Abstract
Background:
Construction of recombinant viruses that can serve as vaccines for the treatment of experimental murine tumors has recently been achieved. The cooperative effects of immune system modulators, including cytokines such as interleukin 12 (IL-12) and costimulatory molecules such as B7-1, may be necessary for activation of cytotoxic T lymphocytes. Thus, we have explored the feasibility and the efficacy of inclusion of these immunomodulatory molecules in recombinant virus vaccines in an experimental antitumor model in mice that uses Escherichia coli β-galactosidase as a target antigen.
Methods:
We developed a “cassette” system in which three loci of the vaccinia virus genome were used for homologous recombination. A variety of recombinant vaccinia viruses were constructed, including one virus, vB7/β/IL-12, that contains the following five transgenes: murine B7-1, murine IL-12 subunit p35, murine IL-12 subunit p40, E. coli lacZ (encodes β-galactosidase, the model antigen), and E. coli gpt (xanthine-guanine phosphoribosyltransferase, a selection gene). The effects of the recombinant viruses on lung metastases and survival were tested in animals that had been given an intravenous injection of β-galactosidase-expressing murine colon carcinoma cells 3 days before they received the recombinant virus by intravenous inoculation.
Results:
Expression of functional B7-1 and IL-12 by virally infected cells was demonstrated in vitro. Lung tumor nodules (i.e., metastases) were reduced in mice by more than 95% after treatment with the virus vB7/β/IL-12; a further reduction in lung tumor nodules was observed when exogenous IL-12 was also given. Greatest survival of tumor-bearing mice was observed in those treated with viruses encoding β-galactosidase and B7-1 plus exogenous IL-12.
Conclusion:
This study shows the feasibility of constructing vaccinia viruses that express tumor antigens and multiple immune cofactors to create unique immunologic microenvironments that can modulate immune responses to cancer.
Tumor antigens that can induce specific cytotoxic T-lymphocyte responses to tumor cells have been identified and cloned (1-3). Tumors that express such antigens infrequently induce cytotoxic T-lymphocyte responses adequate for their destruction, possibly because they lack essential immunologic cofactors. Two such factors, B7-1 (4,5) and interleukin 12 (IL-12) (6), have been expressed separately in recombinant vaccinia virus and appear to improve the efficacy of tumor immunotherapy. Vaccinia virus is a well-characterized expression vector (7,8) that has been used to express a wide variety of recombinant proteins (9). Furthermore, this virus is an ideal vector for the induction of efficient cytotoxic T-lymphocyte responses to recombinant proteins (10), inducing specific protective and therapeutic immune responses to both viral and cellular tumor antigens (11-13).
The costimulatory molecule B7-1 is found on the surface of professional antigen-presenting cells, such as dendritic cells, and interacts with its ligands CD28 and cytotoxic T-lymphocyte antigen-4 expressed on most T cells. The simultaneous interactions of 1) the complex containing the peptide and the major histocompatibility complex with a specific T-cell receptor and of 2) B7-1 with CD28 are essential for the effective stimulation of antigen-specific cytotoxic T lymphocytes, mediated, in part, by the up-regulation and stabilization of interleukin 2 messenger RNA (14). Stimulation via the T-cell receptor without costimulation can result in T-cell anergy or apoptosis. Although many tumors express major histocompatibility complex class I molecules and are able to present antigen, most do not provide costimulation. Transfection of tumor cells with B7-1 can effectively stimulate immunotherapeutic responses (15).
The cytokine IL-12 is a heterodimer composed of two glycoproteins, p40 and p35, and is expressed primarily by activated B cells, monocytes, and macrophages (16). This immunomodulatory cytokine has a variety of functions, including the induction of nonspecific natural killer cells and the maturation of CD8+ T cells into antigen-specific cytotoxic T lymphocytes (16). In addition, IL-12 can stimulate type 1 CD4+ helper T cells that can lead to the production of interferon gamma and the induction of a cell-mediated response that is essential in the vaccine-based immunotherapy of established tumors. Inoculation of mice with a combination of irradiated tumor cells and fibroblast cells expressing IL-12 induced partial protection to subsequent challenge with nonirradiated tumor cells, thus implicating IL-12 as a potential immune adjuvant in the immunotherapy of cancer (17). IL-12 given exogenously at high levels has also been shown to have effective antitumor activity in animal models (18). Several reports (19-21) indicate that the effects of IL-12 are greatly enhanced when B7-1 and IL-12 are added together.
To create a cytokine microenvironment favorable to the antigen-specific activation of cytotoxic T lymphocytes, we examined the efficacy of IL-12 when endogenously expressed by a recombinant vaccinia virus or administered exogenously alone or in combination with B7-1, which augments antitumor responses (5). We have built a series of recombinant viruses that contain as many as five transgenes inserted into three loci of the viral genome to test the effectiveness of recombinant immunogens expressing a model antigen, IL-12, and B7-1. We tested these immunogens by treating a murine colon carcinoma cell line that had been transduced with a model tumor antigen, Escherichia coli β-galactosidase, to create a tumor line called CT26.C25 (22-24).
The construction of these complex viruses required the development of a cassette system that uses three loci within the vaccinia viral genome—loci for hemagglutinin, thymidine kinase, and viral protein 37. We inserted up to five foreign genes into the viral genome by homologous recombination. The genes inserted were as follows: E. coli lacZ (that encodes β-galactosidase, the model antigen), E. coli gpt (xanthine-guanine phosphoribosyltransferase, a selection gene), murine B7-1, and the two subunits of murine IL-12 (p35 and p40). In this study, we explored the toxicity and efficacy of a triple-recombinant vaccinia virus expressing a model tumor-associated antigen alone or in combination with IL-12 and B7-1. We explored the possibility that recombinant viruses encoding specific target antigens and multiple immunostimulatory molecules may enhance the design of recombinant immunogens.
