Abstract
The human immunodeficiency virus type 1 (HIV-1) integrase (IN) protein augments the initiation of reverse transcription. Chimeric HIV-1 containing HIV-2 IN (SG3IN2) is severely impaired in virus infectivity and DNA synthesis. To analyze the nature of this defect, we infected T cells with the chimeric SG3IN2 virus and by continuous passage in cell culture selected for virus with improved replication properties. Viruses from two different time points were chosen for further analysis, an early culture-adapted virus (CF-65) that exhibited an intermediate level of infectivity, and a later-passaged virus (CF-131) that was significantly more infectious. Sequence analysis of multiple clones derived from the CF-65 virus culture demonstrated a diversity of mutations in the reverse transcriptase (RT) and a common V204I IN mutation. Analysis of clones derived from the CF-131 virus indicated the selection of two additional IN mutations, Q96H and K127E, and a fixed V179I RT mutation. By cloning RT and/or IN sequences back into the original SG3IN2 chimeric virus, we demonstrated that mutations in both RT and IN contributed to the improvement in viral fitness. The effect of the HIV-2IN(IN2) mutations on virus DNA synthesis was analyzed by packaging IN2 mutants into HIV-1 as Vpr-IN2 fusion proteins. This analysis revealed that the Q96H, K127E and V204I mutations increased the infectivity of the chimeric virus by augmenting the initiation of viral cDNA synthesis in infected cells. The Q96H and K127E mutations are present in adjacent helical structures on the surface of the IN protein and together account for most of the increase observed in DNA synthesis. Our findings provide evidence that the IN protein augments the initiation of reverse transcription through specific interactions with other viral components comprising the initiation complex. Moreover, they implicate specific regions on the surface of IN that may help to elucidate mechanisms by which the HIV-1 IN protein augments the initiation of HIV-1 reverse transcription in vivo.
Human immunodeficiency virus type 1 (HIV-1) encodes protease (PR), reverse transcriptase (RT), and integrase (IN) as parts of a large Gag-Pol precursor polyprotein (Pr160Gag-Pol). Pr160Gag-Pol plays an important role in virion assembly and is essential for the formation of infectious virions (for a review, see reference 11). Mutagenesis of the C-terminal region of Pr160Gag-Pol (RT and IN domains) has been associated with defects in virion assembly, release, maturation, and protein composition (2, 4, 7, 8, 26, 31). Consequently, these defective viruses may appear to be impaired in early steps of the virus life cycle, such as uncoating and viral DNA synthesis.
Molecular genetic analysis of IN has revealed pleiotropic effects of mutations among different retroviruses. Mutation of nonconserved amino acids within the IN gene of Ty3 (a retrovirus-like element of Saccharomyces cerevisiae) affects multiple stages of the retrotransposition life cycle, including RT and IN expression, 3′-end DNA processing, and nuclear entry (23). These mutations also appear to reduce the level of replicated DNA despite normal levels of exogenous RT activity and capsid maturation (23). Certain point mutations or linker insertion mutations in the Moloney murine leukemia virus (MLV) IN domain impair virion production and proteolytic processing of Gag and Pol (27-29). Similarly, certain HIV-1 IN mutations can cause defects in virion assembly, production, maturation, and nuclear import of the preintegration complex (2-5, 7, 26, 31). Mutations in the HIV-1 IN coding sequence have been shown to impair viral DNA synthesis in infected cells. Mutations that inhibit translation of the entire IN protein or a small portion (22 amino acids) of its carboxy terminus reduce the amount of early viral DNA products detected by PCR, and viruses containing either point mutations in the N-terminal zinc finger or the central domain (F185) exhibit a similar phenotype (7, 8, 18, 20, 37).
Because of the pleiotropic effects of IN mutations, trans-complementation approaches have been exploited to help analyze the role of the mature IN protein in early steps of the virus life cycle. By expressing viral protein R (Vpr)-IN fusion protein (Vpr-IN) in trans with HIV-1, IN can be assembled into progeny virions via the interaction of Vpr with Gag and then liberated by the viral protease (38, 39). Studies with IN mutant viruses that exhibit a defect in DNA synthesis demonstrated efficient complementation by the trans-IN protein (37). trans-Complementation has also been used successfully to study the early IN function of Ty3 and MLV. The DNA synthesis defect of an IN mutant MLV was complemented by expressing MA-CA-IN fusion protein in trans, and the expression of a CA-RT-IN fusion protein complemented transposition and increased the amount of cDNA of some Ty3 IN mutants (16, 22). Similar to HIV-1, trans-complementation of Ty3 and MLV DNA synthesis did not require catalytic activity of the trans-IN protein.
The HIV-2 (strain ST) IN shows 58% identity with the HIV-1 (strain SG3) IN at the amino acid level. Complementation analysis with a heterologous (HIV-2) trans-IN protein (Vpr-IN2) was used to probe for virus- and integrase-specific interactions required for efficient cDNA synthesis. By complementing an IN mutant of HIV-1 that produced a wild-type level of cDNA but was defective in integration, the trans-IN2 protein was shown to integrate (55% of wild-type activity) HIV-1 provirus into the DNA of infected cells (19). This is consistent with in vitro studies that demonstrated that HIV-2 IN supports 3′-end processing and strand transfer of an oligonucleotide substrate that mimics the end of the HIV-1 viral DNA molecule (34). However, the heterologous trans-IN2 protein did not efficiently complement viral DNA synthesis, suggesting that this function requires virus type-specific interactions between the integrase protein and other components of the reverse transcription complex (19, 37).
To further analyze the effect of IN on the initiation of HIV-1 DNA synthesis, we took advantage of a chimeric HIV-1 virus (SG3IN2) in which the HIV-2 IN coding region (IN2) was inserted in place of the cognate IN. This chimeric virus exhibits a severe defect in infectivity and DNA synthesis (19). In this report, we describe the emergence, isolation, and characterization of culture-selected chimeric viruses. Genetic analysis demonstrated that the selected viruses contained mutations in various regions of the viral genome, including the RT and IN2 coding sequences. By expressing the mutated IN2 as a Vpr-IN2 fusion protein in trans with HIV-1 integrase-defective virus, we demonstrated that mutations in IN (Q96H, V204I, and K127E) significantly increased viral DNA synthesis and were principally responsible for the improved fitness of the chimeric virus. The results of this study implicate specific regions on the IN proteins that may play a role in augmenting HIV-1 DNA synthesis.
MATERIALS AND METHODS
Cells and antibodies.
The TZM-bl (previously named JC53-BL) (36), 293T, and JC53 (25) cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U of penicillin per ml, and 0.1 mg of streptomycin per ml (complete DMEM). SupT1 cells were maintained in RPMI 1640 medium supplemented with 15% FBS and 0.1 mg of gentamicin per ml. The anti-HIV-1 capsid monoclonal antibody 183-H12-5C (contributed by B. Chesebro and K. Wehrly) was obtained through the AIDS Research Reference and Reagent Program, National Institutes of Health. The HIV-2 IN protein was detected by using serum (designated 7312A) from an HIV-2-infected individual that was especially reactive to IN by immunoblot analysis.
HIV proviral clones and expression plasmids.
