Abstract
Background:
Evidence that simian virus 40 (SV40) is associated with human mesotheliomas, osteosarcomas, and brain tumors suggests that a recombinant vaccine directed against lethal cancers expressing SV40 T antigen (Tag) could have clinical utility. To address this potential need, we designed a novel vaccinia virus construct that encodes an SV40 Tag in which oncogenic domains were excluded and immunogenic domains were preserved. We named this recombinant construct vaccinia-encoding safety-modified SV40 Tag (vac-mTag).
Methods:
Purified vac-mTag was characterized by DNA sequencing, reverse transcription-coupled polymerase chain reaction, western blot analysis, and immunocytochemical techniques. Induction of Tag-specific immunity was examined by cytolytic T-cell assays, and the efficacy of vac-mTag in protecting animals against Tag-expressing tumors and in treating pre-established microscopic tumors was evaluated in vac-mTag-immunized BALB/c mice.
Results:
The immune response elicited by vac-mTag in C57BL/6 and BALB/c mice included an SV40 Tag-specific cytolytic T-lymphocyte activity against syngeneic (identical genetic background) SV40 Tag-expressing tumor targets. Immunization of mice with a single dose of vac-mTag resulted in potent protection against subsequent challenge with a lethal mouse cancer expressing SV40 Tag. In addition, single-dose vac-mTag immunization coadministered with interleukin 2 produced a possible therapeutic effect against a preadministered microscopic (but lethal) burden of Tag-expressing tumor cells in vivo.
Conclusion:
vac-mTag induces an effective immune response in mice that is specific for a tumor-associated antigen. This response protects against a lethal tumor challenge and results in a possible therapeutic effect against Tag-expressing tumors in vivo. Thus, vac-mTag provides a new avenue for the development of therapies for human cancers thought to be associated with SV40.
Recent developments support the hypothesis that simian virus 40 (SV40) T antigen (Tag) is a relevant target for immunotherapy for human cancers. Contemporary assays for DNA sequences have found SV40 DNA in a majority of human osteosarcomas, bone tumors, ependymomas, choroid plexus tumors, and mesotheliomas that have been evaluated (1-5). Expression of SV40 Tag protein in mesotheliomas and brain tumors has been demonstrated, and functional studies (6,7) have shown that SV40 Tag can interfere with the functions of p53 and retinoblastoma protein in human mesotheliomas. The possible relationship between polio vaccines contaminated with SV40, which were administered to more than 98 million subjects from 1955 through early 1963, and the oncogenesis of these cancers remains controversial (8). Nonetheless, the subsequently increasing occurrence of mesothelioma, a lethal cancer that commonly expresses SV40 Tag and that has limited treatment options, is disconcerting (9). Strategies targeting SV40 Tag represent a rational avenue for developing new therapies for such SV40 Tag-expressing cancers.
SV40 Tag has several attributes that make it potentially useful in recombinant vaccine strategies. First, because SV40 Tag is a genuine tumor-associated antigen that is not present in normal host cells, vaccine therapies targeting this antigen need not overcome tolerance to achieve efficacy and circumvent the potential hazard of autoimmune responses against tumor-associated antigens coexpressed by tumor and normal tissue. Second, apart from its association with certain human cancers, SV40 is also pathogenic in rodent models, providing a unique setting for optimizing potentially clinically relevant recombinant vaccines in animal models (10). Third, the immunogenicity of SV40 Tag has been studied extensively (11-13), providing insight for rational approaches to the development of new therapeutic vaccine strategies targeting this tumor-associated antigen.
Despite these attributes of SV40 Tag, a cancer vaccine strategy providing safe and effective therapy for pre-established SV40 Tag-expressing tumors has previously been elusive. To determine whether recombinant poxvirus vaccines targeting SV40 Tag would provide an avenue for unprecedented therapeutic efficacy against established SV40 Tag-expressing tumors, we have made a novel recombinant vaccinia construct. This vector encodes a safety-modified SV40 Tag sequence (mTag) that excludes retinoblastoma protein binding site, p53 binding site, and the amino-terminal oncogenic CR1 and J domains to optimize potential clinical safety but that preserves immunogenic domains. After confirming expression of the expected SV40 Tag fragment by this novel recombinant vaccinia construct termed recombinant vaccinia-encoding mTag (vac-mTag), studies were undertaken to evaluate its efficacy against SV40 Tag-expressing tumors in vivo. These studies provide evidence that vac-mTag can efficiently prime the immune response to provide effective antigen-specific protection and therapy against SV40 Tag-expressing lethal tumors.
Materials and Methods
Cell Lines
All cell lines were maintained in Dulbecco's modified Eagle medium (Life Technologies, Inc. [GIBCO BRL], Gaithersburg, MD) supplemented with 100 U of penicillin per mL, 100 mg of streptomycin per mL, and 10% fetal bovine serum (Life Technologies, Inc.) at 37 °C in a 5% CO2 incubator. mKSA (provided by J. Butel, Baylor College of Medicine, Houston, TX) and B6wt19 (provided by S. Tevethia, The Pennsylvania State University College of Medicine, Hershey) are SV40 Tag-expressing cell lines derived from BALB/c and C57BL/6 mice, respectively. BSC-1 (a monkey tumor cell line that is known to be a productive host cell for vaccinia infection), YAC-1 (a murine tumor cell line that is exquisitely sensitive to cytolysis mediated by natural killer cells or lymphokine-activated killer cells), and Tag-expressing COS-1 cells were obtained from the American Type Culture Collection (Manassas, VA). RM-1 (provided by T. Thompson, Baylor College of Medicine) is a prostate cancer cell line that lacks Tag and was derived from C57BL/6 mice.