Materials and Methods
Construction of Recombinant Viruses
Three gene loci within the viral genome—viral protein 37 (25), thymidine kinase (7), and hemagglutinin—were used for the construction of the viruses in this study (Fig. 1). The murine B7-1 gene (supplied by R. Germain, National Institutes of Health, Bethesda, MD) and the measles hemagglutinin gene (supplied by S. Rozenblatt, Tel Aviv University, Israel) were cloned into the transfer plasmid pRB21 under control of the vaccinia virus synthetic early/late promoter (26). The protocol devised by Blasco and Moss (25) allowed us to insert the gene of interest, via homologous recombination, into the viral protein 37 loci of a plaque-deficient Western Reserve strain of vaccinia virus to yield the viruses termed vB7-1 and vMHA, respectively. The E. coli lacZ gene (encoding β-galactosidase) was introduced into the genomes of vaccinia B7-1 and vaccinia measles hemagglutinin by homologous recombination with the vaccinia virus transfer plasmid pSC65Δ [a modification of pSC65 (26), in which the vaccinia virus 7.5k early/late promoter drives expression of the lacZ gene]. Recombinant viruses were selected simultaneously for both their thymidine kinase-negative phenotype (7) and their ability to express β-galactosidase (27). The transfer plasmid pGS69 (28) was used to insert the gene encoding nucleoprotein from influenza virus under the control of the vaccinia virus 7.5k promoter. Recombinant virus was selected on the basis of a thymidine kinase-negative phenotype and monolayer-immunostaining analysis. The two subunits of murine IL-12 were cloned into the plasmid pKT under the transcriptional control of vaccinia virus strong late promoters (6) and introduced into the hemagglutinin locus of recombinant vaccinia virus by homologous recombination. Recombinant viruses were isolated by gpt selection as described previously (29).
Fig. 1.
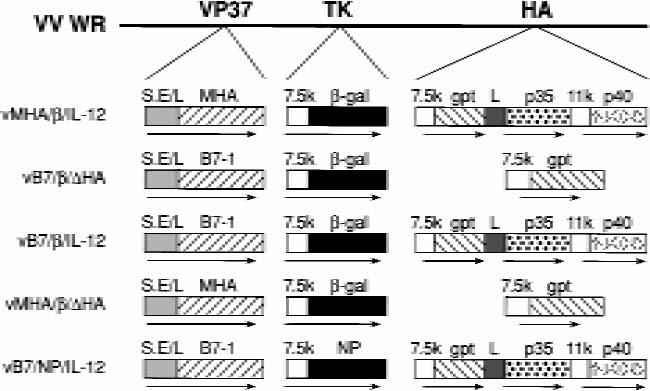
Schematic representation of the genomes of recombinant vaccinia virus (VV) used in this study. The murine B7-1 gene or the control measles hemagglutinin (vMHA) gene was incorporated into the viral protein 37 (VP37) locus of the Western Reserve strain of vaccinia virus (25). Both genes were under the transcriptional control of the synthetic strong early/late promoter (S.E/L). The model tumor-associated antigen β-galactosidase (β-gal) encoded by the Escherichia coli lacZ gene was introduced into the vaccinia virus thymidine kinase (TK) locus, under transcriptional control of the early/late vaccinia virus 7.5k promoter by use of a modified version of the insertion vector pSC65. To control for thymidine kinase disruption and β-galactosidase expression, influenza virus nucleoprotein (NP) was inserted into the vaccinia virus genome by use of the vector pGS69. The two subunits of murine interleukin 12 (IL-12), p35 and p40 (6), were inserted into the hemagglutinin (HA) loci under transcriptional control of the vaccinia virus- and cowpox virus-derived strong late promoters, and hemagglutinin gene disruption by the xanthine-guanine phosphoribosyltransferase (gpt) gene from E. coli was used as a control. vMHA/β/IL-12 = vaccinia virus containing measles hemagglutinin, β-galactosidase, and IL-12; vB7/β/ΔHA = vaccinia virus containing B7-1, β-galactosidase, and a deletion of hemagglutinin; vB7/β/IL-12 = vaccinia virus containing B7-1, β-galactosidase, and IL-12; vMHA/β/ΔHA = vaccinia virus containing measles hemagglutinin, β-galactosidase, and a deletion of hemagglutinin; vB7/NP/IL-12 = vaccinia virus containing B7-1, nucleoprotein, and IL-12.
Homologous recombination was performed by transfection of the transfer plasmid into infected BSC-1 monkey kidney cells as described previously (30). Briefly, cells were infected at an approximate multiplicity of infection of 0.1 and incubated for 1 hour. The cells were washed three times with Opti–MEM serum-free medium (Life Technologies, Inc. [GIBCO BRL], Gaithersburg, MD) and transfected with 2–5 μg of plasmid DNA mixed with Lipofectin (Life Technologies, Inc.) according to the manufacturer's protocol. All recombinant viruses were plaque purified four times under an agar overlay, and virus stocks were purified by centrifugation at 30 000 g for 40 minutes at 4 °C over a sucrose cushion (31). Recombinant virus names have been truncated for clarity. Designations for the viruses constructed are as follows: vaccinia virus containing measles hemagglutinin, β-galactosidase, and IL-12 (vMHA/β/IL-12 [vMCMHA/β/IL-12]); vaccinia virus containing B7-1, β-galactosidase, and a deletion of hemagglutinin (vB7/β/ΔHA [vMCB7/β/ΔHA]); vaccinia virus containing B7-1, β-galactosidase, and IL-12 (vB7/β/IL-12 [vMCB7/β/IL-12]); vaccinia virus containing measles hemagglutinin, β-galactosidase, and a deletion of hemagglutinin (vMHA/β/ΔHA [vMCMHA/β/ΔHA]); and vaccinia virus containing B7-1, nucleoprotein, and IL-12 (vB7/NP/IL-12 [vMCB7/NP/IL-12]).