The HIV-1 pSG3 proviral clone (GenBank accession no. L02317) contains all of the HIV-1 genes with the exception of vpu (10). To facilitate molecular genetic analysis, unique XmaI and BamHI restriction sites were introduced near the beginning (nucleotide 2136) and end (nucleotide 3760) of the RT coding region (38). The chimeric HIV-1 pSG3IN2 proviral clone contains the HIV-2 (ST strain, GenBank accession no. M31113) IN coding sequence and a unique MluI site near the end of IN (nucleotide 4623) in addition to the unique XmaI and BamHI sites described above. The details of its construction were reported earlier (38). The pNL4-3 proviral clone (1), like pSG3, contains natural BssHII and SalI sites at nucleotide positions 258 and 5331, respectively. The chimeric HIV-1 pNL4-3IN2 clone was made by inserting the BssHII-SalI fragment from pSG3IN2 into the BssHII and SalI sites of pNL4-3. Because of the chimeric nature of some viral constructs, the nucleotide positions for all HIV-1 sequences are given as they would appear in the SG3 sequence, while all HIV-2 IN nucleotide positions are given as they would appear in the ST sequence.
pLR2P-vprIN2 expression plasmids were constructed to contain each of the IN2 mutations found in the CF-131 IN gene, either individually or in various combinations. To introduce these mutations, two subgenomic fragments of IN2 were generated by PCR. A first PCR fragment was generated with the sense (5′-AACAAATCAGAAGACTGAG-3′, nucleotides 3512 to 3530, pSG3) and the antisense (5′-CTTCCTGACTAGTGAAGTTGGCACCATT-3′, nucleotides 4429 to 4405, ST) primers. This fragment contains BamHI (nucleotide 3760) and SpeI (nucleotide 4420) restriction sites and encodes amino acids 555 of RT to 123 of IN2. Introduction of the SpeI site was accomplished by changing the TCA codon (S123) to the synonymous AGT codon by PCR with the antisense primer (underlined).
DNA was amplified from pSG3IN2 to obtain a product encoding the wild-type Q96 amino acid, while the 3A17 clone was used as the template to produce a DNA fragment encoding the Q96H mutation. The second PCR product comprised a SpeI (nucleotide 4420) to XhoI (nucleotide 4643) DNA fragment encoding amino acids 122 of IN2 to the stop codon. To produce a SpeI/XhoI DNA fragment containing K127 and V204I, the 3A17 clone was used as the template with the following PCR primers: sense (5′-AACTTCACTAGTCAGGAAGTAAAGATGGTGGCATGG-3′, nucleotides 4414 to 4449, ST) and antisense (5′-TTATCCTCGAGCTAATCCTGTCTACGCGTGTTGG-3′, nucleotides 4643 to 4625, ST). In the sense primer, the SpeI site is underlined and the codon for amino acid K127 is in boldface. The XhoI site is underlined in the antisense primer. The K127- and V204-containing fragment was generated with the same primer pair, but pSG3IN2 was used as the template.
To generate a SpeI-XhoI DNA fragment containing the K127E and V204I mutations, the following primers were used to amplify a fragment from the 3A17 clone: sense (5′-AACTTCACTAGTCAGGAAGTAGAGATGGTGGCATGG-3′, nucleotides 4414 to 4449, ST) and antisense (4643 to 4625) (described above). A DNA fragment encoding K127E was generated with the same primer pair with pSG3IN2 as the template. Ligation of the first and second DNA fragments into BamHI- and XhoI-cut pLR2P-vprIN1 generated pLR2P-vprIN2-SpeI, pLR2P-vprINQ96H, pLR2P-vprINK127E, pLR2P-vprINV204I, pLR2P-vprINQ96H, K127E, pLR2P-vprINQ96H, V204I, pLR2P-vprINK127E, V204I, and pLR2P-vprINQ96H, K127E, V204I, respectively. The mutation(s) in each of the constructs was confirmed by nucleotide sequencing. The construction of pLR2P-vprIN2 and pLR2P-vprIN1 was described earlier (19, 38).
Sequence analysis of CF-65- and CF-131-derived clones.
We sequenced 100 ng of plasmid DNA template with 24 oligonucleotide primers. Each 10-μl sequence reaction included 100 ng of plasmid template, 10 pmol of primer, 1 μl of ABI BigDye terminators version 3.0, and 1.5 μl of 5× buffer. The reactions were cycled in an MJ Tetrad with the following parameters: an initial denaturation at 96°C for 30 s; followed by 25 cycles of 96°C for 10 s, 50°C for 5 s, and 60°C for 4 min. The samples were cleaned by ethanol precipitation: 40 μl of a 62.5% ethanol-1 M potassium acetate mixture was added, and the samples were centrifuged for 45 min at 4,000 rpm at 4°C. The sequence reactions were resuspended in 15 μl of Hi-Di formamide (ABI) and separated on an ABI 3700 capillary electrophoresis machine. Sequence reads were processed through Phred, Phrap, and Consed (9). Potential variations were identified with the Polyphred version 3.4 program (21). All variations were evaluated manually to verify sequence differences.
Transfection and virus preparation.
DNA transfections were performed on 70% confluent monolayer cultures of 293T cells grown in six-well plates (Corning Inc.) by calcium phosphate DNA precipitation as described previously (14). Six micrograms of proviral DNA was transfected. When cotransfected, 4 μg of proviral DNA was used together with 6 μg of the pLR2P-vprIN expression plasmid. Supernatants from the transfected cultures were collected after 60 h, clarified by low-speed centrifugation (1,000 × g, 10 min), and analyzed for HIV-1 p24 antigen concentration by enzyme-linked immunosorbent assay (ELISA) (Beckman-Coulter Inc.).
Analysis of virus infectivity.
Virus infectivity was analyzed with the TZM-bl reporter cell line (previously named JC53-BL) as described earlier (36, 38). This cell line enables quantitative measurements of HIV-1 infection in a single cycle of infection based on activation of an integrated long terminal repeat-β-galactosidase expression cassette. Briefly, supernatants from transfected 293T or infected SupT1 cell cultures were equilibrated for p24 antigen concentration, and four fivefold serial dilutions of each virus were prepared in DMEM containing 1% FBS and 10 μg of DEAE-dextran per ml. Then 200 μl of each dilution was used to infect monolayer cultures of TZM-bl cells. After 48 h, the cell monolayers were washed, fixed, and stained as described earlier (15). Wells containing between 30 and 200 blue cell colonies were used to calculate the number of viral infectious units (IU) per nanogram of p24 antigen. Unless otherwise noted, the data depicted in each figure were derived from at least three independent experiments.
Semiquantitative analysis of viral DNA.
The assay for semiquantitative analysis of viral DNA was performed similarly to that described earlier (35, 37, 41). Briefly, 500 ng (p24 antigen equivalents) of virus derived from SupT1 cell cultures or transfected 293T cells was treated for 1 h at 37°C with RNase-free DNase (20 U/ml, 1 h) (Promega Corp.). The virus was then placed onto a monolayer culture of 106 JC53 cells for 4 h at 37°C in DMEM containing 1% FBS and 10 μg of DEAE-dextran per ml. Then the cell monolayers were washed twice with DMEM, and complete DMEM was added. The cells were lysed 18 h after infection, and then total DNA was extracted. The DNA extracts were resuspended in 200 μl of distilled water and treated with the DpnI restriction endonuclease to digest bacterially derived plasmid DNA (from transfection).