Animals
Six- to 8-week-old male C57BL/6 and BALB/c mice were purchased from Harlan Sprague-Dawley, Inc. (Indianapolis, IN). All experiments were approved by the University of Michigan Committee on Use and Care of Animals and were conducted in accordance with National Institutes of Health guidelines. Mice were followed until death from cancer or were euthanized when an individual who was blinded to the immunization or therapy status of the animals determined that tumors interfered with the animal's well-being, as shown by ungroomed fur, slow movement, or cachexia (as evidenced by wasting and spinal protrusion). Death was confirmed to be tumor-related via postmortem examination by a licensed veterinarian.
Construction of Recombinant Vaccinia Encoding an SV40 Tag Fragment
Polymerase chain reaction (PCR) was performed to amplify mTag from the pBSV-1 plasmid (provided by J. Butel), with 5′ (5′-GGAAGATCTGTCGACCATGGTGTCTGCTATTAATAACTAGC-3′) and 3′(5′-ATACCAATTAATTAACCCGGGTACCTTATTACTCACTGCGTTCCAGGCAATG-3′) primers to adapt the mTag fragment for cloning. The PCR product was electrophoresed in a 1% agarose gel (Boehringer Mannheim Corp., Indianapolis, IN), purified by use of a QIAEX II gel extraction kit (QIAGEN, Santa Clarita, CA), and subcloned in a site adjacent to the synthetic E/L promoter (pS.E/L) in pSC65 (14-16) that had been digested with PacI and BglII. The pSC65-mTag ligation product was used to transform DH5a cells, and pSC65-mTag plasmid DNA was obtained by use of the QIAGEN plasmid preparation kit. Orientation of the mTag insert was confirmed by HindIII digestion, and DNA sequencing confirmed the expected sequence.
vac-mTag was generated by homologous recombination in BSC-1 cells transfected by pSC65-mTag as described (14), with recombinant BSC-1 plaques identified by immunocytostaining with Tag-specific monoclonal antibody Pab204 (provided by J. Pipas, University of Pittsburgh, PA). For immunocytostain, BSC-1 cells were infected with vaccinia control or vac-mTag at a multiplicity of infection of five for 48 hours in eight-well chamber slides and then fixed with a solution of acetone and methanol followed by incubation with Pab204 (1 : 400 dilution). After a 2-hour incubation at room temperature with primary Pab204 anitbody, samples were washed twice and incubated with horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulin G1 antibody (1 : 200 dilution) for 1 hour, after which the substrate diaminobenzidine was added for 5 minutes. Isolation and titer determination of both vac-mTag and control vaccinia vector V69 (encoding influenza nuclear protein) were performed as previously described (14-16).
Reverse Transcription-Coupled PCR
BSC-1 cells were infected with either the control vaccinia vector (V69) or vac-mTag at a multiplicity of infection of five for 48 hours. Total cellular RNA was prepared from infected and uninfected BSC-1 cells, mKSA cells, and B6wt19 cells with the use of RNAzolB (Tel-Test, Friendswood, TX) and quantified by spectrophotometry. Reverse transcription was performed with the Promega reverse transcription system (Promega Corp., Madison, WI). PCR amplification was performed with mTag cloning primers (see above). PCR products were electrophoresed in 1% agarose gels (Boehringer Mannheim Corp.) and visualized with ethidium bromide.
Western Blot Analysis
BSC-1 cells infected with either the control vaccinia vector (V69) or vac-mTag (as described for reverse transcription-coupled PCR above) were harvested and resuspended in protein lysis buffer (1% deoxycholate, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, 0.15 M NaCl, 10 mM NaH2PO4 [pH 7], and 1 mM dithiothreitol; Sigma Chemical Co., St. Louis, MO) with protein inhibitors phenylmethylsulfonyl fluoride, leupeptin, and aprotinin (Boehringer Mannheim Corp.). Protein concentration was determined with Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA). Sodium dodecyl sulfate–polyacrylamide gel electrophoresis in 8% gels was carried out at 250 V for 4 hours at 4 °C with 50 μg of total protein per lane and transferred to nitro-cellulose (Hoefer Semi-Phor™ system; Hoefer Scientific Instruments, San Francisco, CA). After blocking with 5% milk for 1 hour at room temperature, the filter was incubated with primary antibody Pab204 (1 : 5000 dilution), washed three times in Tris-buffered saline containing 0.1% Tween 20, incubated with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G antibody (1 : 10 000 dilution), and visualized by use of ECL detection reagents (Amersham Life Science Inc., Arlington Heights, IL).
SV40 Tag-Specific Cytotoxic T-Lymphocyte (CTL) Activity
Splenocytes from C57BL/6 and BALB/c mice were harvested 3 weeks after intravenous injection of vac-mTag or control vaccinia V69 (5 × 106 plaque-forming units/mouse). CTL activity was evaluated by chromium (51Cr) release assay after 1 week of splenocyte stimulation with mitomycin C-treated syngeneic Tag-expressing tumor cells in vitro. 51Cr-labeled target cells were incubated with splenocytes at ratios of 100, 20, 4, and 0.8 for 4 hours, and lysates were harvested and analyzed as described previously (17). Percent specific lysis was calculated from triplicate samples as follows: [(experimental cpm − spontaneous cpm)/(maximal cpm − spontaneous cpm)] × 100, where cpm = counts per minute. Data included in this report represent all assays in which spontaneous release of labeled target cells was less than 20% of maximal release, and standard deviation of triplicate values were less than 15%.