Immunostaining of Virus Plaques
The immunostaining of virus-infected cell monolayers is similar to that described previously (30). Monolayers of BSC-1 cells were infected with recombinant vaccinia virus. Medium was removed 48 hours after infection, and cells were fixed in a 1 : 1 mixture of acetone and methanol for 2 minutes. Cells were washed in phosphate-buffered saline and incubated with primary antibody diluted in phosphate-buffered saline containing 2% fetal calf serum (phosphate-buffered saline-D) for 1 hour at room temperature. Cells were washed twice with phosphate-buffered saline and incubated with a 1 : 1000 dilution of horseradish peroxidase-conjugated protein A (Amersham Life Science Inc., Arlington Heights, IL) in phosphate-buffered saline-D. After two washes with phosphate-buffered saline, bound immunoglobulin was visualized by the addition of 0.5 mL of substrate solution (10 mL of phosphate-buffered saline containing 10 μL of 30% H2O2 and 200 μL of a dianisidine [Sigma Chemical Co., St. Louis, MO]-saturated ethanol solution). Viruses were checked for stable expression of gene products by a two-step immunostaining procedure similar to that described previously (30). Plaques were initially stained with an antibody recognizing one of the recombinant proteins; positive plaques were counted. In parallel, infected monolayers were stained with a rabbit anti-vaccinia polyclonal antibody. If the numbers of plaques stained in the two assays were similar, the virus was considered to be stable.
Western Blot Analysis
BSC-1 cells were infected at a multiplicity of infection of 10 with the relevant virus and incubated at 37 °C for 24 hours. Cells were harvested in 0.5 mL of solubilization buffer (0.06 M Tris–HCl, 3% sodium dodecyl sulfate, 5% 2-mercaptoethanol, 10% glycine, and 0.002% bromophenol blue). After boiling, samples were separated on a 10% gradient gel and transferred to a nitrocellulose membrane by use of a transfer unit (Bio-Rad Laboratories, Richmond, CA). Proteins were detected by the incubation of membranes in a 1 : 500 dilution of sheep anti-IL-12 antibody (supplied by Stan Wolf, Genetics Institute, Cambridge, MA) and a 1 : 1000 dilution of a rat anti-B7-1 antibody (Pharmingen, San Diego, CA). Incubation with alkaline phosphatase (Pharmingen)-conjugated anti-species antibody (1 : 5000 dilution) and then incubation with a substrate for alkaline phosphatase were used to visualize bound antibody.
Flow Cytometry Analysis
Flow cytometry analysis was similar to the procedure described previously (32). BSC-1 cells were infected for 18 hours at a multiplicity of infection of 10 with either recombinant vaccinia virus expressing B7-1 or control wild-type vaccinia virus. Monolayers were treated with trypsin to obtain a single-cell suspension, and cells were incubated with anti-cytotoxic T-lymphocyte antigen-4 immunoglobulin or the control ligand anti-CD71 immunoglobulin. Fluorescein isothiocyanate-conjugated anti-immunoglobulin was used to quantify ligand interactions.
Tumor Cell Lines
CT26 is an N-nitroso-N-methylurethane-induced undifferentiated colon carcinoma of BALB/c mouse (H-2d) origin. The generation of the β-galactosidase-expressing CT26.CL25 subclone has been described elsewhere (22). Briefly, a clone of CT26 (CT26.WT) was stably transfected with a retrovirus vector containing the lacZ gene under control of the retrovirus long terminal repeat. Sub-clones were evaluated for β-galactosidase expression and their ability to be lysed in a 51Cr release assay by cytotoxic T lymphocytes specific for β-galactosidase. The subclone CT26.CL25 was used in all studies. CT26.WT and CT26.CL25 were maintained in RPMI-1640 medium containing 10% heat-inactivated fetal calf serum (Biofluids, Rockville, MD), 0.03% l-glutamine, streptomycin (100 μg/mL), penicillin (100 μg/mL), and gentamicin sulfate (50 μg/mL) (National Institutes of Health Media Center, Bethesda, MD). CT26.CL25 was maintained in medium containing bioactive G418 (400 μg/mL) (Life Technologies, Inc.).
Treatment of Established Tumors
Female BALB/c (H-2d) mice, 8–12 weeks old, obtained from the Frederick Cancer Research and Development Center (Frederick, MD) were used for all animal experiments. Mice were inoculated intravenously with a lethal dose of tumor cells (5 × 105 cells). Three days later, mice were inoculated intravenously with 5 × 107 plaque-forming units of recombinant vaccinia virus. Indicated groups received 0.5 μg of recombinant murine IL-12 (from Stan Wolf) delivered intraperitoneally for 5 consecutive days after virus inoculation. All mice were randomly assigned to experimental groups and ear tagged before receiving virus. On day 12 after tumor inoculation, mouse lungs were removed and stained with India ink, and tumor nodules were counted by an individual who was blinded to the status of the animals as described previously (33). Mice were cared for in accord with institutional guidelines. The number of mice in each of the 10 treatment groups is indicated at the bottom of Fig. 3. Similarly treated groups of mice were followed for survival. No mice were killed early because of tumor burden. No assessable mice were excluded from the analysis. The number of mice that could be evaluated in each of the six treatment groups shown in Fig. 4, A, was as follows: 15 mice in the group infected with vaccinia virus containing B7-1, β-galactosidase, and IL-12 (vB7/β/IL-12) or with vaccinia virus containing B7-1, nucleoprotein, and IL-12 (vB7/NP/IL-12); 14 mice in the group infected with vaccinia virus containing B7-1, β-galactosidase, and a deletion of hemagglutinin (vB7/β/ΔHA) or with vaccinia virus containing measles hemagglutinin, β-galactosidase, and a deletion of hemagglutinin (vMHA/β/ΔHA); and 11 mice in the group infected with vaccinia virus containing measles hemagglutinin/β-galactosidase/IL-12 (vMHA/β/IL-12) or with phosphate-buffered saline. The number of mice that could be evaluated in each treatment groups, shown in Fig. 4, B, is as follows: 16 mice in the group infected with vaccinia virus containing measles hemagglutinin, β-galactosidase, and a deletion of hemagglutinin (vMHA/β/ΔHA) or with vaccinia virus containing B7-1, β-galactosidase, and IL-12 (vB7/β/IL-12); 13 mice in the group infected with vaccinia virus containing B7-1, nucleoprotein, and IL-12 (vB7/NP/IL-12); 12 mice in the group infected with vaccinia virus containing B7-1, β-galactosidase, and a deletion of hemagglutinin; and 11 mice in the group infected with vaccinia virus containing measles hemagglutinin, β-galactosidase, and IL-12 (vMHA/β/IL-12) or with phosphate-buffered saline.
