates can be obscured since the rate of mineral apposition naturally slows as a forming osteon nears completion. Therefore, the osteonal mineral apposition rate was normalized (On.MARN) by %W.Wi.UF to account for the non-linear refilling rate of osteons in order to observe any biological changes in remodeling rates caused by hibernation. Activation frequency (Ac.f) and filling period (FP) were calculated using Equations 2 and 3, respectively [33, 34]:
| (2) |
| (3) |
Statistics
Paired t-tests (pairing the hibernating to active bears based on age and sex as described in Table 1) were conducted to look for differences in each bone property between hibernating and active bears. Cortical thickness, porosity and bone remodeling parameters were compared between anatomical quadrants using one-factor ANOVA and two-factor ANOVA with interaction (factor 1: activity state (hibernating or active), factor 2: anatomical quadrant) to determine whether hibernation (like other forms of disuse) induced localized changes in the femoral cortex [35].
Results
Mechanical, Geometrical, and Mineral Properties
Hibernation did not compromise cortical bone geometry; there were no (p > 0.374) differences in cortical thickness, moments of inertia, section modulus, periosteal area, or cortical area between active and hibernating bears, and there was no evidence of marrow cavity expansion (Table 3). Furthermore, cortical thickness was not different between anatomical quadrants for active (p = 0.940) or hibernating (p = 0.907) bears, suggesting that hibernation did not preferentially affect cortical geometry in one region of the cross-section.
Table 3.
Geometrical and mechanical properties were not different between hibernating and active bears, but ash fraction was significantly increased during hibernation. Means with standard deviations in parentheses are presented. See Table 2 for explanations of abbreviations.
| Property | Active | Hibernating | p-value |
|---|---|---|---|
| Ps.Ar (mm2) | 608 (432) | 589 (339) | 0.704 |
| Ct.Ar (mm2) | 412 (315) | 403 (256) | 0.786 |
| Es.Ar (mm2) | 196 (117) | 186 (85) | 0.638 |
| Es.Ar/Ps.Ar (%) | 35 (5) | 34 (6) | 0.643 |
| Ct.Th (mm) | 5.4 (2.6) | 5.4 (2.2) | 0.856 |
| IML (mm4) | 32,213 (39,061) | 26,205 (27,776) | 0.374 |
| IAP (mm4) | 43,142 (51,856) | 38,949 (41,829) | 0.466 |
| Imax (mm4) | 43,406 (51,990) | 39,035 (41,842) | 0.455 |
| SM (mm3) | 2,097 (2117) | 1,833 (1,539) | 0.439 |
|
| |||
| E (GPa) | 9.8 (2.5) | 11.4 (3.0) | 0.241 |
| σU (MPa) | 189 (16) | 202 (31) | 0.227 |
| U (J) | 52 (32) | 49 (42) | 0.676 |
| u (J / mm3) | 7.4 (3.9) | 5.0 (1.4) | 0.259 |
|
| |||
| Ash fraction | 0.671 (0.019) | 0.683 (0.018) | 0.001 |
Hibernation also did not compromise whole bone mechanical properties or mineralization. There were no differences (p > 0.227) in elastic modulus, ultimate stress, energy to failure, or modulus of toughness between active and hibernating grizzly bears (Table 3), and interestingly, the ash fraction of hibernating grizzly bear femurs was greater (p = 0.001) than active grizzly bear femurs (Table 3).
Static and Dynamic Histomorphometry
Cortical bone microstructure was preserved in the hibernating bear femurs; intracortical porosity was 30% lower (p = 0.0008) in the hibernating bears (Table 4), and porosity was not different between anatomical quadrants for active (p = 0.545) or hibernating (p = 0.449) bears. Cortical bone turnover decreased during hibernation; labeled osteon density was 64% lower (p = 0.031) and activation frequency was 75% lower (p = 0.032) in the hibernating compared to active bears. Few resorption cavities were observed in the juvenile bears, and therefore resorption cavity density was only quantified for the adult bears. Refilling and resorption cavity density also showed a decreasing trend during hibernation; refilling cavity density was 55% lower (p = 0.065) and resorption cavity density was 68% lower in the hibernating bears (p = 0.108) (Table 4). Osteonal mineral apposition rate was 36% lower (p = 0.015) in the hibernating bears, but the normalized osteonal mineral apposition rate and filling period were not different (Table 4). These data indicate that fewer osteons are actively remodeling during hibernation, and active osteons refill (form bone) at a normal rate.