Vaccine Protection and Therapy Against Tumor Challenge
For experiments evaluating protection against tumor challenge after immunization, BALB/c mice were immunized with V69 or vac-mTag at 5 × 106 plaque-forming units/mouse via tail-vein injection. Three weeks later, 106 mKSA tumor cells were injected subcutaneously in the right flank. Animals were monitored four to six times weekly for onset and progression of tumors that were measurable by calipers and for survival by an individual blinded to the immunization status of the animals.
To evaluate therapy of pre-established microscopic tumors in BALB/c mice, 106 mKSA tumor cells were injected subcutaneously in the right flank. Therapy was begun 2 days later by injecting into the tail vein vac-mTag or V69 (control vaccinia vector) at 5 × 106 plaque-forming units/mouse combined with intraperitoneal injection of recombinant interleukin 2 at 90 000 IU per mouse (Hoffmann-La Roche Inc., Nutley, NY) once daily for an additional 3 days. Animals were monitored four to six times weekly for onset and progression of tumors measurable by calipers and for survival by an individual blinded to the immunization status of the animals.
Statistical Analysis
Statistical significance of survival data was evaluated by Kaplan–Meier plots and logrank analysis was performed with Statistica software (StatSoft, Inc., Tulsa, OK). All P values are two-sided.
Results
Construction and Characterization of vac-mTag, a Recombinant Vaccinia Virus Encoding SV40 Tag Immunogenic Domains and Oncogenic Domains
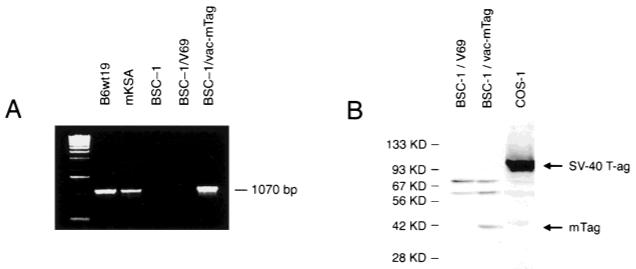
A strategy was undertaken to construct a poxvirus vaccine that would encode sequences from SV40 Tag, would preserve as many potential T-cell epitopes from SV40 Tag as possible, would allow abundant expression regulated by a vaccinia promoter, and would also have multiple safety features for potential human use. For this purpose, a region of SV40 Tag was selected that excludes the following pivotal oncogenic domains of SV40 Tag: the retinoblastoma protein binding site, the second site of the bipartite p53 binding region, and the transforming CR1 and J domains (18-21). The nononcogenic region was extended to include defined murine CTL epitopes for relevant preclinical efficacy testing (13); to include a majority of the 23 peptides in SV40 Tag that are candidates for presentation by common human HLA A, B, or C subtypes based on their low dissociation rates (half-time of dissociation >2 hours) calculated with available software (vide infra) (22,23); and to concurrently exclude two cryptic motifs (T5NT sites, where N is any nucleotide) that could interfere with vaccinia-directed translation (24) (Fig. 1). These criteria delimited a fragment of SV40 Tag encoding amino acids 205–566, and mTag, the modified SV40 Tag gene encoding this region, was positioned under the control of the vaccinia synthetic early and late promoter (pS.E/L in pSC65), which has been shown to have favorable capacity for inducing tumor-associated antigen-specific antitumor immune responses compared with other vaccinia promoters (14,16). After cloning mTag complementary DNA into pSC65 plasmid, which was then integrated into vaccinia virus, plaques of BSC-1 cells producing vac-mTag were identified by immunocytostaining with anti-Tag monoclonal antibody Pab204 (data not shown). This monoclonal antibody specifically binds to amino acids 453–469 of the Tag protein (25). Expression of the expected mTag transcript was confirmed by reverse transcription-coupled PCR (Fig. 2, A). Primers corresponding to sequences flanking the ends of the 1070-base-pair transcript were used to amplify a complementary DNA of the expected size in vac-mTag-infected BSC-1 cells; this transcript was not detected in uninfected or control vaccinia-infected BSC-1 cells. Translation of the truncated Tag protein fragment was confirmed by western blotting with anti-Tag antibody Pab204, which demonstrated the expected 40-kd band in vac-mTag-infected BSC-1 cells (Fig. 2, B). Thus, a unique recombinant vaccinia virus encoding an SV40 Tag region has been constructed and isolated that leads to expression in infected primate cells of a truncated SV40 Tag protein that lacks defined oncogenic domains but preserves putative T-cell epitopes.
Fig. 1.