Fig. 3.
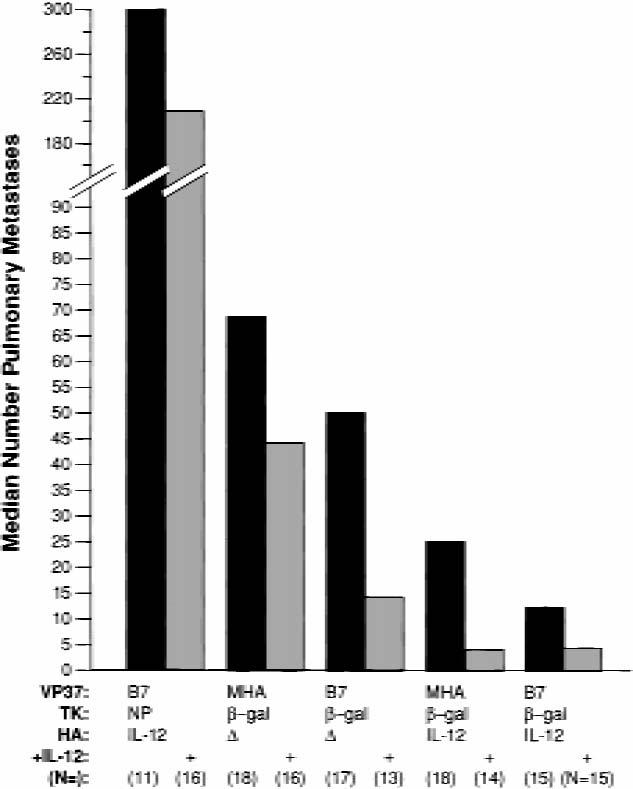
Active treatment of established metastases with recombinant vaccinia virus encoding a tumor-associated antigen, murine B7-1 (B7), and murine interleukin 12 (IL-12). The following data have been compiled from three identical experiments; the total number of mice in each group is shown in parentheses. Mice were inoculated with 5 × 105 CT26.CL25 tumor cells and treated 3 days later with 5 × 107 plaque-forming units of the indicated recombinant vaccinia virus. Groups of mice marked “+” received exogenous IL-12 (0.5 μg of recombinant murine IL-12 intraperitoneally) for 5 consecutive days after virus inoculation. Nine days after virus inoculation, mice were killed, their lungs were removed, and the metastases were counted. Solid bars indicate results from animals treated with the indicated recombinant viruses; shaded bars indicate results from animals treated with the indicated viruses and exogenous IL-12. VP37 = viral protein 37; MHA = measles hemagglutinin; TK = thymidine kinase; NP = nucleoprotein; β-gal = β-galactosidase; HA = hemagglutinin.
Fig. 4.
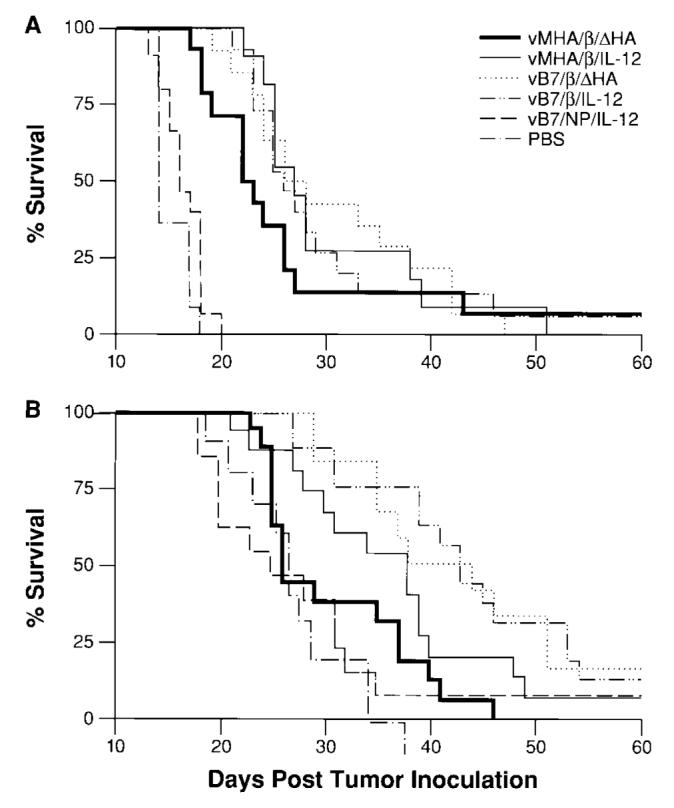
Effects of exogenous and endogenous delivery of interleukin 12 (IL-12) on the survival of mice with established metastases. The following data have been calculated from groups of at least 10 mice. Mice were inoculated with 5 × 105 CT26.CL25 tumor cells and treated 3 days later with 5 × 107 plaque-forming units of the indicated recombinant vaccinia virus. As indicated, some groups of mice were treated with 0.5 μg of recombinant murine IL-12 intraperitoneally for 5 consecutive days after virus inoculation. A) Coexpression of B7-1 and IL-12 in vaccinia virus (vB7/β/IL-12) did not have a statistically significant effect on the survival of mice with established β-galactosidase-positive tumors compared with β-galactosidase expression alone (vMHA/β/ΔHA). B) Mice receiving combined treatment with exogenous IL-12 and recombinant vaccinia virus coexpressing B7-1 and IL-12 (vB7/β/IL-12) had statistically significantly improved survival compared with mice receiving combined treatment with exogenous IL-12 and recombinant vaccinia virus expressing β-galactosidase (vMHA/β/ΔHA) See legend to Fig. 1 for explanation of abbreviations of treatment groups. PBS = phosphate-buffered saline.