The total DNA concentration was determined by measuring optical density at 260 nm, and an equal amount of each DNA extract was subjected to 30 cycles of PCR amplification with primers designed to detect the early (R-U5) product of reverse transcription: sense, 5′-GGCTAGCTAGGGAACCCACTG-3′ (nucleotides 43 to 63) (41), and antisense, 5′-CTGCTAGAGATTTTCCACACTGAC-3′ (nucleotides 183 to 159) (37). The PCR products were separated on a 1.5% agarose gel and visualized by ethidium bromide staining. The staining intensity of each amplified DNA product was measured with a Bio-Rad GelDoc imaging system. With Quality One software (Bio-Rad, version 4.1.1), the amount of amplified DNA was determined by extrapolation against known concentrations of pSG3 proviral DNA analyzed in parallel. Note that data were obtained from at least three independent experiments, the results were reproducible, and representative data are depicted.
Immunoblot analysis.
Virions were concentrated from the supernatant of infected cells by ultracentrifugation through cushions of 20% sucrose with a Beckman SW41 rotor (125,000 × g, 2 h). Pellets were solubilized in loading buffer (62.5 mM Tris-HCl [pH 6.8], 0.2%sodium dodecyl sulfate, 5% 2-mercaptoethanol, 10% glycerol), boiled, and separated on sodium dodecyl sulfate-containing 12.5% polyacrylamide gels. Following electrophoresis, proteins were transferred to nitrocellulose (0.2-μm pore size) by electroblotting. The nitrocellulose blots were incubated for 1 h at room temperature in blocking buffer (5% nonfat dry milk in phosphate-buffered saline) and then with constant rocking for 1 h with primary antibodies. Protein-bound antibodies were detected by chemiluminescence with horseradish peroxidase-conjugated species-specific secondary antibodies (Southern Biotechnology Associates, Inc.) according to the recommendations of the manufacturer (Amersham Biosciences).
RESULTS
Selection of replication-competent SG3IN2 chimeric virus.
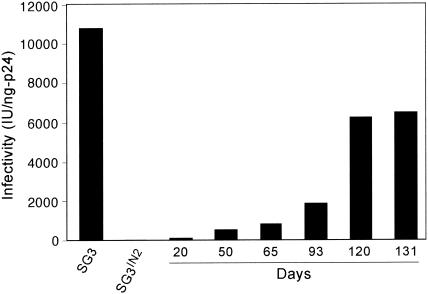
The chimeric SG3IN2 virus is severely impaired in replication, and our initial attempts to establish a productive infection in SupT1 cells with transfection-derived virus were unsuccessful. To increase infectivity, a virus stock was prepared by cotransfecting 293T cells with Vpr-IN and pSG3IN2. SupT1 cells were infected, and the cell culture was passaged every 4 to 5 days by transferring 106 cells to a new culture flask containing an equal number of uninfected SupT1 cells. After 104 days of culture, the virus was able to establish a productive infection by cell-free transmission to uninfected SupT1 cells. Subsequently, the virus was continuously cell-free passaged and periodically analyzed for infectivity with the TZM-bl reporter cell line. After 65 days of cell-free passage (CF-65), a substantial increase in virus infectivity was detected (845 infectious units per ng of p24 antigen) and on day 131 (CF-131) the virus reached 6,521 IU/ng of p24 (Fig. 1). This represented a 130-fold increase in infectivity over that of the original SG3IN2 virus and a level that was approximately 60% of that of the wild-type SG3 virus (10,812 IU/ng of p24). Continued passage of the culture for an additional 80 days did not lead to a further increase in virus infectivity. By PCR with HIV-2 IN-specific primers, we confirmed that the culture-adapted virus retained the HIV-2 IN coding region (data not shown).
FIG. 1.
Selection of SG3IN2 virus with increased infectivity. Five-hundred-nanogram equivalents (HIV-1 p24 antigen) of the SG3IN2 virus collected from SupT1 cells after 104 days of continuous culture were clarified by low-speed centrifugation, filtered through a 0.45-μm sterile filter, and used to infect 106 uninfected SupT1 cells. Every 4 to 5 days thereafter, culture supernatant was collected and filtered through a 0.45-μm sterile filter, and serial tenfold dilutions were prepared and used to challenge 106 uninfected SupT1 cells. At the time points indicated, the cultures were analyzed for HIV-1 p24 antigen concentration by ELISA (Beckman-Coulter Inc.) and virus infectivity with the TZM-bl reporter cell line as described in Materials and Methods. As a control, transfection-derived SG3IN2 and SG3 obtained from chronically infected cultures of SupT1 cells were also analyzed for p24 and infectivity. Infectivity (in infectious units [IU]) is expressed by standardization to 1 ng of p24 antigen.
Increased DNA synthesis of culture-adapted SG3IN2 virus.
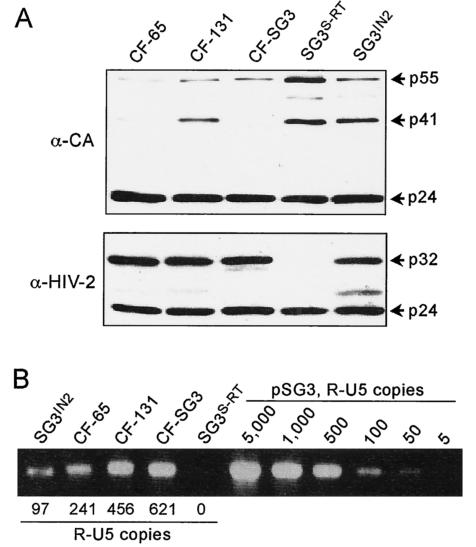
Since the original SG3IN2 and the culture-adapted CF-65 and CF-131 viruses express a chimeric Gag-Pol polyprotein that might fold differently and cause late-stage defects, virus collected from culture supernatants was analyzed by Western blot analysis. With a monoclonal antibody reactive with the HIV-1 capsid protein, only minimal differences in proteolytic protein processing were detected between the SG3IN2, CF-65, CF-131, and wild-type viruses. Relative to wild-type SG3, the SG3IN2 and CF-131 viruses appeared to contain a greater amount of incompletely processed p41 protein (Fig. 2A). By using serum from an individual infected with HIV-2 as a probe, similar amounts of the IN proteins were detected. A protein with a mass of approximately 27 kDa was also detected for the SG3IN2 virus and to a lesser extent for the CF-65 and CF-131 viruses.
FIG. 2.
Analysis of culture-adapted virus. (A) Immunoblot analysis of SG3IN2 and culture-adapted viruses. Supernatants from SupT1 cell cultures infected with the CF-65, CF-131, and SG3 viruses were collected for analysis. Culture supernatants were also collected for analysis from 293T cells transfected with pSG3S-RT and pSG3IN2. Virus was concentrated by ultracentrifugation (125,000 × g, 2 h) through cushions of 20% sucrose. Viral pellets were lysed, and immunoblots were prepared and probed with anti-CA monoclonal antibody or serum from an HIV-2-infected individual. (B) Analysis of viral cDNA synthesis in infected cells; 500 ng (p24 antigen equivalents) of CF-65, CF-131, SG3, SG3S-RT, and SG3IN2 was used to infect 5 × 105 JC53 cells for 4 h at 37°C. The SG3S-RT and SG3IN2 viruses were obtained by transfection of 293T cells. Eighteen hours after infection, total DNA extracts were prepared, and 20 ng of each was analyzed by PCR for the R-U5 DNA product of reverse transcription. A standard curve was created with standards ranging from 5 to 5,000 DNA copies of pSG3. PCR-amplified DNA was resolved on a 1.5% agarose gel and stained with ethidium bromide, and the R-U5 product was quantified as described in Materials and Methods.