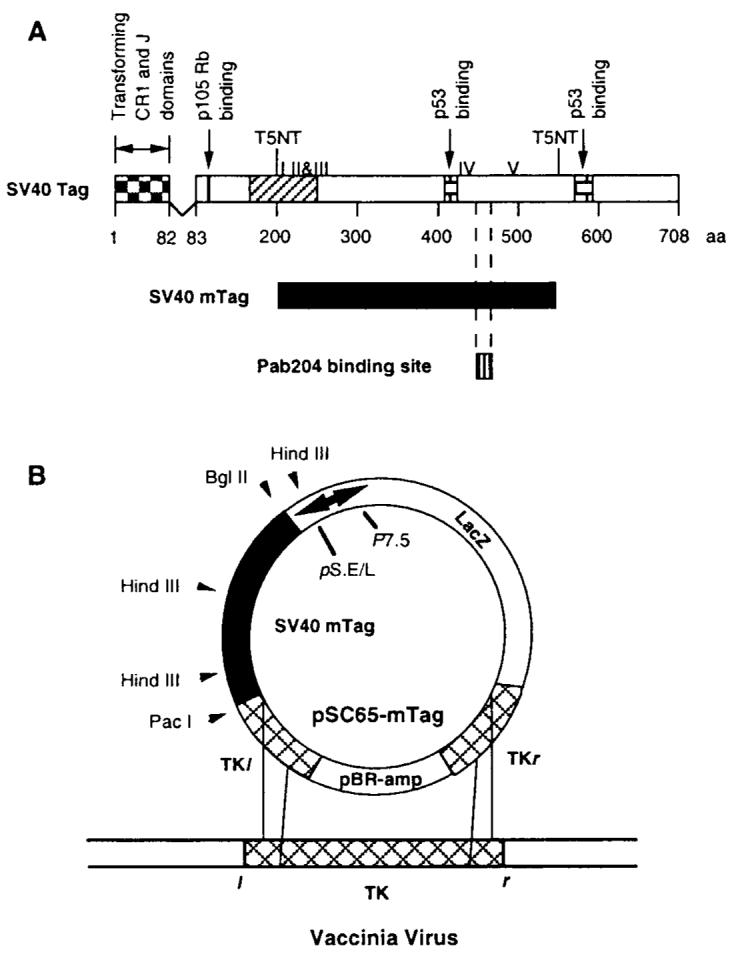
Design and construction of a recombinant vaccinia vector that encodes a simian virus 40 (SV40) T antigen (Tag) fragment that excludes oncogenic domains but preserves putative T-cell recognition sites. A) mTag, a 1070-base-pair fragment of SV40 Tag complementary DNA encoding amino acids 205–566, excludes oncogenic domains (retinoblastoma protein binding, p53 binding, and amino-terminal CR1 and J region transforming domains) but preserves five murine cytotoxic T lymphocyte epitopes (I–V) and multiple candidate peptides for presentation by human leukocyte antigens HLA A, HLA B, or HLA C. B) After mTag was cloned into pSC65 vaccinia shuttle vector in a position regulated by the synthetic early/late promoter (pS.E/L), recombination at homologous thymidine kinase (TK) sequences yielded the recombinant virus named vac-mTag.
Fig. 2.
Infection by vac-mTag, a recombinant vaccinia-encoding safety-modified simian virus 40 (SV40) T antigen (Tag), leads to abundant expression in host cells of a truncated Tag protein. BSC-1 monkey tumor cells (2 × 105 cells) were infected with either control vaccinia (V69) or vac-mTag. Total cellular RNA and protein lysates were prepared from the infected and uninfected BSC-1 cells, Tag-expressing B6wt19 mouse cells, Tag-expressing mKSA mouse cells, and Tag-expressing COS-1 monkey cells. A) Reverse transcription-coupled polymerase chain reaction (RT–PCR) was performed to detect the expected transcript in vac-mTag-infected BSC-1 cells compared with uninfected or control vaccinia (V69)-infected BSC-1 cells. RNA from Tag-expressing B6wt19 and mKSA cells was used to confirm sensitivity of the RT–PCR for detecting expressed Tag sequences. B) Western blot analysis was performed with anti-Tag monoclonal antibody Pab204. Fifty micrograms of total protein was loaded in each lane. SV40 Tag-expressing COS-1 cells were used as a positive control to confirm the sensitivity of the antibody Pab204. The expected 93-kd SV40 Tag protein in COS-1 cells and the expected 40-kd mTag protein in vac-mTag-infected BSC-1 cells are indicated by arrows. Control vaccinia (V69)-infected BSC-1 cells do not express mTag. KD = kilodaltons; bp = base pairs.
Immunization With vac-mTag Induces Specific CTL Activity Against SV40 Tag
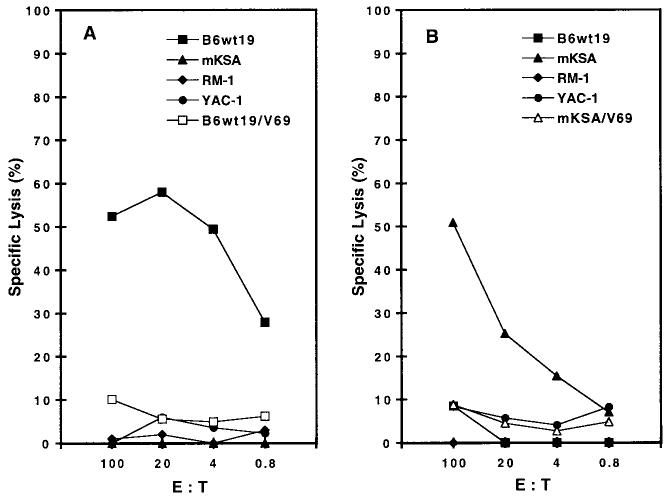
We next sought to evaluate the ability of vac-mTag to induce SV40 Tag-specific CTLs in vivo. These experiments were undertaken in C57BL/6 and BALB/c mice due to the availability of syngeneic transformed cells from these mouse strains (B6wt19 and mKSA cells) that express Tag. SV40 Tag-specific CTL activity was evaluated with effector CTLs derived from splenocytes of vac-mTag-immunized C57BL/6 mice against C57BL/6-derived Tag-expressing B6wt19 target cells (Fig. 3, A). This CTL activity was major histocompatibility complex (MHC) class I-restricted, as shown by the absence of cytolytic activity against BALB/c-derived mKSA tumor cells. Antigenic specificity to Tag of the CTL response induced by vac-mTag was demonstrated as follows: Tag-expressing B6wt19 tumor cells were not lysed by control CTLs from mice immunized with a control vaccinia vector lacking Tag sequences (V69), syngeneic RM-1 tumor cells lacking Tag were not lysed by Tag-specific CTLs derived from C57BL/6 mice immunized with vac-mTag, and natural killer cell-sensitive YAC-1 target cells were likewise not killed by the vac-mTag-primed CTLs. Conversely, CTLs from vac-mTag-immunized BALB/c mice only killed Tag-expressing target cells of BALB/c origin (mKSA) without substantial lysis of B6wt19 cells, confirming MHC class I restriction of the CTL response (Fig. 3, B). Thus, vac-mTag immunization induced MHC class I-restricted Tag-specific CTLs.