Statistical Analysis
Lungs containing more than 300 metastases were defined as too numerous to count. Therefore, these data were analyzed with the nonparametric two-tailed Kruskal–Wallis test. The Mantel–Cox test was used to determine the statistical significance of the survival data. The statistical data were analyzed for overall difference among the groups, and then we reanalyzed the individual comparisons by adjusting P values by using a Bonferonni method to reflect the fact that we were making multiple pairwise comparisons. All P values are two-sided.
Results
Design, Construction, and Analysis of Recombinant Vaccinia Virus Expressing Multiple Gene Products
We constructed a panel of viruses to elucidate the specific antitumor effects of coexpression of a model tumor-associated antigen with the immune cofactors IL-12 and B7-1, either individually or in combination. The panel of recombinant vaccinia viruses (Fig. 1) was designed so that all recombinant viruses would express identical levels of model tumor-associated antigen and immune cofactors, because identical plasmids were used for homologous recombination. In addition, to control for the effects of viral open reading frame disruption (34) and high-level expression of foreign genes on viral replication in vitro and in vivo, we inserted control genes into the designated loci. The measles hemagglutinin gene was used to control for the effects of recombinant vaccinia virus expression of B7-1 because it is a glycosylated protein of similar molecular weight. No “triple control virus” (measles hemagglutinin/nucleoprotein/gpt) was constructed or used in these studies. We have shown (22,33) that wild-type fowlpox and vaccinia virus immunogens have no effect in this mouse model. A triple control virus (measles hemagglutinin/nucleoprotein/gpt) would almost certainly behave like the wild-type virus, known to mediate the expression of approximately 200 gene products. In other words, the expression of three irrelevant genes by large viruses, such as those in the poxvirus family, would be unlikely to alter its immunogenicity. Viruses were checked for stable expression of all recombinant gene products by use of a two-step immunostaining protocol, and all recombinant vaccinia viruses (including those expressing all five foreign genes) were found to be stable.
Specific vaccinia virus promoters were selected for expression of each recombinant gene. We used a vaccinia virus early/late promoter to drive expression of our model tumor-associated antigen, β-galactosidase, because early expression has been shown to be beneficial for induction of cytotoxic T-lymphocyte and immunotherapeutic responses (35). A synthetic early/late promoter was used for expression of B7-1 because we hypothesized that a high level of expression of the costimulatory protein may be more efficacious. The use of promoters for expression of the IL-12 subunits was dictated by the expression level requirements necessary for stability of the heterodimer (6). In addition, cytokines often function in a dose-dependent manner; therefore, poxvirus promoters yielding high levels of protein were used to drive expression of p35 and p40. We used the 7.5k promoter to drive expression of the model tumor-associated antigen β-galactosidase, as well as the selection gene gpt. The two 7.5k promoters were positioned so that, if homologous recombination and subsequent gene deletion occurred between these identical sequences, the resulting virus would not be viable because the intervening DNA region contains genes essential for vaccinia virus replication.
The expression of each of the transgenes was analyzed to ensure their fidelity before their use in vivo. Western blot analysis was carried out on all recombinant viruses (Fig. 2, A). Murine B7-1 was observed as a diffuse band of approximately 55 kilodaltons (kDa) (Fig. 2, A; lane 1). The two subunits of murine IL-12 were bands of approximately 40 and 35 kDa (Fig. 2, A; lane 3). The expression of biologically intact B7-1 was verified by its ability to bind one of its natural ligands, cytotoxic T-lymphocyte antigen-4. Anti-cytotoxic T-lymphocyte antigen-4 immunoglobulin could bind to the surface of the majority of cells infected with recombinant virus expressing B7-1, as shown by flow cytometry analysis (Fig. 2, B). The activity of murine IL-12 expressed by recombinant vaccinia virus was determined by measurement of the proliferative response of phorbol 12-myristate 13-acetate-activated murine splenocytes in the presence of culture supernatants from cells infected with recombinant vaccinia virus, as described previously (6). From these studies, it was calculated that the rate of in vitro expression of biologically active murine IL-12 was approximately 1.5 μg/106 cells per 24 hours (6). β-Galactosidase was determined to be enzymatically active by staining cells with 5-bromo-4-chloro-3-indolyl β-d-galactoside.
Fig. 2.

Analysis of viral expression of recombinant proteins. A) Western blot analysis of murine interleukin 12 (IL-12) and murine B7-1 is shown. Cells were infected with viruses encoding B7-1 (lane 1); the Western Reserve strain of vaccinia virus (lane 2); viruses encoding B7-1, Escherichia coli β-galactosidase, and IL-12 (lane 3); or the Western Reserve strain (lane 4). B) Flow cytometry analysis of the interaction of recombinant vaccinia virus expressed B7-1 with its ligand anti-cytotoxic T-lymphocyte antigen-4 immunoglobulin (CTLA4Ig) is shown. Infected BSC-1 monkey kidney cells were incubated with cytotoxic T-lymphocyte antigen-4 immunoglobulin or control CD7 immunoglobulin (CD7Ig). Ligand binding was detected by treatment with fluorescein isothiocyanate-conjugated anti-immunoglobulin. The y-axis represents cell number, and the x-axis gives fluorescence intensity. kDa 4 kilodaltons.
Thus, we developed a “cassette” system in which three loci of the vaccinia viral genome were used for homologous recombination. The resulting triple-recombinant vaccinia viruses that were constructed mediated the expression of a model tumor antigen, β-galactosidase, expressed alone or in combination with B7-1 and/or IL-12. The resultant recombinant vaccinia virus contained the following five transgenes: B7-1, IL-12 (p35 and p40), lacZ (the model antigen), and gpt (selection gene), all of which were indeed expressed in their full-length functional forms.