To examine whether the SG3IN2 virus was impaired in DNA synthesis and if adaptation of the CF-65 and CF-131 viruses compensated for this defect, JC53 cells were infected with 500 ng (p24 antigen equivalents) of SG3IN2, CF-65, CF-131, SG3, or SG3S-RT virus. Total cellular and viral DNA was extracted 18 h after infection and analyzed by PCR with the R-U5 primer pair to detect the minus-strand strong-stop DNA. The SG3IN2 virus produced approximately sixfold less strong-stop DNA compared to wild-type virus. Compared with SG3IN2, the CF-65 and CF-131 viruses produced 2.5- and 4.7-fold more strong-stop DNA (Fig. 2B). Viral DNA was not detected in cells infected with the RT- and IN-deficient SG3S-RT virus that was included as a negative control. The same relative differences in viral DNA synthesis were found with PCR primers that detected intermediate and late products of reverse transcription (data not shown). These results indicated that the CF-65 and CF-131 viruses were able to produce more viral DNA than the SG3IN2 virus and that this relative increase was similar in magnitude to the improvement in virus infectivity.
Analysis of subgenomic DNA fragments.
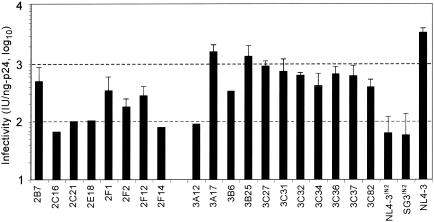
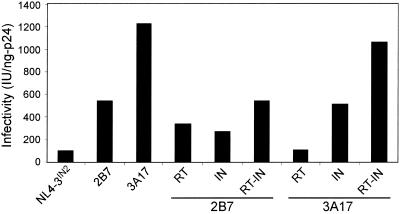
To identify genetic changes that were selected in the CF-65 and CF-131 viruses, a gag-pol-containing DNA fragment was PCR amplified from DNA extracts of infected SupT1 cells and cloned into the naturally existing BssHII and SalI sites of the pNL4-3 proviral clone. Multiple clones were picked and screened by transfection into 293T cells, followed by p24 antigen and infectivity analyses of the progeny virus. Thirteen of 24 clones generated from the CF-65 virus were p24 antigen positive, eight of which exhibited greater infectivity than the NL4-3IN2 control (Fig. 3). The NL4-3IN2 clone was substituted for SG3IN2 solely to facilitate cloning of the BssHII-SalI DNA fragment. The infectivity of NL4-3IN2 was similar to that of the original SG3IN2. Clone 2B7 was the most infectious and produced on average 495 IU/ng of p24, a sixfold increase over that of NL4-3IN2 and 15% of that of NL4-3.
FIG. 3.
Analysis of a 5′ subgenomic DNA fragment derived from culture-adapted virus. SupT1 cells were infected with the CF-65 or CF-131 virus, and 2 days later high-molecular-weight DNA extracts were prepared. A 5.3-kb DNA fragment was amplified from each DNA extract by PCR and ligated into a pNL4-3 proviral backbone, from which multiple clones were derived. Then 6 μg of DNA of each clone was transfected into 293T cells by calcium phosphate DNA precipitation. The pNL4-3IN2, pSG3IN2, and pNL4-3 clones were included as controls. The culture supernatants were collected 48 h later and analyzed for HIV-1 p24 antigen concentration by ELISA and for infectivity with TZM-bl reporter cells. Infectivity is expressed by standardization to 1 ng of p24 antigen. The four most infectious clones derived from CF-65 (2B7, 2F1, 2F2, and 2F12) and the nine most infectious clones derived from CF-131 (3A17, 3B25, 3C27, 3C31, 3C32, 3C34, 3C36, 3C37, and 3C82) were analyzed in three separate experiments, and standard deviations were calculated.
In the case of clones derived from the CF-131 virus culture, 16 of 21 were p24 antigen positive, and 11 were more infectious than NL4-3IN2 (Fig. 3). Clones 3A17 and 3B25 had the greatest infectivity, producing on average 1,598 IU/ng of p24 (a 28-fold increase, or 51% of that of NL4-3) and 1,333 IU/ng of p24 (a 23-fold increase or 42% of that of NL4-3). As expected, the transfection-derived viruses were overall less infectious calculated per nanogram of p24 compared with the viruses derived from chronically infected cells. However, relative to the wild-type virus (transfection-derived NL4-3), the 2B7 and 3A17 viruses exhibited a level of infectivity similar to that of the CF-65 and CF-131 viruses, respectively, from which they were derived. Western blot analysis indicated that there was no difference between the 2B7 and 3A17 viruses and that from which they were derived (data not shown). This suggested that the BssHII/SalI DNA fragment cloned into pNL4-3 was principally responsible for the improvement in virus infectivity and DNA synthesis.
Sequence analysis of CF-65 and CF-131 clones.
The BssHII/SalI DNA fragments of multiple infectious proviral clones derived from the CF-65 and CF-131 virus cultures were sequenced. Sequence analysis of four clones derived from the CF-65 virus (2B7, 2F1, 2F2, and 2F12) indicated that each one represented a distinct genotype present in the virus culture at the time (Table 1). Conserved among each clone was a valine-to-isoleucine mutation in IN at amino acid 204 (V204I). The only other mutation conferring an amino acid change in IN resulted in an isoleucine-to-threonine substitution at position 200 (I200T) of the 2F2 clone. Greater polymorphism among the CF-65 clones was found in the RT coding region, and none of these mutations appeared to be predominant among the virus population. Sequence analysis of regions outside of RT and IN revealed two addition mutations present only in the 2B7 clone. These included a valine-to-isoleucine mutation at amino acid 230 (V230I) of the capsid (CA) and an arginine-to-glycine mutation at amino acid 87 of the protease (R87G).
TABLE 1.
Mutations present in the CF-65 and CF-131 clones
| Clone | Mutationa
|
|||||||
|---|---|---|---|---|---|---|---|---|
| MA | CA | NC | PR | RT | IN | Vif | Vpr | |
| 2B7 | nf | V230I | nf | R87G | K102R, D237N | V204I | nf | nf |
| 2F1 | nf | nf | nf | nf | A62T, D320N | V204I | nf | nf |
| 2F2 | nf | nf | nf | nf | R83K | V204I, I200T | nf | nf |
| 2F12 | nf | nf | nf | nf | Y127C, V179I, Q373K | V204I | nf | nf |
| 3A17 | V35I, E40K | V27I, V230I | R10G | nf | V179I | Q96H, K127E, V204I | R157Gb | V60R |
| 3B25 | V35I, E40K | V27I, V230I | R10G | nf | V179I | Q96H, K127E, V204I | R157G | V60R |
| 3C27 | V35I, E40K | V27I, V230I | R10G | nf | V179I | Q96H, K127E, V204I | H43Q, R157G | V60R |
| 3C32 | V35I, E40K | V27I, V230I | R10G | nf | V179I, A360D | Q96H, K127E, V204I | R157G | V60R |
| 3C34 | V35I, E40K | V27I, V230I | R10G | nf | V179I, S519K | Q96H, K127E, V204I | V141A | V60R |
| 3C36 | V35I, E40K, Q55R | V27I, V230I | R10G | nf | V179I | Q96H, K127E, V204I | R157G | V60R |
| 3C37 | V35I, E40K | V27I, V230I | nf | nf | V179I | Q96H, K127E, V204I | R157G | V60R |
| 3C82 | V35I, E40K | V27I, V230I | R10G | nf | D67N, V179I | Q96H, K127E, V204I | R157G | V60R |
nf, no mutation found in that region.