Table 4.
Whole cross-section averages for microstructural and remodeling properties indicate decreased turnover with balanced formation and resorption in hibernating grizzly bear femurs. Means with standard deviations in parentheses are presented. See Table 2 for explanations of abbreviations.
| Property | Active | Hibernating | p-value |
|---|---|---|---|
| Por (%) | 7.6 (0.5) | 5.3 (0.4) | 0.0008 |
| Rf.Ca.Dn (#/mm2) | 0.9 (0.2) | 0.4 (0.2) | 0.065 |
| Rp.Ca.Dn (#/mm2) | 0.3 (0.02) | 0.1 (0.03) | 0.108 |
| L.On.Dn (#/mm2) | 7.0 (3.1) | 2.5 (0.8) | 0.031 |
| On.MAR (μm/day) | 1.1 (0.1) | 0.7 (0.1) | 0.015 |
| On.MARN (μm/day) | 3.4 (1.1) | 3.3 (1.5) | 0.911 |
| Ac.f (#/mm2/year) | 24.6 (11.9) | 6.2 (3.3) | 0.032 |
| FP (days) | 59.7 (16.5) | 81.0 (17.6) | 0.131 |
Resorption cavity density in the active bears was the only cortical bone remodeling parameter that varied by quadrant (p = 0.043); the highest values were found in the anterior quadrant, followed by the posterior and medial quadrants, with the lowest values in the lateral quadrant (Figure 3). There were no differences between quadrants in any other remodeling parameters for hibernating or active bears (p > 0.184).
Figure 3.
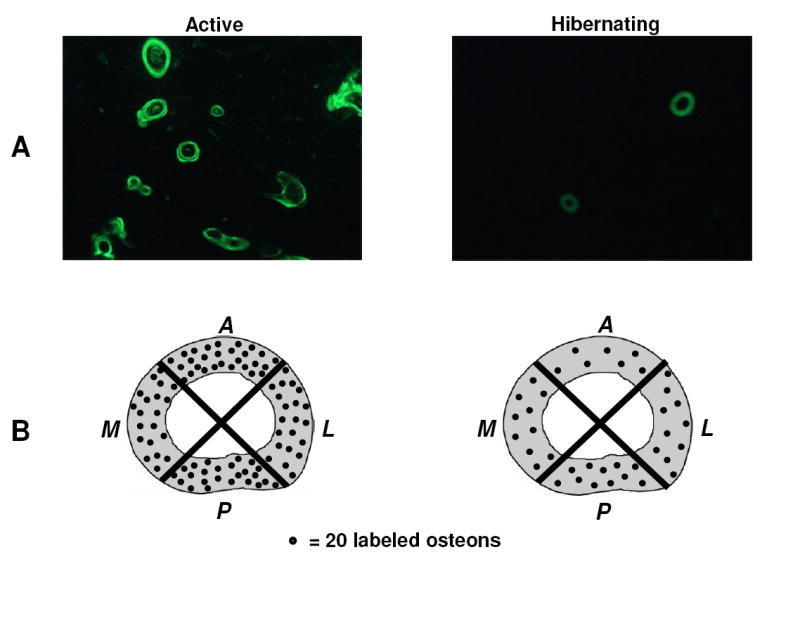
A: Labeled osteon density was decreased (p = 0.031) in hibernating compared to active bears (40x original magnification). B: There were no differences in labeled osteon density (p > 0.198) between quadrants in either hibernating or active bears.
In summary, hibernating grizzly bear femurs were less porous and more mineralized, and did not demonstrate losses of whole bone mechanical properties or geometry compared to active bear femurs. Hibernation decreases cortical bone turnover, preserves balanced formation and resorption, and does not preferentially affect localized regions of the bone cortex.