Active Immunotherapy of Established Pulmonary Metastases With Multiple Recombinant Vaccinia Virus
To determine the antitumor effects of IL-12 and B7-1 when expressed independently or simultaneously with the model tumor-associated antigen, we treated mice bearing 3-day-old pulmonary tumors with 5 × 107 plaque-forming units of the respective recombinant vaccinia viruses. The results from three consecutive and identical experiments were pooled and analyzed (Fig. 3). No mice were excluded from this analysis. Treatment with a recombinant vaccinia virus expressing tumor-associated antigen alone had a statistically significant effect compared with treatment with a control recombinant vaccinia virus, reducing the number of pulmonary metastases from more than 300 to 68 (P<.001). Coexpression of B7-1 and the tumor-associated antigen did not improve therapy in a statistically significant manner compared with tumor-associated antigen alone.
The group of mice receiving virus encoding IL-12 and tumor-associated antigen had fewer lung metastases than the group immunized with a virus containing tumor-associated antigen alone (median number of metastases = 68 versus 24), although this result was not statistically significant when adjusted for the number of pairwise comparisons. This antitumor effect appeared to be specific for tumor-associated antigen because treatment with recombinant vaccinia virus expressing tumor-associated antigen and IL-12 was statistically significantly better than treatment with recombinant vaccinia virus expressing IL-12 and B7-1 with a control antigen, nucleoprotein (P<.001). Coexpression of both immune cofactors with β-galactosidase did not statistically significantly improve active therapy compared with coexpression of tumor-associated antigen and IL-12 alone.
Effects of B7-1 and IL-12 Coexpression on Survival of Mice Bearing 3-Day-Old Pulmonary Metastases
To determine whether treatment with recombinant vaccinia virus expressing tumor-associated antigen and immune modulators could prolong the survival of mice with 3-day established pulmonary metastases, we treated the animals with recombinant vaccinia virus encoding B7-1 and/or IL-12 (Fig. 4, A). Treatment with recombinant vaccinia virus encoding tumor-associated antigen alone resulted in a statistically significant (P<.001) improvement in survival compared with treatment with recombinant vaccinia virus encoding an irrelevant tumor-associated antigen, nucleoprotein, with B7-1 and IL-12. Coexpression of tumor-associated antigen with B7-1 or IL-12 either independently or simultaneously did not increase average survival times of mice in a statistically significant manner compared with tumor-associated antigen alone.
Because the addition of exogenous recombinant murine IL-12 to recombinant vaccinia virus-based therapy further decreased lung metastases (Fig. 3), the effect of recombinant murine IL-12 on survival time was examined (Fig. 4, B). Treatment with exogenous recombinant murine IL-12 alone was found to improve survival in a statistically significant manner when administered with or without control virus (P<.001) to mice bearing established CT26.C25 pulmonary tumors. Indeed, the effects of the exogenous IL-12 appeared to mask the effects of the recombinant vaccinia virus expressing tumor-associated antigen because there was no statistically significant difference between this virus and one expressing the control antigen nucleoprotein with B7-1 and IL-12. However, in the presence of exogenous IL-12, treatment with recombinant vaccinia virus containing B7-1 and tumor-associated antigen increased survival in a statistically significant manner compared with treatment with tumor-associated antigen alone (P = .02).
Discussion
This study shows the feasibility of constructing vaccinia viruses that express tumor antigens and multiple immune cofactors to create unique immunologic microenvironments that can modulate immune responses to cancer. We have generated a set of triple-recombinant vaccinia viruses that use a “cassette” system for their construction. Multiply recombinant anticancer vaccines based on poxviruses can be constructed in this fashion because three different loci within the vaccinia virus genome are used. We have constructed a panel of recombinant vaccinia viruses that enabled us to study the activities and contributions of tumor-associated antigen, B7-1, and IL-12 alone and in combination. Importantly, all viruses stably expressed biologically active IL-12, B7-1, and/or tumor-associated antigen. An advantage of the cassette system described in this article is the ability to rapidly screen immunoregulatory molecules for their function in cancer immunotherapy. Homologous recombination of one or more complementary DNAs could yield a recombinant vaccinia virus encoding tumor-associated antigen and the molecule(s) of interest.
Manipulation of the immune response may be a useful addition to current treatment options for human cancer. Lymphocytes can recognize and destroy tumor cells in vitro (2,3) and in vivo (36). It is likely that a proper immunologic microenvironment will promote the induction and maintenance of an antitumor immune response. Two immunostimulatory molecules that have been implicated in the antitumor immune response are IL-12 and B7-1. In this article, we show the successful antigen-specific treatment of a murine cancer by coexpressing tumor-associated antigen, IL-12, and B7-1 in a recombinant vaccinia virus. The treatment showed no obvious toxicity and was effective in reducing established lung metastases. In addition, when exogenous IL-12 was administered with a virus coexpressing tumor-associated antigen and B7-1, the treatment could increase the survival time of mice over that of mice that received exogenous IL-12 and a virus expressing tumor-associated antigen alone.
IL-12 has recently been shown (17) to have distinct antitumor effects in several model systems. It has also been shown to be effective when expressed by a recombinant vaccinia virus, although only in a preliminary model (6). The therapeutic effectiveness of IL-12 expressed by recombinant vaccinia virus, determined to produce 1.5 μg/106 cells per 24 hours in vitro for approximately 5 days [(6); Restifo NP: unpublished data], appeared no different from that of treatment with exogenous IL-12 at 0.5 μg per mouse per day. We observed a statistically significant improvement in survival of mice that were treated with exogenous IL-12 and recombinant vaccinia virus expressing B7-1 and β-galactosidase. This effect was distinct from the antigen-independent effects of IL-12 that were observed in both sets of experiments. A statistically significant reduction in the number of lung metastases was observed when mice were treated with a control virus expressing an irrelevant tumor-associated antigen and exogenous IL-12 (Fig. 3). In addition, mice bearing tumors established for 3 days survived statistically significantly longer than untreated mice when administered exogenous IL-12 alone (Fig. 4). This nonspecific effect was further illustrated by our ability to treat a tumor lacking the model antigen (the CT26 cell line) with the administration of exogenous IL-12 (data not shown). Surprisingly, coexpression of B7-1 did not enhance the treatment effect of tumor-associated antigen or IL-12 in the lung metastases model as we have reported (5,21). This difference may be due to the fivefold higher dose of virus given in this study, which could have resulted in the immunostimulatory effect of the tumor-associated antigen overwhelming the effects of B7-1 and IL-12. Also, it should be noted that the function of B7-1 was demonstrated by binding to cytotoxic T-lymphocyte antigen-4, whereas the binding efficiency of B7-1 to CD28 is 20-fold lower (37). In the survival model, however, when B7-1 was expressed in a virus along with tumor-associated antigen, an enhanced effect compared with the effect of tumor-associated antigen alone was observed when exogenous IL-12 was used, suggesting that the B7-1 was capable of engaging a positive signaling ligand, likely to be CD28. This finding is in agreement with results from survival experiments performed by Rao et al. (21).