Fig. 3.
Immunization with vac-mTag, a recombinant vaccinia-encoding safety-modified simian virus 40 (SV40) T antigen (Tag), facilitates induction of major histocompatibility complex class I-restricted Tag-specific cytotoxic T-lymphocyte (CTL) activity. C57BL/6 mice (A) or BALB/C mice (B) were immunized with V69 (control vaccinia) or vac-mTag at 5 × 106 plaque-forming units/mouse, administered by tail-vein injection. Three weeks after immunization, splenocytes were harvested and assayed for Tag-specific CTL activity by chromium release assay after 7 days of in vitro stimulation with mitomycin C-treated syngeneic (identical genetic background) Tag-expressing tumor cells. Targets include Tag-expressing B6wt19 (H-2b) cells, Tag-expressing mKSA (H-2d) cells, and RM-1 (H-2b) cells, which do not express Tag. YAC-1 cells were also included to evaluate cytolytic activity of natural killer cells. Open symbols represent nonspecific syngeneic CTL activity induced by the vaccinia vector control V69. Individual conditions were performed in triplicate, and standard deviation at each measurement did not exceed 15%. This experiment was repeated three times with similar results. E : T = effector cell to target cell ratio.
Antitumor Efficacy of vac-mTag Administered In Vivo
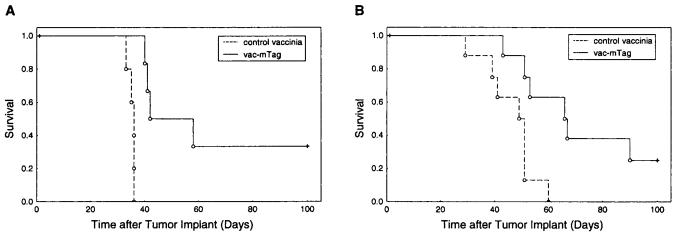
After demonstrating that vac-mTag was able to induce Tag-specific CTL activity, we undertook studies to characterize the in vivo antitumor efficacy of vac-mTag immunization against growth and progression of Tag-expressing tumors. The tumorigenic and highly lethal mKSA model system was used for these studies (10), instead of the B6wt19 system, because B6wt19 cells were not consistently tumorigenic in control assays. First, the protective effect of vac-mTag immunization against subsequent tumor challenge was evaluated by immunization of BALB/c mice with vac-mTag or control vaccinia followed with mKSA tumor challenge 3 weeks later. Statistically significant protection against tumor challenge was seen in vac-mTag-immunized mice compared with control vaccinia vector-immunized mice, as measured by survival (Fig. 4, A; P = .02). A similar protective effect of vac-mTag immunization was seen when tumor-free survival, reflecting time to onset of measurable tumors, was evaluated as an end point. Survival, however, was used as the principal end point because it was found that mKSA tumors can metastasize to distant sites (including lung and lymph nodes; data not shown), which were not readily measurable in the absence of postmortem examination, and metastases render uncertain the utility of measuring changes in the primary tumor volume in this model.
Fig. 4.
Immunization with vac-mTag, recombinant vaccinia-encoding safety-modified simian virus 40 (SV40) T antigen (Tag), has statistically significant protective and therapeutic antitumor efficacy in vivo. A) Protective effect of vac-mTag against a Tag-expressing mKSA tumor in BALB/c mice is shown. Mice were immunized with V69 or vac-mTag at 5 × 106 plaque-forming units/mouse 3 weeks before tumor challenge with 106 mKSA cells that were administered subcutaneously. Animals were monitored four to six times weekly for onset and progression to lethal tumors by an individual blinded to the immunization status. The y axis shows percent overall survival. All lethal events were due to tumor progression (two-sided P = .02, logrank test; n = 11 mice, with six receiving control vaccinia and five receiving vac-mTag; confirmed in three similar experiments). B) Therapeutic effect of vac-mTag against preadministered mKSA tumor burden in BALB/c mice. Mice were subcutaneously injected with 106 mKSA tumor cells. Two days later, mice were treated with vac-mTag or V69 at 5 × 106 plaque-forming units/mouse administered by tail-vein injection once and interleukin 2 at 90 000 IU/mouse injected intraperitoneally once daily for 3 days. Animals were monitored four to six times weekly for lethal tumor progression and survival by an individual blinded to the immunization status. The y axis shows percent overall survival. All lethal events were due to tumor progression (two-sided P = .02, logrank test; n = eight mice in each therapy group).