Discussion
Disuse uncouples bone formation from resorption [2, 3], leading to increased porosity, decreased bone geometrical properties, and decreased bone mineral content which compromises bone mechanical properties and increases fracture risk [5, 10, 14-16, 36] (Figure 5A, B). Spaceflight decreases trabecular indices of bone formation in the iliac crest of monkeys [37, 38], and human astronauts experience an imbalance in bone resorption and bone formation during spaceflight [2, 39], which probably causes the cortical bone loss that is observed after 4-6 months in space [40, 41]. Similarly, patients with acute spinal cord injury (< 6 months post-injury) experience increased bone resorption and increased urinary calcium excretion, which likely explains why spinal cord injury patients lose bone structural properties and demonstrate increased rates of bone fracture [12, 16, 42, 43]. In contrast, we showed decreased turnover with balanced formation and resorption in hibernating grizzly bear femurs (Table 4) which reduced porosity and maintained bone geometrical and mechanical properties during hibernation (Tables 3 and 4, Figure 5C, D). These findings are in agreement with a previous study which showed that cross-sectional area of the humerus is not different in pre- and post-hibernation black bears [23]. Furthermore, age-related changes in black bear cortical bone are not deleterious despite the fact that black bears are inactive for approximately 6 months annually [19, 21, 22, 44]. Taken together, these results suggest that bears maintain skeletal integrity with age because they do not lose cortical bone during hibernation.
Figure 5.
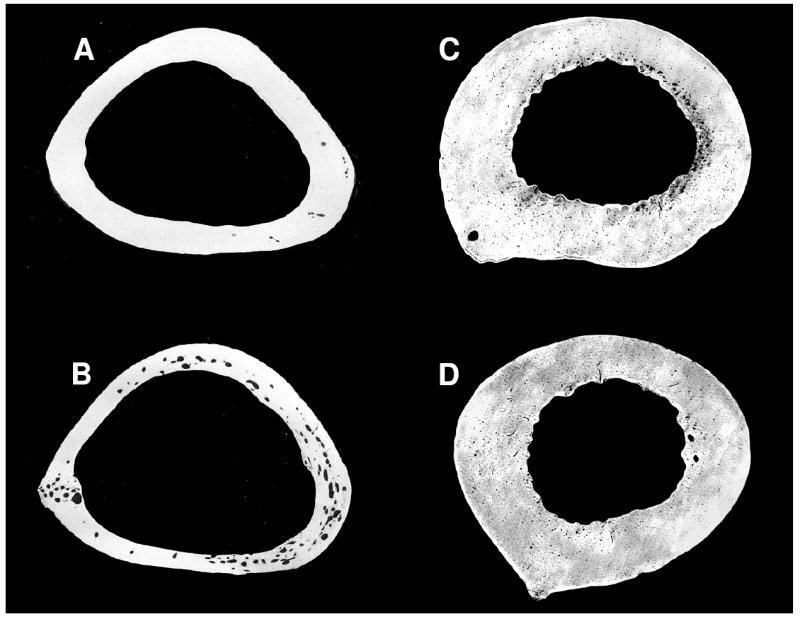
Bone geometry and intracortical porosity do not respond similarly to disuse in turkeys (a, b) and grizzly bears (c, d). A: control turkey ulna, B: turkey ulna immobilized for 8 weeks, C: active grizzly bear femur, D: grizzly bear femur after 17 weeks of hibernation. Large intracortical pores and thinning of the bone cortex, which occur during disuse in the turkey ulna, are not seen in the hibernating grizzly bear femur. In contrast, grizzly bear bone becomes less porous during physical inactivity. Turkey ulnae images are reproduced from: J Biomechanics 17(12), Lanyon, L. E. & Rubin, C. T., “Static vs dynamic loads as an influence on bone remodeling,” pp. 897-905, copyright Elsevier (1984).
Decreased bone formation occurs in other animal models of disuse, but it is frequently accompanied by increased bone resorption which leads to bone loss via increased intracortical porosity, increased endosteal expansion, or a combination of these changes [8, 9, 36]. Though bone formation indices (labeled osteon density, refilling cavity density) declined in hibernating grizzly bears, porosity and resorption cavity density were decreased (Table 4) and endosteal area did not change (Table 3). Resorption and refilling cavity densities were decreased by approximately the same amount (-68% and -55%, respectively) in the hibernating bears (Table 4), suggesting that the two processes remained in balance. Normalized mineral apposition rate was unchanged between active and hibernating bears. Therefore, the current study suggests that hibernation caused decreased turnover with balanced formation and resorption in grizzly bears which prevented bone loss. This is in contrast to the bone loss which occurs due to unbalanced relative increases in resorption over formation during limb immobilization in dogs [5], and increased resorption and decreased formation associated with spaceflight in humans and limb immobilization in rats [2, 3]. The decrease in cortical bone remodeling during hibernation likely explains why ash fraction was significantly greater in the hibernating bears (Table 3), since older bone is more mineralized than newly remodeled bone.