Because β-galactosidase is a xenogeneic antigen that is derived from E. coli and can be down-regulated in vivo over time (38), the model described in this article can be paralleled only to tumors that express non-self antigens such as viral antigens or mutated “self” antigens. Many of the tumor-associated antigens recently characterized in melanoma are nonmutated self antigens (39). However, it does appear that recombinant vaccinia virus can induce immune responses that can overcome tolerance to self antigens, as was shown for a recombinant vaccinia virus expressing the tyrosinase-related protein-1 in a C57BL/6 mouse model system. Two inoculations with the recombinant vaccinia virus were able to induce autoimmunity to melanocytes (Overwijk WW: unpublished data). Also, a related vaccinia virus strain, ectromelia, expressing a zona pellucida glycoprotein can induce infertility in mice (40).
As a result of the effort of the World Health Organization's campaign to eliminate smallpox, many people born before 1970 will have recall immune responses to vaccinia virus, possibly decreasing the duration of expression in vivo. In recent years, alternative agents such as recombinant fowlpox virus (22) and naked DNA (41) have been developed as recombinant vectors. Although we and others continue to explore the use of recombinant vaccinia virus in clinical trials, the use of immunodulatory molecules such as B7-1 and IL-12, as well as others including interleukin 2 (33), should be explored by use of alternative vectors, including DNA, expressing cloned tumor-associated antigen such as MART-1/MelanA, gp100, and tyrosinase.
Acknowledgments
We thank Martha Blalock for graphical expertise, Don White for statistical analyses, David Jones for help with animal procurement, and Karen Hathcock for help with the B7-1/anti-cytotoxic T-lymphocyte antigen-4 flow cytometry analysis.
References
- 1.Chen L, Mizuno MT, Singhal MC, Hu SL, Galloway DA, Hellstrom I, et al. Induction of cytotoxic T lymphocytes specific for a syngeneic tumor expressing the E6 oncoprotein of human papillomavirus type 16. J Immunol. 1992;148:2617–21. [PubMed] [Google Scholar]
- 2.Boon T, Coulie PG, Van den Eynde B. Tumor antigens recognized by T cells. Immunol Today. 1997;18:267–8. doi: 10.1016/s0167-5699(97)80020-5. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA. Cancer vaccines based on the identification of genes encoding cancer regression antigens. Immunol Today. 1997;18:175–82. doi: 10.1016/s0167-5699(97)84664-6. [DOI] [PubMed] [Google Scholar]
- 4.Hodge JW, Abrams S, Schlom J, Kantor JA. Induction of antitumor immunity by recombinant vaccinia viruses expressing B7-1 or B7-2 costimulatory molecules. Cancer Res. 1994;54:5552–5. [PubMed] [Google Scholar]
- 5.Chamberlain RS, Carroll MW, Bronte V, Hwu P, Warren S, Yang JC, et al. Costimulation enhances the active immunotherapy effect of recombinant anticancer vaccines. Cancer Res. 1996;56:2832–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Meko JB, Yim JH, Tsung K, Norton JA. High cytokine production and effective antitumor activity of a recombinant vaccinia virus encoding murine interleukin 12. Cancer Res. 1995;55:4765–70. [PubMed] [Google Scholar]
- 7.Mackett M, Smith GL, Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. 1982 [classical article] Biotechnology. 1992;24:495–9. [PubMed] [Google Scholar]
- 8.Panicali D, Paoletti E. Construction of poxviruses as cloning vectors: insertion of the thymidine kinase gene from herpes simplex virus into the DNA of infectious vaccinia virus. Proc Natl Acad Sci U S A. 1982;79:4927–31. doi: 10.1073/pnas.79.16.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moss B. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc Natl Acad Sci U S A. 1996;93:11341–8. doi: 10.1073/pnas.93.21.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennink JR, Yewdell JW, Smith GL, Moller C, Moss B. Recombinant vaccinia virus primes and stimulates influenza haemagglutinin-specific cytotoxic T cells. Nature. 1984;311:578–9. doi: 10.1038/311578a0. [DOI] [PubMed] [Google Scholar]
- 11.Borysiewicz LK, Fiander A, Nimako M, Man S, Wilkinson GW, Westmoreland D, et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347:1523–7. doi: 10.1016/s0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 12.Kantor J, Irvine K, Abrams S, Kaufman H, DiPietro J, Schlom J. Antitumor activity and immune responses induced by a recombinant carcinoembryonic antigen–vaccinia virus vaccine. J Natl Cancer Inst. 1992;84:1084–91. doi: 10.1093/jnci/84.14.1084. [DOI] [PubMed] [Google Scholar]
- 13.Meneguzzi G, Cerni C, Kieny MP, Lathe R. Immunization against human papillomavirus type 16 tumor cells with recombinant vaccinia viruses expressing E6 and E7. Virology. 1991;181:62–9. doi: 10.1016/0042-6822(91)90470-v. [DOI] [PubMed] [Google Scholar]
- 14.Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173:721–30. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993;259:368–70. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- 16.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–27. [PubMed] [Google Scholar]
- 17.Tahara H, Zeh HJ, 3rd, Storkus WJ, Pappo I, Watkins SC, Gubler U, et al. Fibroblasts genetically engineered to secrete interleukin 12 can suppress tumor growth and induce antitumor immunity to a murine melanoma in vivo. Cancer Res. 1994;54:182–9. [PubMed] [Google Scholar]
- 18.Nastala CL, Edington HD, McKinney TG, Tahara H, Nalesnik MA, Brunda MJ, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994;153:1697–706. [PubMed] [Google Scholar]