Although protection against postimmunization tumor challenge has been previously shown with other vehicles for SV40 Tag immunization (such as peptides, plasmid DNA, and transformed Tag-expressing cellular vaccines) (10-13,26), these immunization strategies targeting Tag have not been shown previously to exhibit therapeutic efficacy in treating pre-established tumors. The utility of vac-mTag for therapy for preadministered microscopic tumor burden of Tag-expressing mKSA tumors was, therefore, evaluated. Tag-specific vaccine therapy, achieved by coadministration of vac-mTag intravenously with recombinant interleukin 2 introperitoneally [interleukin 2 has been demonstrated previously to enhance therapeutic efficacy of recombinant poxvirus in pretreating established pulmonary tumors (15)], improved the survival of mice with preadministered mKSA tumor cells as compared with control therapy using vaccinia control vector (V69) with recombinant interleukin 2 (Fig. 4, B). The vac-mTag therapy also induced permanent tumor remission in a small subset of mice. These findings demonstrate that vac-mTag induced a potent antigen-specific immune response in vivo, resulting in statistically significant protection against Tag tumor challenge and in a possible therapeutic effect against a preadministered microscopic burden of SV40 Tag-expressing tumor cells.
Discussion
This report describes the construction and characterization of vac-mTag, a unique recombinant vaccinia virus, that, after immunization with a single dose in vivo, confers protection against subsequent tumor challenge and provides therapeutic efficacy against pre-established SV40 Tag-expressing tumors in a lethal SV40 Tag-expressing cancer model system. Expression of the truncated SV40 Tag after vac-mTag infection was demonstrated by immunocytostain (data not shown), reverse transcription-coupled PCR, and western blotting. SV40 Tag-specific, MHC class I-restricted CTL activity was demonstrated in vac-mTag-immunized mice.
Previously, immunization strategies for inducing SV40 Tag-specific immune responses have focused on delivering SV40 Tag protein, peptides, SV40-transformed cells, or recombinant vaccines in the form of full-length SV40 Tag DNA (10-13,26,27). In general, these approaches have succeeded in inducing protection against subsequent tumor challenge, but therapeutic efficacy against pre-established SV40 tumors has not been shown previously. Recombinant vaccine strategies against SV40 Tag have themselves been limited to naked plasmid SV40 Tag DNA immunization (10) and recent use of vaccinia encoding full-length Tag or murine MHC class I-specific peptides for studying mechanisms of mouse antigen presentation in vitro (28). Although immunization with these constructs encoding full-length Tag induced Tag-specific CTLs in mice, the oncogenic potential of full-length SV40 constructs hinders potential applicability in human subjects. In addition to lacking human safety modifications, such vaccinia vectors encoding short mouse MHC-specific Tag peptides or full-length Tag (though studied for their abilities of presenting and generating CTL activity in vitro) have not been reported previously to show efficacy in protecting against or treating tumor growth in vivo.
Two desirable components addressing safety optimization for potential clinical use were sought in designing a novel SV40 recombinant vaccine for the current study: first, the choice of a vector for vaccine delivery minimizing likelihood of persistent SV40 Tag oncogene expression in host cells and, second, modification of the tumor-associated antigen to exclude oncogenic components.
In selecting a viral vector, several unique properties of poxvirus vectors such as vaccinia are desirable for an oncogene-specific recombinant vaccine such as one targeting SV40 Tag. Poxvirus carries its own transcription machinery for RNA synthesis and does not integrate into the host cell genome. Viral transcription factors are thus required to express the inserted gene (29). Persistent oncogene expression when an oncogene tumor-associated antigen is delivered by vaccinia is, therefore, expected to be highly unlikely, and this rationale has been applied previously to support the clinical development of recombinant vaccinia encoding human papillomavirus E6/E7 genes as a cervical cancer vaccine (30,31). Despite these hypothetical attributes, however, prior reports of therapeutic efficacy of vaccinia vectors targeting oncogenic tumor-associated antigens have been previously accomplished only in a rodent polyoma model that lacks human clinical correlates (32). These rationale supported the use of vaccinia as the base vector for an SV40 Tag-specific recombinant vaccine.
The oncogenic capacity of SV40 Tag is largely due to its ability to bind and inactivate the products of a number of tumor suppressor genes, including p53 and members of the retinoblastoma family. In addition, the region of SV40 Tag encoded by the first exon can contribute to transformation via a mechanism independent of p53 and retinoblastoma protein (18-21). Safety concerns regarding these oncogenic domains were addressed by selecting a region of SV40 Tag (amino acids 205–566) that excludes the retinoblastoma protein binding site and the transforming CR1 and J domains in the amino terminus and excludes the second site of the bipartite region required for p53 binding (18-21). In addition, two T5NT sequences in Tag gene were excluded to avoid premature termination of transcripts by the vaccinia RNA polymerase (24). A complete SV40 Tag open reading frame between these oncogenic sites was included in vac-mTag to maximally preserve nononcogenic tumor-associated antigen epitopes. We found that in the mKSA tumor model in syngeneic BALB/c mice, the resulting vac-mTag vector showed statistically significant antitumor efficacy in vivo.
The suggestion that vac-mTag may be useful for clinical studies targeting human tumors expressing SV40 Tag requires comment regarding the relevance of vac-mTag with regard to candidate peptides in the mTag sequence for specific human HLA types. For this purpose, the predicted dissociation rates of Tag peptides for the most common HLA types in three North American races (Caucasian, black, and Oriental) were calculated with software from Parker et al. (22,23). The most common North American HLA types (based on prevalence >20% in a specific racial group) are as follows: Caucasians, A1, A2, A3, B7, B44, and Cw3; blacks, A2, A30, Bw53, and Cw4; and Orientals, A2, A11, A24, B13, Bw60, and Cw3 (33). An earlier study (34) suggests that peptides with half-times of dissociation from MHC class I equal to 2 hours or greater are associated with effective antigenic function when delivered by vaccinia vectors. Analysis of the entire SV40 Tag sequence identified 23 candidate antigenic peptides fitting these dissociation rate criteria for the HLA subtypes common in North Americans. Fourteen of these 23 peptide motifs are present within the Tag fragment encoded by vac-mTag. Moreover, a recent study evaluating Tag-specific T cell activity in a patient with a Tag-expressing mesothelioma has identified a dominant Tag peptide at Tag position 285 (Bright R: personal communication), a motif that is also encoded within the vac-mTag vector. These findings support the concept that vac-mTag is a rational substrate for clinical studies targeting Tag-expressing human cancers.