Disuse can cause detrimental changes in bone structure in localized regions of the diaphyseal cortex. For example, in immobilized turkey radii, more than half (+58%) of the disuse-induced increase in porosity occurred within the ventral/caudal quadrant of the cortex [35]. Similarly, hindlimb suspension decreased the cortical thickness of the anterior quadrant (but not other quadrants) in the femur of hindlimb suspended rats which lowered whole bone strength [45]. In contrast, there were no significant differences in porosity or cortical thickness between anatomical quadrants for hibernating or active grizzly bears, suggesting that hibernation did not cause localized bone structural changes in a focused region of the femoral cortex. Most remodeling indices also showed no variation between quadrants in either hibernating or active bears.
Serum markers of bone turnover suggest that bone remodeling is increased, but balanced, in black bears during hibernation [26], which is in contrast to the decreased cortical bone remodeling observed in the current study. Both serum carboxy-terminal telopeptide of type-I collagen (ICTP, a bone resorption marker) and serum osteocalcin (a bone formation marker) are elevated in hibernating bears [26]. At present, this discrepancy cannot be resolved. Serum markers of bone turnover represent global skeletal remodeling, and thus could reflect bone turnover from another skeletal location (e.g., trabecular bone); previous work suggests that trabecular remodeling indices (e.g., osteoclast surface, mineralizing surface, bone formation rate) in the ilium are increased, but balanced, in hibernating compared to active bears [24]. Alternatively, the increase in serum remodeling markers during hibernation could reflect serum marker accumulation due to decreased renal activity in hibernating bears even if bone remodeling is unchanged or decreased. Hibernation is a mechanism to survive prolonged periods of famine when food is scarce; metabolic energy is conserved by reducing many homeostatic processes (e.g., heart rate is reduced). Decreased bone remodeling would be beneficial from an energy conservation perspective. Despite the discrepancy in the observed magnitudes of remodeling, the prior serum marker studies in bears and the current histological work all suggest bears maintain balanced bone resorption and formation during hibernation, which likely explains how bears are able to maintain homeostatic serum calcium levels since bears do not intake or excrete calcium during hibernation [24].
The mechanism by which bears prevent bone loss during hibernation is unknown, but previous work suggests the involvement of parathyroid hormone (PTH) [26]. PTH is the primary regulator of blood calcium levels, and therefore likely plays a major role in maintaining homeostatic serum calcium levels in bears during hibernation. Serum levels of osteocalcin (a marker of bone formation) and PTH are elevated and positively correlated in hibernating bears [26]. However, previous measurements of PTH levels in bears were made with an assay that detects both intact (PTH 1-84) and large C-terminal fragments of the hormone (C-PTH). C-PTH fragments are known to accumulate in humans and animals during renal failure since they cannot be cleared by the kidneys [46, 47], and may contribute to the development of adynamic (low turnover) bone disease in these subjects [48]. Bears do not urinate during hibernation, and therefore may experience a similar phenomenon. Large C-PTH fragments, acting through a CPTH receptor found on osteoblasts and osteocytes, have an anti-resorptive effect in vitro and a hypocalcaemic effect in vivo [49-51]. A possible mechanism for how PTH could explain the cortical bone remodeling observed in the current study is shown in Figure 6. Intact PTH (PTH 1-84), acting through the PTH1 receptor on osteocytes and osteoblasts, could prevent the increased apoptosis and decreased bone formation rates normally associated with disuse [3, 52, 53]. Prevention of osteocyte apoptosis would prevent increases in targeted remodeling. Concomitantly, large C-terminal PTH fragments (C-PTH) may bind to CPTH receptors on osteoblasts and decrease osteoclastogenesis via decreased production of RANK ligand (RANKL) and/or increased production of osteoprotegrin (OPG) [49]. This would also decrease bone resorption, indicated by the decreased resorption cavity density and activation frequency in hibernating bears (Table 4). Bone formation would continue at normal rates on active surfaces until they neared completion (indicated by the lack of change in normalized mineral apposition rate during hibernation (Table 4)). Bone formation indices would then decrease in balance with bone resorption indices as active surfaces were completed, resulting in lower values for refilling cavity and labeled osteon densities (Table 4). The result of decreased, balanced turnover during hibernation would be decreased porosity (Table 4), increased mineral (Table 3), and the preservation of bone geometry and strength (Table 3).
Figure 6.