R157G is the result of an alanine deletion at position 5059, resulting in a frameshift and an ocher stop codon in the reading frame at amino acid position 158.
Eight clones derived from the CF-131 virus were sequenced. Three common mutations were found in IN: glutamine to histidine at amino acid residue 96 (Q96H), lysine to glutamic acid at residue 127 (K127E), and valine to isoleucine at residue 204 (V204I) (Table 1). No other amino acid mutation were found in IN. In RT, all eight clones contained a valine-to-isoleucine mutation at position 179 (V179I). This V179I mutation was present in the 2F12 clone, indicating that it was a polymorphism present in the CF-65 virus culture that appeared to become fixed in the CF-131 population. Other sporadic mutations in RT included aspartic acid to asparagine at residue 67 (clone 3C82), alanine to aspartic acid at residue 360 (clone 3C32), and a serine to lysine at residue 519 (clone 3C34).
Sequence analysis of the regions outside of RT and IN identified additional mutations among the clones derived from the CF131 virus. All of the clones contained the V35I and E40K MA mutations and the V27I and V230I CA mutations. The 3C36 clone had an additional glutamine to arginine at position 55 (Q55R) in MA. Seven of eight clones contained an arginine to glycine mutation at position 10 (R10G) in the nucleocapsid. The absence of this mutation in 3C37 appeared to reduce virus infectivity. In Vif, seven of eight clones contained an arginine to glycine mutation at position 157 (R157G) that was the result of a frameshift caused by the deletion of adenine at position 5059. This frameshift produced a TAA stop codon at position 158. All eight clones contained a valine to arginine mutation at position 60 (V60R) in Vpr.
Analysis of the RT and IN coding regions.
Earlier reports indicated that the retroviral IN protein augments the initiation of viral DNA synthesis (16, 22, 32, 33, 37). Consistent with this effect, the IN and RT proteins of HIV-1 (13, 37) and MLV (12) have been shown to interact physically. To determine the extent to which the RT and IN mutations were responsible for the increased infectivity of the CF-65 and CF131 viruses, we inserted the RT and/or IN coding region from the most infectious CF-65 (2B7) and CF-131 (3A17) clones into pNL4-3IN2 as described in Materials and Methods. Transfection-derived virions were normalized for p24 antigen concentration and used to infect TZM-bl reporter cells.
In the case of the 2B7 clone, RT and IN increased infectivity to 340 and 276 IU/ng of p24, respectively (Fig. 4). Together, the 2B7 RT and IN coding regions (RT-IN) increased infectivity to 548 IU/ng of p24, or 101% of that of the 2B7 virus. In contrast to 2B7, virus containing the 3A17 IN exhibited a marked increase in infectivity compared with virus containing the 3A17 RT. By itself, the 3A17 RT (containing V179I) had little effect on infectivity. However, the 3A17 RT-IN appeared to have a synergistic effect, increasing infectivity to 1,065 IU/ng of p24, or 87% of that of the 3A17 clone. None of the RT, IN, or RT-IN recombinant constructs had any apparent effect on virion production (p24 antigen) or proteolytic processing of viral proteins (data not shown). These results suggested that the selection of mutations in both RT and IN was important for the early adaptation of the chimeric virus and the selection of additional mutations in IN2 led to a further improvement in virus infectivity.
FIG. 4.
Infectivity of virus containing the RT and/or IN region derived from the 2B7 and 3A17 clones. DNA fragments containing either RT, IN, or RT and IN derived from the 2B7 and 3A17 clones were inserted into the genome of pNL4-3IN2, and sequences were confirmed. Virus was derived from each recombinant plasmid by transfection of 293T cells and analyzed for p24 antigen concentration and infectivity with TZM-bl reporter cells. Infectivity is expressed by standardization to 1 ng of p24 antigen. This experiment was repeated twice, and data representative of both experiments are depicted.
Analysis of the culture adapted IN by trans-complementation.
To further analyze how changes specific to IN affected virus infectivity, we exploited a Vpr-IN trans-complementation approach. This and similar trans-complementation approaches have been used to uncouple the pleiotropic effect of IN/Pr160Gag-Pol mutations (16, 22, 37) and thus help specifically analyze the effect of the mature IN protein on the initiation of reverse transcription. To analyze the effects of the three IN mutations on virus infectivity and DNA synthesis, each mutation was engineered, individually and in different combinations, into the pLR2P-vprIN expression plasmid as described in Materials and Methods. These expression plasmids were cotransfected into 293T cells with pSG3S-IN (SG3 IN-minus proviral DNA), and progeny virions were analyzed for IN2 content, infectivity, and DNA synthesis. As controls, the pLR2P-vprIN1 and pLR2P-vprIN2 expression plasmids were transfected with pSG3S-IN, and the progeny virus was analyzed.
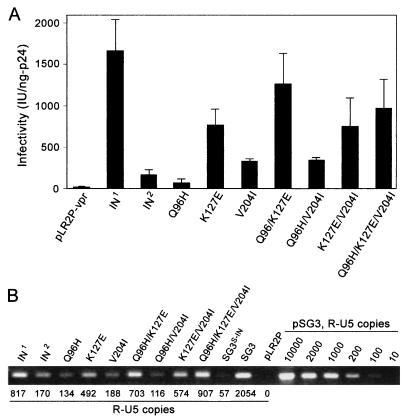
Consistent with earlier results (19), immunoblot analysis confirmed efficient virion incorporation for each of the Vpr-IN2 mutants and liberation of the IN from Vpr (data not shown). Individually, the K127E mutation conferred the greatest increase in infectivity: Vpr-INK127E-complemented virus produced 755 IU/ng of p24, a fivefold increase over that of Vpr-IN2-complemented virus (152 IU/ng of p24) (Fig. 5A). Virus complemented with the trans-INv204I and trans-INQ96H mutants generated 314 and 54 IU/ng of p24, respectively, a 2.4-fold increase and 2.8-fold decrease in infectivity, respectively. The combination of V204I with either Q96H or K127E increased infectivity to 332 and 742 IU/ng of p24, respectively. The combination of all three mutations (V204, K127E, and Q96H) increased infectivity to 971 IU/ng of p24, or 6.4-fold. Interestingly, the Q96H/K127E combination, which did not exist among the various clones that were sequenced, exhibited the greatest level of infectivity, suggesting that these two mutations may be largely responsible for the improved phenotype detected in the 3A17 clone and CF-131 virus.
FIG. 5.
Analysis of mutations selected in the IN2 coding region by trans-complementation; 4 μg of pSG3S-IN proviral DNA was cotransfected into 293T cells with 6 μg of pLR2P-vpr, pLR2P-vprIN1, or pLR2P-vprIN2 (controls) or with 6 μg of the pLR2P-vprIN expression plasmids containing mutations in the IN coding region, as depicted. Forty-eight hours after transfection, the culture supernatants were collected, clarified by low-speed centrifugation, filtered through 0.45-μm sterile filters, and analyzed for virus concentration by HIV-1 p24 antigen ELISA. (A) Analysis of virus infectivity. Fivefold serial dilutions of each virus were prepared and analyzed for infectivity with TZM-bl reporter cells. Infectivity was standardized to 1 ng of p24 antigen. (B) Analysis of viral DNA synthesis; 500 ng (p24 antigen equivalents) of each virus was analyzed as described for Fig. 2B. A standard curve was created with standards ranging from 10 to 10,000 copies of pSG3 DNA.