- 19.Kubin M, Kamoun M, Trinchieri G. Interleukin 12 synergizes with B7/CD28 interaction in inducing efficient proliferation and cytokine production of human T cells. J Exp Med. 1994;180:211–22. doi: 10.1084/jem.180.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy EE, Terres G, Macatonia SE, Hsieh CS, Mattson J, Lanier L, et al. B7 and interleukin 12 cooperate for proliferation and interferon gamma production by mouse T helper clones that are unresponsive to B7 costimulation. J Exp Med. 1994;180:223–31. doi: 10.1084/jem.180.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao JB, Chamberlain RS, Bronte V, Carroll MW, Irvine KR, Moss B, et al. IL-12 is an effective adjuvant to recombinant vaccinia virus-based tumor vaccines: enhancement by simultaneous B7-1 expression. J Immunol. 1996;156:3357–65. [PMC free article] [PubMed] [Google Scholar]
- 22.Wang M, Bronte V, Chen PW, Gritz L, Panicali D, Rosenberg SA, et al. Active immunotherapy of cancer with a nonreplicating recombinant fowl-pox virus encoding a model tumor-associated antigen. J Immunol. 1995;154:4685–92. [PMC free article] [PubMed] [Google Scholar]
- 23.Specht JM, Wang G, Do MT, Lam JS, Royal RE, Reeves ME, et al. Dendritic cells retrovirally transduced with a model antigen gene are therapeutically effective against established pulmonary metastases. J Exp Med. 1997;186:1213–21. doi: 10.1084/jem.186.8.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song W, Kong HL, Carpenter H, Torii H, Granstein R, Rafii S, et al. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J Exp Med. 1997;186:1247–56. doi: 10.1084/jem.186.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blasco R, Moss B. Selection of recombinant vaccinia viruses on the basis of plaque formation. Gene. 1995;158:157–62. doi: 10.1016/0378-1119(95)00149-z. [DOI] [PubMed] [Google Scholar]
- 26.Chakrabarti S, Sisler JR, Moss B. Compact, synthetic, vaccinia virus early/late promoter for protein expression. Biotechniques. 1997;23:1094–7. doi: 10.2144/97236st07. [DOI] [PubMed] [Google Scholar]
- 27.Chakrabarti S, Brechling K, Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985;5:3403–9. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith GL, Levin JZ, Palese P, Moss B. Synthesis and cellular location of the ten influenza polypeptides individually expressed by recombinant vaccinia viruses [published erratum appears in Virology 1988;163:259] Virology. 1987;160:336–45. doi: 10.1016/0042-6822(87)90004-3. [DOI] [PubMed] [Google Scholar]
- 29.Falkner FG, Moss B. Escherichia coli gpt gene provides dominant selection for vaccinia virus open reading frame expression vectors. J Virol. 1988;62:1849–54. doi: 10.1128/jvi.62.6.1849-1854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carroll MW, Moss B. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in a nonhuman mammalian cell line. Virology. 1997;238:198–211. doi: 10.1006/viro.1997.8845. [DOI] [PubMed] [Google Scholar]
- 31.Earl PL, Cooper N, Moss B. Preparation of cell cultures and vaccinia virus stocks. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, editors. Current protocols in molecular biology. John Wiley & Sons; New York (NY): 1991. pp. 16.16.1–16.16.7. [Google Scholar]
- 32.Hathcock KS, Laszlo G, Pucillo C, Linsley P, Hodes RJ. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med. 1994;180:631–40. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bronte V, Tsung K, Rao JB, Chen PW, Wang M, Rosenberg SA, et al. IL-2 enhances the function of recombinant poxvirus-based vaccines in the treatment of established pulmonary metastases. J Immunol. 1995;154:5282–92. [PMC free article] [PubMed] [Google Scholar]
- 34.Buller RM, Smith GL, Cremer K, Notkins AL, Moss B. Decreased virulence of recombinant vaccinia virus expression vectors is associated with a thymidine kinase-negative phenotype. Nature. 1985;317:813–5. doi: 10.1038/317813a0. [DOI] [PubMed] [Google Scholar]
- 35.Bronte V, Carroll MW, Goletz TJ, Wang M, Overwijk WW, Marincola F, et al. Antigen expression by dendritic cells correlates with the therapeutic effectiveness of a model recombinant poxvirus tumor vaccine. Proc Natl Acad Sci U S A. 1997;94:3183–8. doi: 10.1073/pnas.94.7.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melief CJ, Kast WM. T-cell immunotherapy of tumors by adoptive transfer of cytotoxic T lymphocytes and by vaccination with minimal essential epitopes. Immunol Rev. 1995;145:167–77. doi: 10.1111/j.1600-065x.1995.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 37.Kariv I, Truneh A, Sweet RW. Analysis of the site of interaction of CD28 with its counter-receptors CD80 and CD86 and correlation with function. J Immunol. 1996;157:29–38. [PubMed] [Google Scholar]
- 38.Carroll MW, Overwijk WW, Chamberlain RS, Rosenberg SA, Moss B, Restifo NP. Highly attenuated modified vaccinia virus Ankara (MVA) as an effective recombinant vector: a murine tumor model. Vaccine. 1997;15:387–94. doi: 10.1016/s0264-410x(96)00195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–86. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson RJ, Maguire DJ, Hinds LA, Ramshaw IA. Infertility in mice induced by a recombinant ectromelia virus expressing mouse zona pellucida glycoprotein 3. J Biol Reprod. 1998;58:152–9. doi: 10.1095/biolreprod58.1.152. [DOI] [PubMed] [Google Scholar]
- 41.Irvine KR, Rao JB, Rosenberg SA, Restifo NP. Cytokine enhancement of DNA immunization leads to effective treatment of established pulmonary metastases. J Immunol. 1996;156:238–45. [PMC free article] [PubMed] [Google Scholar]