Proposed mechanism for how parathyroid hormone (PTH) could decrease cortical bone remodeling in hibernating grizzly bears.
The present study provides further support for the idea that bears possess a unique regulatory mechanism that prevents bone loss during disuse. Further research into this mechanism may result in the development of improved treatments (e.g., novel PTH peptides) for osteoporosis. For example, bear PTH 1-34 promotes greater anti-apoptotic gene expression in osteoblasts than human PTH 1-34 [54].
Figure 4.

Resorption cavity density for the active bears was the only remodeling parameter that varied significantly by quadrant. Quadrants with the same letter are not significantly different from each other (p > 0.05). Means with SE bars are presented.
Table 2.
List of all geometrical, mechanical, and histomorphometry variables, their abbreviations, and units
| Property | Abbreviation | Units |
|---|---|---|
| periosteal area | Ps.Ar | mm2 |
| cortical area | Ct.Ar | mm2 |
| endosteal area | Es.Ar | mm2 |
| endosteal area fraction | Es.Ar/Ps.Ar | % |
| cortical thickness | Ct.Th | mm |
| mediolateral moment of inertia | IML | mm4 |
| anteroposterior moment of inertia | IAP | mm4 |
| maximum moment of inertia | Imax | mm4 |
| section modulus | SM | mm3 |
| whole bone elastic modulus | E | GPa |
| ultimate stress | σU | MPa |
| energy to failure | U | J |
| modulus of toughness | u | J / mm3 |
| porosity | Por | % |
| refilling cavity density | Rf.Ca.Dn | #/mm2 |
| resorption cavity density | Rp.Ca.Dn | #/mm2 |
| labeled osteon density | L.On.Dn | #/mm2 |
| osteonal mineral apposition rate | On.MAR | μm/day |
| normalized osteonal mineral apposition rate | On.MARN | μm/day |
| activation frequency | Ac.f | #/mm2/year |
| filling period | FP | days |
Acknowledgments
This publication was made possible by Grant Number AR050420 from NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Additional funding was received from the National Science Foundation Graduate Research Fellowship Program, Michigan Space Grant Consortium, Michigan Technological University Department of Educational Opportunity, and Timothy Floyd, M.D. The authors thank Dr. David Burr for his advice on calcein labeling procedures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Takata S, Yasui N. Disuse osteoporosis. J Med Invest. 2001;48:147–56. [PubMed] [Google Scholar]
- 2.Caillot-Augusseau A, Lafage-Proust MH, Soler C, Pernod J, Dubois F, Alexandre C. Bone formation and resorption biological markers in cosmonauts during and after a 180-day space flight (Euromir 95) Clin Chem. 1998;44:578–85. [PubMed] [Google Scholar]
- 3.Weinreb M, Rodan GA, Thompson DD. Osteopenia in the immobilized rat hind limb is associated with increased bone resorption and decreased bone formation. Bone. 1989;10:187–94. doi: 10.1016/8756-3282(89)90052-5. [DOI] [PubMed] [Google Scholar]
- 4.Zerwekh JE, Ruml LA, Gottschalk F, Pak CY. The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. J Bone Miner Res. 1998;13:1594–601. doi: 10.1359/jbmr.1998.13.10.1594. [DOI] [PubMed] [Google Scholar]
- 5.Li CY, Price C, Delisser K, Nasser P, Laudier D, Clement M, Jepsen KJ, Schaffler MB. Long-term disuse osteoporosis seems less sensitive to bisphosphonate treatment than other osteoporosis. J Bone Miner Res. 2005;20:117–24. doi: 10.1359/JBMR.041010. [DOI] [PubMed] [Google Scholar]
- 6.Shackelford LC, LeBlanc AD, Driscoll TB, Evans HJ, Rianon NJ, Smith SM, Spector E, Feeback DL, Lai D. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol. 2004;97:119–29. doi: 10.1152/japplphysiol.00741.2003. [DOI] [PubMed] [Google Scholar]
- 7.Vico L, Chappard D, Alexandre C, Palle S, Minaire P, Riffat G, Morukov B, Rakhmanov S. Effects of a 120 day period of bed-rest on bone mass and bone cell activities in man: attempts at countermeasure. Bone Miner. 1987;2:383–94. [PubMed] [Google Scholar]