Accumulating evidence that there is an association between SV40 Tag and human mesotheliomas, osteosarcomas, ependymomas, and choroid plexus tumors indicates potential clinical utility for a SV40 Tag-specific recombinant vaccine that can be administered practically and safely to human subjects. As many as 60% of these human tumors have been found to carry SV40 sequences in contemporary studies (1-7,9) and up to two thirds of evaluable specimens have been found to express SV40 Tag messenger RNA or protein (7). In this paper, we have demonstrated that vac-mTag, a novel recombinant vaccinia construct encoding mTag DNA, can induce a potent SV40 Tag-specific immune response in vivo, suggesting a possible therapeutic effect in a lethal SV40 Tag-associated murine cancer model and providing evidence of a potentially preventive and therapeutic approach for treating SV40-associated human cancers.
Acknowledgments
Supported by Public Health Service grant CA71532 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services. M. G. Sanda is the recipient of an American Cancer Society Clinical Career Development Award.
We thank Linda Charles for her technical assistance, Drs. John Lednicky and Janet Butel (Baylor College of Medicine, Houston, TX) for their helpful discussions of T-antigen cloning strategies, and Cathy Schleif for her expert assistance in the preparation of the manuscript.
Footnotes
Note: While this manuscript was in review, the presence of SV40 Tag in human mesothelioma was confirmed in a multi-institutional study reported by Testa et al. (35).
References
- 1.Carbone M, Rizzo P, Procopio A, Giuliano M, Pass HI, Gebhardt MC, et al. SV40-like sequences in human bone tumors. Oncogene. 1996;13:527–35. [PubMed] [Google Scholar]
- 2.Bergsagel DJ, Finegold MJ, Butel JS, Kupsky WJ, Garcea RL. DNA sequences similar to those of simian virus 40 in ependymomas and choroid plexus tumors of childhood. N Engl J Med. 1992;326:988–93. doi: 10.1056/NEJM199204093261504. [DOI] [PubMed] [Google Scholar]
- 3.Carbone M, Pass HI, Rizzo P, Marinetti M, Di Muzio M, Mew DJ, et al. Simian virus 40-like DNA sequences in human pleural mesothelioma. Oncogene. 1994;9:1781–90. [PubMed] [Google Scholar]
- 4.Lednicky JA, Garcea RL, Bergsagel DJ, Butel JS. Natural simian virus 40 strains are present in choroid plexus and ependymoma tumors. Virology. 1995;212:710–7. doi: 10.1006/viro.1995.1529. [DOI] [PubMed] [Google Scholar]
- 5.Geissler E. SV40 and human brain tumors. Prog Med Virol. 1990;37:211–22. [PubMed] [Google Scholar]
- 6.De Luca A, Baldi A, Esposito V, Howard CM, Bagella L, Rizzo P, et al. The retinoblastoma gene family pRb/p105, p107, pRb2/p130 and simian virus-40 large T-antigen in human mesotheliomas. Nature Med. 1997;3:913–6. doi: 10.1038/nm0897-913. [DOI] [PubMed] [Google Scholar]
- 7.Carbone M, Rizzo P, Grimley PM, Procopio A, Mew DJ, Shridhar V, et al. Simian virus-40 large-T antigen binds p53 in human mesotheliomas. Nat Med. 1997;3:908–12. doi: 10.1038/nm0897-908. [DOI] [PubMed] [Google Scholar]
- 8.Shah K, Nathanson N. Human exposure to SV40: review and comment. Am J Epidemiol. 1976;103:1–12. doi: 10.1093/oxfordjournals.aje.a112197. [DOI] [PubMed] [Google Scholar]
- 9.Carbone M, Rizzo P, Pass HI. Simian virus 40, poliovaccines and human tumors: a review of recent developments. Oncogene. 1997;15:1877–88. doi: 10.1038/sj.onc.1201375. [DOI] [PubMed] [Google Scholar]
- 10.Bright RK, Beames B, Shearer MH, Kennedy RC. Protection against a lethal challenge with SV40-transformed cells by the direct injection of DNA-encoding SV40 large tumor antigen. Cancer Res. 1996;56:1126–30. [PubMed] [Google Scholar]
- 11.Gooding LR, O'Connell KA. Recognition by cytotoxic T lymphocytes of cells expressing fragments of the SV40 tumor antigen. J Immunol. 1983;131:2580–6. [PubMed] [Google Scholar]
- 12.Knowles BB, Koncar M, Pfizenmaier K, Solter D, Aden DP, Trinchieri G. Genetic control of the cytotoxic T cell response to SV40 tumor-associated specific antigen. J Immunol. 1979;122:1798–806. [PubMed] [Google Scholar]
- 13.Tevethia SS. Recognition of simian virus 40 T antigen by cytotoxic T lymphocytes. Mol Biol Med. 1990;7:83–96. [PubMed] [Google Scholar]
- 14.Earl PL, Cooper N, Moss B. Preparation of cell cultures and vaccinia virus stocks. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith LA, et al., editors. Current protocols in molecular biology. Vol. 2. Greene Publishing Associates and Wiley Interscience; New York (NY): 1991. pp. 16.16.1–16.16.7. [Google Scholar]
- 15.Bronte V, Tsung K, Rao JB, Chen PW, Wang M, Rosenberg SA, et al. IL-2 enhances the function of recombinant poxvirus-based vaccines in the treatment of established pulmonary metastases. J Immunol. 1995;154:5282–92. [PMC free article] [PubMed] [Google Scholar]