The effect of these mutations on viral DNA synthesis was examined by infecting JC53 cells with each virus and 18 h later extracting the total DNA for quantitative analysis. For the single-amino-acid mutants, the relative changes (compared with IN2) in minus-strand strong-stop DNA synthesized were similar to the changes in infectivity. Compared with the IN2 control, less DNA was detected with the INQ96H mutant, approximately the same amount was produced by the INV204I mutant, and the INK127E mutant produced 2.8-fold more (Fig. 5B). In the case of the Q96H/V204I mutant, DNA synthesis decreased slightly, whereas that of the K127E/V204I mutant increased by 3.4-fold. Unlike our infectivity results, virus complemented with the V204/K127E/Q96H IN mutant exhibited the greatest increase in viral DNA synthesis (5.3-fold). This may help explain why the V204I mutation was maintained following acquisition of the Q96H and K127E mutations.
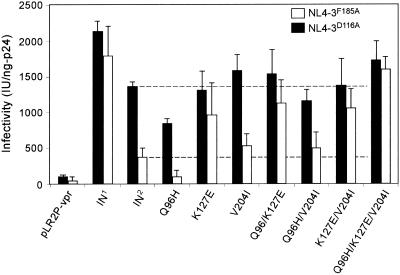
To analyze the effect of the different IN mutations on integration, we exploited the catalytically defective NL4-3D116A mutant provirus. After virus entry into the TZM-bl reporter cells, the noninfectious NL4-3D116A virus synthesizes wild-type levels of DNA. Therefore, it is possible to assess the enzymatic activity of IN mutations by Vpr-IN complementation analysis with the NL4-3D116A virus. Similarly, by exploiting the NL4-3F185A mutant virus, which encodes an IN protein defective in DNA synthesis but competent for integration, we can analyze the effect of IN mutations on DNA synthesis independently of their effect on integration.
NL4-3D116A (black bars) expressed with Vpr-IN1 produced 2,129 IU/ng of p24 (Fig. 6), confirming efficient complementation of the NL4-3D116A virus. Complementation with Vpr-IN2 produced 1,256 IU/ng of p24 (59% of that of trans-IN1). This result was consistent with data reported earlier (19) and suggested that the IN2 protein supports HIV-1 DNA integration reasonably well. The mutant trans-IN proteins did not increase infectivity markedly beyond that attained with Vpr-IN2 (Fig. 6), suggesting that these mutations do not affect the enzymatic activity of the IN3A17 protein. A notable exception was Q96H, which reduced infectivity compared with IN2. In the case of NL4-3F185A virus (open bars), complementation with the mutant trans-IN proteins (except for Q96H) increased infectivity. Similar to our results with minus-strand strong-stop DNA synthesis (Fig. 5B), the K127E, Q96H/K127E, K127E/V204I, and Q96H/K127E/V204I mutants exhibited the greatest complementation. NL4-3F185A complemented with IN2 containing all three mutations (Q96H/K127E/V204I) produced 1,581 IU/ng of p24, or 86% of the amount generated by Vpr-IN1. This suggested that the nearly wild-type level of DNA synthesis was restored by the selection of these three mutations and that their affect was exerted by the mature IN2 mutant protein.
FIG. 6.
Analysis of integration activity by Vpr-IN trans-complementation; 4 μg of pNL4-3D116A (black bars) or pNL4-3F185A (open bars) proviral DNA was cotransfected into 293T cells with 6 μg of pLR2P-vpr, pLR2P-vprIN1, or pLR2P-vprIN2 (controls) or with 6 μg of pLR2P-vprIN expression plasmids containing mutations in the IN2 coding region, as depicted. Infectivity was determined as described for Fig. 5.
DISCUSSION
In this study, we use a chimeric virus that was severely defective in DNA synthesis to select viruses with improved replication fitness. Analysis of the most replication-fit virus demonstrated that improved fitness was largely due to compensatory amino acid changes selected in IN2. These mutations were shown to increase virus fitness largely by augmenting the initiation of reverse transcription. Importantly, our trans-complementation analysis indicate that the IN mutations enhanced DNA synthesis by an effect on the mature IN2 protein itself on an early step in the virus life cycle. Considering the high degree of structural homology between the HIV-1 and HIV-2 IN proteins, the mutations selected in IN2 may implicate regions on the HIV-1 IN protein that are important for the initiation of DNA synthesis.
Our analysis was focused on viruses that were derived after 65 and 131 days of cell-free passage. The CF-131 virus was studied because it was the most infectious, while the CF-65 virus was significantly less infectious and represented a biological and temporal intermediate form of chimeric virus. To facilitate molecular genetic analysis of these viruses, a 5.3-kb DNA fragment comprising most of the 5′ end of the viral genome was cloned into the naturally existing BssHII and SalI restriction sites of the pNL4-3 clone. The most infectious clones derived at each time point exhibited a level of infectivity similar to that of the respective parental virus. This result indicated that the 5′ DNA segment of the chimeric virus was largely responsible for the improved viral fitness.
Sequence analysis of four clones derived from the CF-65 virus identified amino acid mutations that were mostly restricted to RT and IN2. Mutations in RT were heterogeneous, while each clone contained the V204I IN mutation, suggesting that it had become fixed in the cell-free chimeric virus population. These results may suggest that the selection of mutations in RT and/or IN enabled cell-free transmission and productive infection of the CF-65 chimeric virus. This idea was in part validated when these two regions were analyzed in cis and found to improve infectivity (Fig. 4). In the evolution of the CF-131 virus, the Q96H, K127E, and V204I IN mutations were selected and appeared to be principally responsible for the increased level of infectivity and DNA synthesis. Interestingly, with the exception of V179I, most of the other CF-65 RT mutations were lost during the evolution of the CF-131 virus. Analysis of the CF-131 RT in cis indicated that the V179I mutation had little or no effect on virus infectivity by itself but together with the three IN mutations (Q96H, K127E, and V204I) augmented infectivity to a level close to that of the 3A17 clone (Fig. 4). While the RT and IN mutations appear to account for most of the improvement in virus infectivity, mutations detected in other regions of the virus genome do warrant further study.
By expressing and packaging IN in trans, it is possible to discriminate between the effect of IN mutations on early versus late events of the virus life cycle (38). Therefore, several studies of IN function have exploited trans-expression approaches to help elucidate its role in augmenting viral DNA synthesis (22, 30, 33, 37, 40). In this study, the 3A17 virus contained multiple mutations that could possibly affect different viral proteins and different steps of the virus life cycle. By expressing the IN mutations found in CF-65 and CF-131 viruses in trans, we were able to analyze how changes specific to the IN protein affected virus infectivity and DNA synthesis without introducing these mutations into the Gag-Pol (IN domain) precursor protein.
Consistent with our analysis in cis, the trans-INQ96H, K127E, V204I mutant rescued virus infectivity by approximately 58% compared with the homologous trans-IN1 (Fig. 5A) and complemented strong-stop DNA synthesis at least as efficiently as the homologous trans-IN1 (Fig. 5B). Analysis of the different IN mutations revealed a strong correlation between their effects on infectivity and on DNA synthesis. One exception was observed when comparing INKQ96H, K127E with INQ96H, K127E, V204I. While trans-INQ96H, K127E, V204I reproducibly rescued DNA synthesis better than INKQ96H, K127E, the opposite was true for virus infectivity (compare Fig. 5A and 5B). The V204I mutation was selected early and remained fixed during the evolution of the chimeric virus. Since the presence of this mutation in cis appeared to increase relative infectivity greater than when provided in trans, this mutation may correct a late-stage event. The INQ96H mutation reduced DNA synthesis and integration, but in combination with INK127E, both were increased. This could suggest that the Q96H and K127E mutations are synergistic and that the IN2 surface region containing these two residues may be important for augmenting DNA synthesis.