- 8.Wronski TJ, Morey ER. Inhibition of cortical and trabecular bone formation in the long bones of immobilized monkeys. Clin Orthop Relat Res. 1983:269–76. [PubMed] [Google Scholar]
- 9.Bain SD, Rubin CT. Metabolic modulation of disuse osteopenia: endocrine-dependent site specificity of bone remodeling. J Bone Miner Res. 1990;5:1069–75. doi: 10.1002/jbmr.5650051011. [DOI] [PubMed] [Google Scholar]
- 10.Rubin C, Gross T, Qin YX, Fritton S, Guilak F, McLeod K. Differentiation of the bone-tissue remodeling response to axial and torsional loading in the turkey ulna. J Bone Joint Surg Am. 1996;78:1523–33. doi: 10.2106/00004623-199610000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Lang TF, Leblanc AD, Evans HJ, Lu Y. Adaptation of the proximal femur to skeletal reloading after long-duration spaceflight. J Bone Miner Res. 2006;21:1224–30. doi: 10.1359/jbmr.060509. [DOI] [PubMed] [Google Scholar]
- 12.Modlesky CM, Slade JM, Bickel CS, Meyer RA, Dudley GA. Deteriorated geometric structure and strength of the midfemur in men with complete spinal cord injury. Bone. 2005;36:331–9. doi: 10.1016/j.bone.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Garber MA, McDowell DL, Hutton WC. Bone loss during simulated weightlessness: a biomechanical and mineralization study in the rat model. Aviat Space Environ Med. 2000;71:586–92. [PubMed] [Google Scholar]
- 14.Kaneps AJ, Stover SM, Lane NE. Changes in canine cortical and cancellous bone mechanical properties following immobilization and remobilization with exercise. Bone. 1997;21:419–23. doi: 10.1016/s8756-3282(97)00167-1. [DOI] [PubMed] [Google Scholar]
- 15.Kanis J, Oden A, Johnell O. Acute and long-term increase in fracture risk after hospitalization for stroke. Stroke. 2001;32:702–6. doi: 10.1161/01.str.32.3.702. [DOI] [PubMed] [Google Scholar]
- 16.Vestergaard P, Krogh K, Rejnmark L, Mosekilde L. Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord. 1998;36:790–6. doi: 10.1038/sj.sc.3100648. [DOI] [PubMed] [Google Scholar]
- 17.Weinreb M, Patael H, Preisler O, Ben-Shemen S. Short-term healing kinetics of cortical and cancellous bone osteopenia induced by unloading during the reloading period in young rats. Virchows Arch. 1997;431:449–52. doi: 10.1007/s004280050122. [DOI] [PubMed] [Google Scholar]
- 18.Leblanc AD, Schneider VS, Evans HJ, Engelbretson DA, Krebs JM. Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res. 1990;5:843–50. doi: 10.1002/jbmr.5650050807. [DOI] [PubMed] [Google Scholar]
- 19.Harvey KB, Donahue SW. Bending properties, porosity, and ash fraction of black bear (Ursus americanus) cortical bone are not compromised with aging despite annual periods of disuse. J Biomech. 2004;37:1513–20. doi: 10.1016/j.jbiomech.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Donahue SW, McGee ME, Harvey KB, Vaughan MR, Robbins CT. Hibernating bears as a model for preventing disuse osteoporosis. J Biomech. 2006;39:1480–1488. doi: 10.1016/j.jbiomech.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 21.McGee ME, Magic KW, Miller DL, Maki AJ, Donahue SW. Black bear femoral porosity decreases and mechanical properties increase with age despite annual periods of disuse (hibernation) Eng Frac Mech. 2007;74:1942–1952. [Google Scholar]
- 22.McGee ME, Miller DL, Auger J, Black HL, Donahue SW. Black bear femoral geometry and cortical porosity are not adversely affected by ageing despite annual periods of disuse (hibernation) J Anat. 2007;210:160–9. doi: 10.1111/j.1469-7580.2006.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pardy CK, Wohl GR, Ukrainetz PJ, Sawers A, Boyd SK, Zernicke RF. Maintenance of bone mass and architecture in denning black bears (Ursus americanus) J Zool Lond. 2004;263:359–364. [Google Scholar]