- 16.Bronte V, Carroll MW, Goletz TJ, Wang M, Overwijk WW, Marincola F, et al. Antigen expression by dendritic cells correlates with the therapeutic effectiveness of a model recombinant poxvirus tumor vaccine. Proc Natl Acad Sci U S A. 1997;94:3183–8. doi: 10.1073/pnas.94.7.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanda MG, Restifo NP, Walsh JC, Kawakami Y, Nelson WG, Pardoll DM, et al. Molecular characterization of defective antigen processing in human prostate cancer. J Natl Cancer Inst. 1995;87:280–5. doi: 10.1093/jnci/87.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu J, Rice PW, Gorsch L, Abate M, Cole CN. Transformation of a continuous rat embryo fibroblast cell line requires three separate domains of simian virus 40 large T antigen. J Virol. 1992;66:2780–91. doi: 10.1128/jvi.66.5.2780-2791.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peden KW, Spence SL, Tack LC, Cartwright CA, Srinivasan A, Pipas JM. A DNA replication-positive mutant of simian virus 40 that is defective for transformation and the production of infectious virions. J Virol. 1990;64:2912–21. doi: 10.1128/jvi.64.6.2912-2921.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pipas JM, Peden KW, Nathans D. Mutational analysis of simian virus 40 T antigen: isolation and characterization of mutants with deletions in the T-antigen gene. Mol Cell Biol. 1983;3:203–13. doi: 10.1128/mcb.3.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stubdal H, Zalvide J, DeCaprio JA. Simian virus 40 large T antigen alters the phosphorylation state of the RB-related proteins p130 and p107. J Virol. 1996;70:2781–8. doi: 10.1128/jvi.70.5.2781-2788.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994;152:163–75. [PubMed] [Google Scholar]
- 23.Parker KC. HLA peptide binding predictions software [computer program] National Cancer Institute; Bethesda (MD): 1998. http://bimas.dcrt.nih.gov/cgi-bin/molbio/ken-parker-comboform. [Google Scholar]
- 24.Earl PL, Hugin AW, Moss B. Removal of cryptic poxvirus transcription termination signals from the human immunodeficiency virus type 1 envelope gene enhances expression and immunogenicity of a recombinant vaccinia virus. J Virol. 1990;64:2448–51. doi: 10.1128/jvi.64.5.2448-2451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mole SE, Gannon JV, Anton IA, Ford MJ, Lane DP. Host proteins that bind to or mimic SV40 large T antigen: using antibodies to look at protein interactions and their significance. Immunol Suppl. 1989;2:80–5. [PubMed] [Google Scholar]
- 26.Anderson JL, Martin RG, Chang C, Mora PT, Livingston DM. Nuclear preparations of SV40-transformed cells contain tumor-specific transplantation antigen activity. Virology. 1977;76:420–5. doi: 10.1016/0042-6822(77)90314-2. [DOI] [PubMed] [Google Scholar]
- 27.Bright RK, Shearer MH, Kennedy RC. SV40 large tumor antigen associated synthetic peptides define native antigenic determinants and induce protective tumor immunity in mice. Mol Immunol. 1994;31:1077–87. doi: 10.1016/0161-5890(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 28.Fu TM, Mylin LM, Schell TD, Bacik I, Russ G, Yewdell JW, et al. An endoplasmic reticulum-targeting signal sequence enhances the immunogenicity of an immunorecessive simian virus 40 large T antigen cytotoxic T-lymphocyte epitope. J Virol. 1998;72:1469–81. doi: 10.1128/jvi.72.2.1469-1481.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moss B. Vaccinia virus: a tool for research and vaccine development. Science. 1991;252:1662–7. doi: 10.1126/science.2047875. [DOI] [PubMed] [Google Scholar]
- 30.Borysiewicz KL, Fiander A, Nimako M, Man S, Wilkinson GW, Westmoreland D, et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347:1523–7. doi: 10.1016/s0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 31.Meneguzzi G, Cerni C, Kieny MP, Lathe R. Immunization against human papillomavirus type 16 tumor cells with recombinant vaccinia viruses expressing E6 and E7. Virology. 1991;181:62–9. doi: 10.1016/0042-6822(91)90470-v. [DOI] [PubMed] [Google Scholar]
- 32.Lathe R, Kieny MP, Gerlinger P, Clertant P, Guizani I, Cuzin F, et al. Tumor prevention and rejection with recombinant vaccinia. Nature. 1987;326:878–80. doi: 10.1038/326878a0. [DOI] [PubMed] [Google Scholar]
- 33.Lee TD. Distribution of HLA antigens in North American Caucasians, North American blacks, and Orientals. In: Lee J, editor. The HLA system: a new approach. Springer-Verlag; New York (NY):: 1990. [Google Scholar]
- 34.van der Burg SH, Visseren MJ, Brandt RM, Kast WM, Melief CJ. Immunogenicity of peptides bound to MHC class I molecules depends on the MHC-peptide complex stability. J Immunol. 1996;156:3308–14. [PubMed] [Google Scholar]
- 35.Testa JR, Carbone M, Hirvonen A, Khalili K, Krynska B, Linnainmaa K, et al. A multi-institutional study confirms the presence and expression of Simian Virus 40 in human malignant mesotheliomas. Cancer Res. 1998;58:4505–9. [PubMed] [Google Scholar]