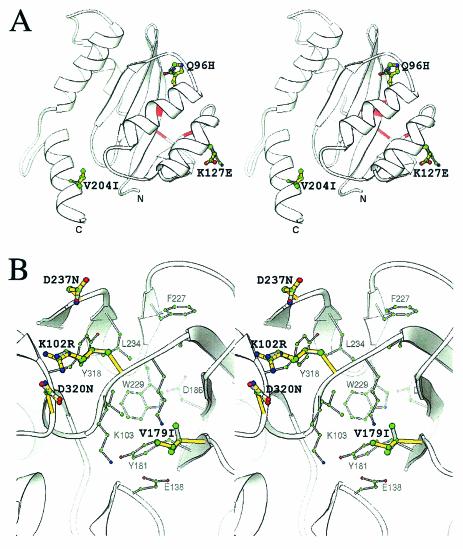
To further understand the effect of mutations on IN2, we modeled its secondary structure. The catalytic domain of HIV-2ST IN (residues 55 to 208) shows 90% sequence identity (98% homology) with IN from simian immunodeficiency virus (INSIV). We used the crystal structure of the catalytic domain of IN from SIV (PDB/1C6V) as the starting model in the program Swiss-Model, and the resultant coordinates were subjected to Procheck analysis (17) to assess the final quality of the model. All three mutations in IN2 were present on a surface that is opposite that of the catalytic triad (shaded red on the ribbon diagram in Fig. 7A). Two mutations, Q96H and K127E, were present on adjacent helices and separated by a distance of 12.56 Å (between Cα's). Furthermore, residue K127E brings about a contrast in the net local charge, from positive to negative, while the Q96H mutation marginally changes the net potential. Interestingly, the V204I mutation converts this residue back to the HIV-1 and HIV-2 consensus, and the surface in this region closely resembles that of HIV-1 IN.
FIG. 7.
Modeled structure of both mutated and unmutated IN2 and RT. (A) Stereo view of the IN2 and INQ96H, K127E, V204I ribbon structure. The ribbon structure was derived from the SIV crystal structure (PDB/1C6V) with Swiss-Model. Residues 96, 127, and 204 are represented by the ball-and-stick illustration, where the green, blue, and red spheres represent carbon, nitrogen, and oxygen atoms, respectively. All bonds of the unmutated IN2 are colored gray, while the overlapping yellow bonds are those of the Q96H, K127E, and V204I mutations. The catalytic triad position on the ribbon structure is shown in red. All diagrams were generated with the program Ribbons (6). (B) Stereo view of the NNRTI binding pocket of RT (PDB1/DLO). The residues of the NNRTI binding pocket are shaded gray, and the atoms are colored as explained above. The thicker bonds shaded in yellow represent the mutated residues R102, I179, N237, and N320, whereas the grayish overlapping bonds represent the original K102, V179, D237, and D320 residues. Residues known to contribute to the NNRTI pocket are depicted in gray, and their atoms and bonds are also displayed in a reduced size.
Our analysis of the CF-65 virus indicated that changes in RT augmented viral DNA synthesis (Fig. 5). Sequence analysis of the CF-65 virus revealed that three of the four clones had mutations (K102R, V179I, D237N, and D320N) that clustered around the nonnucleoside RT inhibitor (NNRTI) binding pocket on RT (Fig. 7B). Except for V179I, a conserved hydrophobic mutation which is a part of the NNRTI binding pocket, the K102R, D237N, and D320N mutations are present on the outer surface of this pocket in very close proximity and invoke a net change in the potential on the surface. Our data indicate that these represent compensatory mutations that improve the infectivity of the CF-65 virus (Fig. 4). Their presence on the outer surface of the NNRTI binding site could imply a role for this region in triggering DNA synthesis. However, there is no clear evidence at the moment to corroborate this argument, and it certainly warrants further study.
Our data clearly show that changes in IN and RT affect DNA synthesis. In other studies with recombinant proteins in pulldown assays, IN and RT as well as MLV RT and IN have been shown to interact physically in vitro (12, 13, 32, 37). A recent study demonstrated that monoclonal antibodies generated against the minimal DNA binding domain in the C terminus of IN block the interaction of recombinant IN and RT (13). Biochemical analysis demonstrating that HIV-1 RT and IN inhibit each other's function (24, 32) perhaps suggests interaction between these proteins during early stages of the virus life cycle. In our study, it was notable that many of the CF-65 clones contained RT mutations proximal to the hydrophobic pocket, conferring a slight change in charge. Except for V179I, these mutations were lost while additional mutations were selected in IN2, ultimately leading to a fitter virus. These results give a snapshot of how the RT and IN2 genes appeared to coevolve to ultimately generate a virus with a greater capacity to synthesize viral DNA in infected cells.
Taken together, our findings indicate an important biological role for IN in the initiation of reverse transcription. It is consistent with our model that specific interactions between IN and other components that comprise the initiation complex are required to prevent premature initiation, thus ensuring association of IN with the nuclear preintegration complex and integration of the provirus upon the completion of reverse transcription.
Acknowledgments
We thank Tony Klon and Steve Harvey for assistance with the modeling of HIV and SIV IN.