- 24.Floyd T, Nelson RA, Wynne GF. Calcium and bone metabolic homeostasis in active and denning black bears (Ursus americanus) Clin Orthop. 1990;8:301–9. [PubMed] [Google Scholar]
- 25.McGee ME, Castillo AB, Nelson OL, Robbins CT, Donahue SW. 52nd Annual Meeting of the Orthopaedic Research Society. Chicago, IL: Orthopaedic Research Society; 2006. The effects of disuse (hibernation) on trabecular architecture and mineral density in grizzly bear (Ursus arctos horribilis) femurs; p. Poster #1609. [Google Scholar]
- 26.Donahue SW, Galley SA, Vaughan MR, Patterson-Buckendahl P, Demers LM, Vance JL, McGee ME. Parathyroid hormone may maintain bone formation in hibernating black bears (Ursus americanus) to prevent disuse osteoporosis. J Exp Biol. 2006;209:1630–8. doi: 10.1242/jeb.02185. [DOI] [PubMed] [Google Scholar]
- 27.Blanchard BM. Size and growth patterns of the Yellowstone grizzly bear. International Conference on Bear Research and Management; 1987. pp. 99–107. [Google Scholar]
- 28.Troyer WA, Hensel RJ. U.S. Department of the Interior, Bureau of Sport Fish and Wildlife, Branch of Wildlife Refuges. 1969. The brown bear of Kodiak Island. [Google Scholar]
- 29.Pearson AM. The northern grizzly bear Ursus arctos L (Report Series No. 34) Ottawa: Canadian Wildlife Service; 1975. [Google Scholar]
- 30.Schwartz CC, Miller SD, Haroldson MA. Grizzly Bear. In: Feldhamer GA, Thompson BC, Chapman JA, editors. Wild Mammals of North America: Biology, Management, and Conservation. Baltimore: Johns Hopkins University Press; 2003. pp. 556–586. [Google Scholar]
- 31.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 32.Hori M, Takahashi H, Konno T, Inoue J, Haba T. A classification of in vivo bone labels after double labeling in canine bones. Bone. 1985;6:147–54. doi: 10.1016/8756-3282(85)90047-x. [DOI] [PubMed] [Google Scholar]
- 33.Metz LN, Martin RB, Turner AS. Histomorphometric analysis of the effects of osteocyte density on osteonal morphology and remodeling. Bone. 2003;33:753–9. doi: 10.1016/s8756-3282(03)00245-x. [DOI] [PubMed] [Google Scholar]
- 34.Vajda EG, Kneissel M, Muggenburg B, Miller SC. Increased intracortical bone remodeling during lactation in beagle dogs. Biol Reprod. 1999;61:1439–44. doi: 10.1095/biolreprod61.6.1439. [DOI] [PubMed] [Google Scholar]
- 35.Gross TS, Rubin CT. Uniformity of resorptive bone loss induced by disuse. J Orthop Res. 1995;13:708–14. doi: 10.1002/jor.1100130510. [DOI] [PubMed] [Google Scholar]
- 36.Jaworski ZF, Liskova-Kiar M, Uhthoff HK. Effect of long-term immobilisation on the pattern of bone loss in older dogs. J Bone Joint Surg Br. 1980;62-B:104–10. doi: 10.1302/0301-620X.62B1.6985912. [DOI] [PubMed] [Google Scholar]
- 37.Zerath E, Grynpas M, Holy X, Viso M, Patterson-Buckendahl P, Marie PJ. Spaceflight affects bone formation in rhesus monkeys: a histological and cell culture study. J Appl Physiol. 2002;93:1047–56. doi: 10.1152/japplphysiol.00610.2001. [DOI] [PubMed] [Google Scholar]
- 38.Zerath E, Novikov V, Leblanc A, Bakulin A, Oganov V, Grynpas M. Effects of spaceflight on bone mineralization in the rhesus monkey. J Appl Physiol. 1996;81:194–200. doi: 10.1152/jappl.1996.81.1.194. [DOI] [PubMed] [Google Scholar]
- 39.Smith SM, Wastney ME, O’Brien KO, Morukov BV, Larina IM, Abrams SA, Davis-Street JE, Oganov V, Shackelford LC. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the mir space station. J Bone Miner Res. 2005;20:208–18. doi: 10.1359/JBMR.041105. [DOI] [PubMed] [Google Scholar]
- 40.Keyak J, Koyama A, LeBlanc A, Lu Y, Lang T. 53rd Annual Meeting of the Orthopaedic Research Society. San Diego, CA: Orthopaedic Research Society; 2007. Reduction in proximal femoral strength after long-duration spaceflight; p. 15. [Google Scholar]
- 41.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–12. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 42.Maimoun L, Couret I, Micallef JP, Peruchon E, Mariano-Goulart D, Rossi M, Leroux JL, Ohanna F. Use of bone biochemical markers with dual-energy x-ray absorptiometry for early determination of bone loss in persons with spinal cord injury. Metabolism. 2002;51:958–63. doi: 10.1053/meta.2002.34013. [DOI] [PubMed] [Google Scholar]
- 43.de Bruin ED, Herzog R, Rozendal RH, Michel D, Stussi E. Estimation of geometric properties of cortical bone in spinal cord injury. Arch Phys Med Rehabil. 2000;81:150–6. [PubMed] [Google Scholar]
- 44.Harvey KB, Drummer TD, Donahue SW. The tensile strength of black bear (Ursus americanus) cortical bone is not compromised with aging despite annual periods of hibernation. J Biomech. 2005;38:2143–50. doi: 10.1016/j.jbiomech.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Shaw SR, Zernicke RF, Vailas AC, DeLuna D, Thomason DB, Baldwin KM. Mechanical, morphological and biochemical adaptations of bone and muscle to hindlimb suspension and exercise. J Biomech. 1987;20:225–34. doi: 10.1016/0021-9290(87)90289-2. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen-Yamamoto L, Rousseau L, Brossard JH, Lepage R, Gao P, Cantor T, D’Amour P. Origin of parathyroid hormone (PTH) fragments detected by intact-PTH assays. Eur J Endocrinol. 2002;147:123–31. doi: 10.1530/eje.0.1470123. [DOI] [PubMed] [Google Scholar]
- 47.Brossard JH, Cloutier M, Roy L, Lepage R, Gascon-Barre M, D’Amour P. Accumulation of a non-(1-84) molecular form of parathyroid hormone (PTH) detected by intact PTH assay in renal failure: importance in the interpretation of PTH values. J Clin Endocrinol Metab. 1996;81:3923–9. doi: 10.1210/jcem.81.11.8923839. [DOI] [PubMed] [Google Scholar]
- 48.Monier-Faugere MC, Geng Z, Mawad H, Friedler RM, Gao P, Cantor TL, Malluche HH. Improved assessment of bone turnover by the PTH-(1-84)/ large C-PTH fragments ratio in ESRD patients. Kidney Int. 2001;60:1460–8. doi: 10.1046/j.1523-1755.2001.00949.x. [DOI] [PubMed] [Google Scholar]
- 49.Divieti P, John MR, Juppner H, Bringhurst FR. Human PTH-(7-84) inhibits bone resorption in vitro via actions independent of the type 1 PTH/PTHrP receptor. Endocrinology. 2002;143:171–6. doi: 10.1210/endo.143.1.8575. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen-Yamamoto L, Rousseau L, Brossard JH, Lepage R, D’Amour P. Synthetic carboxyl-terminal fragments of parathyroid hormone (PTH) decrease ionized calcium concentration in rats by acting on a receptor different from the PTH/PTH-related peptide receptor. Endocrinology. 2001;142:1386–92. doi: 10.1210/endo.142.4.8093. [DOI] [PubMed] [Google Scholar]
- 51.Langub MC, Monier-Faugere MC, Wang G, Williams JP, Koszewski NJ, Malluche HH. Administration of PTH-(7-84) antagonizes the effects of PTH-(1-84) on bone in rats with moderate renal failure. Endocrinology. 2003;144:1135–8. doi: 10.1210/en.2002-221026. [DOI] [PubMed] [Google Scholar]
- 52.Dufour C, Holy X, Marie PJ. Skeletal unloading induces osteoblast apoptosis and targets alpha5beta1-PI3K-Bcl-2 signaling in rat bone. Exp Cell Res. 2007;313:394–403. doi: 10.1016/j.yexcr.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 53.Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, Bellido T. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res. 2006;21:605–15. doi: 10.1359/jbmr.060107. [DOI] [PubMed] [Google Scholar]
- 54.McGee ME, Galley SA, Nelsen MP, Tsai CJ, Donahue SW. 53rd Annual Meeting of the Orthopaedic Research Society. San Diego, CA: Orthopaedic Research Society; 2007. Synthetic black bear (Ursus americanus) PTH 1-34 upregulates c-fos and decreases the ratio of Bax/Bcl-2 in MC-3T3 osteoblastic cells; p. Poster #1289. [Google Scholar]