This research was supported by National Institutes of Health grants CA73470 and AI47714 and the facilities of the Central AIDS Virus, Genetic Sequencing, and Flow Cytometry Cores of the Birmingham Center for AIDS Research (P30-AI-27767). This research was also supported by a Merit Review Award funded by the Office of Research and Development, Medical Research Services, Department of Veterans Affairs.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansari-Lari, M. A., L. A. Donehower, and R. A. Gibbs. 1995. Analysis of human immunodeficiency virus type 1 integrase mutants. Virology 211:332-335. [DOI] [PubMed] [Google Scholar]
- 3.Ansari-Lari, M. A., and R. A. Gibbs. 1996. Expression of human immunodeficiency virus type 1 reverse transcriptase in trans during virion release and after infection. J. Virol. 70:3870-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukovsky, A., and H. Göttlinger. 1996. Lack of integrase can markedly affect human immunodeficiency virus type 1 particle production in the presence of an active viral protease. J. Virol. 70:6820-6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon, P. M., E. D. Byles, S. M. Kingsman, and A. J. Kingsman. 1996. Conserved sequences in the carboxyl terminus of integrase that are essential for human immunodeficiency virus type 1 replication. J. Virol. 70:651-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carson, M. 1997. RIBBONS. Methods Enzymol. 277:493-505. [PubMed] [Google Scholar]
- 7.Engelman, A., G. Englund, J. M. Orenstein, M. A. Martin, and R. Craigie. 1995. Multiple effects of mutations in human immunodeficiency virus type 1 integrase on viral replication. J. Virol. 69:2729-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engelman, A., Y. Liu, H. Chen, M. Farzan, and F. Dyda. 1997. Structure-based mutagenesis of the catalytic domain of human immunodeficiency virus type 1 integrase. J. Virol. 71:3507-3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ewing, B., L. Hillier, M. C. Wendl, and P. Green. 1998. Base-calling of automated sequencer traces with phred. I. Accuracy assessment. Genome Res. 8:175-185. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh, S. K., P. N. Fultz, E. Keddie, M. S. Saag, P. M. Sharp, B. H. Hahn, and G. M. Shaw. 1993. A molecular clone of HIV-1 tropic and cytopathic for human and chimpanzee lymphocytes. Virology 194:858-864. [DOI] [PubMed] [Google Scholar]
- 11.Goff, S. P. 2001. Retroviridae: the retroviruses and their replication, p. 1871-1939. In D. M. Knipe and P. M. Howley (ed.), Virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 12.Hu, S. C., D. L. Court, M. Zweig, and J. G. Levin. 1986. Murine leukemia virus pol gene products: Analysis with antisera generated against reverse transcriptase and endonuclease fusion proteins expressed in Escherichia coli. J. Virol. 60:267-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishikawa, T., N. Okui, N. Kobayashi, R. Sakuma, T. Kitamura, and Y. Kitamura. 1999. Monoclonal antibodies against the minimal DNA-binding domain in the carboxyl-terminal of human immunodeficiency virus type 1 integrase. J. Virol. 73:4475-4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kappes, J. C., X. Wu, and J. C. Wakefield. 2003. Production of trans-lentiviral vector with predictable safety, p. 449-465. In C. A. Machida (ed.), Viral vectors for gene therapy. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 15.Kimpton, J., and M. Emerman. 1992. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated B-galactosidase gene. J. Virol. 66:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai, L., X. Wu, and J. C. Kappes. 2001. Moloney murine leukemia virus integrase protein augments viral DNA synthesis in infected cells. J. Virol. 75:11365-11372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laskowski, R. A., M. W. MacArthur, D. S. Moss, and J. M. Thornton. 1993. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26:283-291. [Google Scholar]
- 18.Leavitt, A. D., G. Robles, N. Alesandro, and H. E. Varmus. 1996. human immunodeficiency virus type 1 integrase mutants retain in vitro integrase activity yet fail to integrate viral DNA efficiently during infection. J. Virol. 70:721-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, H., X. Wu, H. Xiao, and J. C. Kappes. 1999. Targeting human immunodeficiency virus type 2 (HIV-2) integrase protein into HIV-1. J. Virol. 73:8831-8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masuda, T., V. Planelles, P. Krogstad, and I. S. Y. Chen. 1995. Genetic analysis of human immunodeficiency virus type 1 integrase and the U3 att site: unusual phenotype of mutants in the zinc finger-like domain. J. Virol. 69:6687-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nickerson, D. A., V. O. Tobe, and S. L. Taylor. 1997. PolyPhred: automating the detection and genotyping of single nucleotide substitutions with fluorescence-based resequencing. Nucleic Acids Res. 25:2745-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nymark-McMahon, M. H., N. S. Beliakova-Bethell, J.-L. Darlix, S. F. J. Le Grice, and S. B. Sandmeyer. 2002. Ty3 integrase is required for initiation of reverse transcription. J. Virol. 76:2804-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nymark-McMahon, M. H., and S. B. Sandmeyer. 1999. Mutations in nonconserved domains of Ty3 integrase affect multiple stages of the Ty3 life cycle. J. Virol. 73:453-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oz, I., O. Avidan, and A. Hizi. 2002. Inhibition of the integrases of human immunodeficiency viruses type 1 and type 2 by reverse transcriptases. Biochem. J. 361:557-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Platt, E., J., K. Wehrly, S. E. Kuhmann, B. Chesebro, and D. Kabat. 1998. Effects of CCR5 and CD4 cell surface concentration on infection by macrophage tropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quillent, C., A. M. Borman, S. Paulous, C. Dauguet, and F. Clavel. 1996. Extensive regions of pol are required for efficient human immunodeficiency virus polyprotein processing and particle maturation. Virology 219:29-36. [DOI] [PubMed] [Google Scholar]
- 27.Roth, M. J. 1991. Mutational analysis of the carboxy terminus of the Moloney murine leukemia virus integration protein. J. Virol. 65:2141-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roth, M. J., P. Schwartzberg, N. Tanese, and S. P. Goff. 1990. Analysis of mutations in the integration function of Moloney murine leukemia virus: effects on DNA binding and cutting. J. Virol. 64:4709-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartzberg, P., J. Colicelli, and S. P. Goff. 1984. Construction and analysis of deletion mutations in the pol gene of Moloney leukemia virus: a new viral function required for productive infection. Cell 37:1043-1052. [DOI] [PubMed] [Google Scholar]
- 30.Shehu-Xhilaga, M., M. Hill, J. A. Marshall, J. Kappes, S. M. Crowe, and J. Mak. 2002. The conformation of the mature dimeric human immunodeficiency virus type 1 RNA genome requires packaging of Pol protein. J. Virol. 76:4331-4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin, C.-G., B. Taddeo, W. A. Haseltine, and C. M. Farnet. 1994. Genetic analysis of the human immunodeficiency virus type 1 integrase protein. J. Virol. 68:1633-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tasara, T., G. Maga, M. O. Hottiger, and U. Hubscher. 2000. HIV-1 reverse transcriptase and integrase enzymes physically interact and inhibit each other. FEBS Lett. 507:39-44. [DOI] [PubMed] [Google Scholar]
- 33.Tsurutani, N., M. Kubo, Y. Maeda, T. Ohashi, N. Yamamoto, M. Kannagi, and T. Masuda. 2000. Identification of critical amino acid residues in human immunodeficiency virus type 1 IN required for efficient proviral DNA formation at steps prior to integration in dividing and nondividing cells. J. Virol. 74:4795-4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Gent, D. C., C. Vink, A. A. M. Oude Groeneger, and R. H. A. Plasterk. 1993. Complementation between HIV integrase proteins mutated in different domains. EMBO J. 12:3261-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Schwedler, U., J. Song, C. Aiken, and D. Trono. 1993. Vif is crucial for human immunodeficiency virus type 1 proviral DNA synthesis in infected cells. J. Virol. 67:4945-4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei, X., J. M. Decker, H. M. Liu, Z. Zhang, R. B. Arani, J. M. Kilby, M. S. Saag, X. Wu, G. M. Shaw, and J. C. Kappes. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu, X., H. Liu, H. Xiao, J. A. Conway, E. Hehl, G. V. Kalpana, V. Prasad, and J. C. Kappes. 1999. Human immunodeficiency virus type 1 integrase protein promotes reverse transcription through specific interactions with the nucleoprotein reverse transcription complex. J. Virol. 73:2126-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu, X., H. Liu, H. Xiao, J. A. Conway, E. Hunter, and J. C. Kappes. 1997. Functional RT and IN incorporated into HIV-1 particles independently of the Gag-Pol precursor protein. EMBO J. 16:5113-5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu, X., H. Liu, H. Xiao, J. Kim, P. Seshaiah, G. Natsoulis, J. D. Boeke, B. H. Hahn, and J. C. Kappes. 1995. Targeting foreign proteins to human immunodeficiency virus particles via fusion with Vpr and Vpx. J. Virol. 69:3389-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yung, E., M. Sorin, A. Pal, E. Craig, A. Morozov, O. Delattre, J. Kappes, D. Ott, and G. V. Kalpana. 2001. Inhibition of HIV-1 virion production by a transdominant mutant of integrase interactor 1. Nat. Med. 7:920-926. [DOI] [PubMed] [Google Scholar]
- 41.Zack, J. A., S. J. Arrigo, S. R. Weitsman, A. S. Go, A. Haislip, and I. S. Y. Chen. 1990. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 61:213-222. [DOI] [PubMed] [Google Scholar]