Abstract
Sir2, a NAD-dependent deacetylase, modulates lifespan in yeasts, worms and flies. The SIRT1, mammalian homologue of Sir2, regulates signaling for favoring survival in stress. But whether SIRT1 has the function to influence cell viability and senescence under non-stressed conditions in human diploid fibroblasts is far from unknown. Our data showed that enforced SIRT1 expression promoted cell proliferation and antagonized cellular senescence with the characteristic features of delayed Senescence-Associated β-galactosidase (SA-β-gal) staining, reduced Senescence-Associated Heterochromatic Foci (SAHF) formation and G1 phase arrest, increased cell growth rate and extended cellular lifespan in human fibroblasts, while dominant-negative SIRT1 allele (H363Y) did not significantly affect cell growth and senescence but displayed a bit decreased lifespan.. Western blot results showed that SIRT1 reduced the expression of p16INK4A and promoted phosphorylation of Rb. Our data also exposed that overexpression of SIRT1 was accompanied by enhanced activation of ERK and S6K1 signaling. These effects were mimicked in both WI38 cells and 2BS cells by concentration-dependent resveratrol, a SIRT1 activator. It was noted that treatment of SIRT1-.transfected cells with Rapamycin, a mTOR inhibitor, reduced the phosphorylation of S6K1 and the expression of Id1, implying that SIRT1-induced phosphorylation of S6K1 may be partly for the decreased expression of p16INK4A and promoted phosphorylation of Rb in 2BS. It was also observed that the expression of SIRT1 and phosphorylation of ERK and S6K1 was declined in senescent 2BS. These findings suggested that SIRT1-promoted cell proliferation and antagonized cellular senescence in human diploid fibroblasts may be, in part, via the activation of ERK/ S6K1 signaling.
Introduction
Cellular senescence, a process of cell aging in which primary cells in culture lose their ability to divide, is accompanied by a specific set of changes including growth cessation, morphological changes, appearance of senescence-associated beta-galactosidase (SA-β-gal) activity and increased expression of cyclin-dependent kinase inhibitors (CDKIs). Though lack of a clear correlation between organismal aging with cellular growth viability, the study of mammalian cell aging in vitro has enormous potential for telling us how human aging works [1]. Besides, it is noteworthy that cellular senescence is well regarded as one of cellular mechanisms to prevent oncogenesis [2].
The silent information regulator 2 (Sir2) is an NAD-dependent deacetylase. It is well known that overexpression of Sir2 or its orthologs can extend organismal life span in a wide range of lower eukaryotes, including yeasts [3], [4], worms [5] and flies [6]. In mammalians, Sir2 is represented by seven homologues (SIRTs 1∼7), of which SIRT1 is the most closely related to the yeast Sir2 and intensively studied. Recent studies have demonstrated that Sirt1 played an important role in the regulation of cell survival by inhibiting apoptosis induced by stresses [7]–[9]. Therefore, it is speculated that SIRT1 can also reduce cell aging. But study of overexpression of seven human sirtuins (SIRT1∼7) failed in demonstrating the effects on replicative life span in skin-derived human cells or prostate epithelial cells [10]. In addition, SIRT1 silencing by RNAi or specific inhibitors did not affect cell viability and was not sufficient to induce activation of endogenous p53 in the absence of applied stress [11]–[13]. However some studies also showed that SIRT1 protein decreased significantly with serial cell passage both in human cells and murine cells and found a significant positive correlation between the level of SIRT1 and cell proliferation and observed an inverse association between SIRT1 and SA-β-gal activity [14]. Besides, there were studies showed that SIRT1 silencing by RNAi could be more sensitive to induce cell arrest in cancer cells than in normal cells [11], [15]. These inconsistencies on the function of SIRT1 in the process of cellular senescence may be associated with cell-type-specific context and different molecular mechanisms involved.
One important mechanism responsible for the replicative senescence of human cells is the erosion and eventual dysfunction of telomeres [16]. However, in certain fibroblasts, e.g. MRC5, WI38 and IMR90, immortalization could not be efficiently obtained only by telomerase transfection [17]. In these cell lines, the accumulation of p16INK4A was noted as another important mechanism that contributes to replicative senescence in these cell lines [18]. Recent studies discovered that the accumulation of p16INK4A may also, in part, contributed to the physiological aging in vivo, for instance the deterioration of age-associated Haematopoietic stem cells (HSC) functions [19], the declines of olfactory bulb neurogenesis [20] and the restraints of islet regenerative potential [21]. Moreover, it was worthy of note that the accumulation of p16INK4A was reported as a robust biomarker in mammals and could be attenuated by caloric restriction (CR) [21]. Therefore it was presumed that the diminished expression of p16INK4A by CR could contribute to the decreased pathology of organs aging. Since SIRT1 acts as a key regulator orchestrating the response to caloric restriction in mammals [22], it enforced us to speculate that the expression of p16INK4A could be partly modulated by SIRT1.
In our study, we aimed at investigating whether increased expression of SIRT1 can regulate cell proliferation and cellular senescence in normal human diploid fibroblasts with feature of p16INK4A accumulation at late passage. Our research demonstrated that SIRT1 could delay cellular senescence and extend cell life span with promoted phosphorylations of ERK and S6K1, which may be partly associated with the expression of p16INK4A in human diploid fibroblasts. Besides, our data revealed that SIRT1/ERK/S6K1 signaling was suppressed in senescent cells, implying the important role in the process of cellular senescence.
Results
SIRT1 overexpression led to a reduction of senescence-associated biomarkers
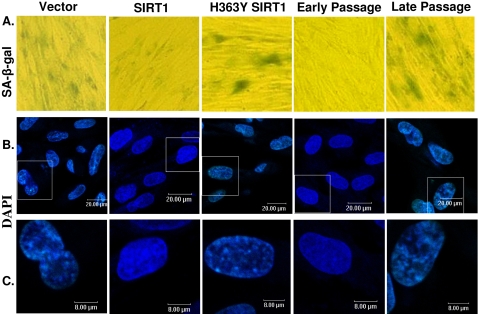
Cellular senescence is characterized by elevated level of an endogenous β-galactosidase activity at pH 6.0. To confirm our hypothesis that SIRT1 is associated with cellular senescence, vectors expressing wild type SIRT1 and dominant negative form of SIRT1 (H363YSIRT1), as well as mock vector (pcDNA 3.1) were separately transfected into 2BS, a strain of human embryonic lung fibroblast. After a two-week G418 selection to eliminate untransfected cells, cell populations underwent serial confluent culture with 50 µg/ml G418 until one of them reached notable growth arrest. Subsequently, SA-β-gal activity was measured among different stable transformation pools. Early passage (23 PDs) and late passage (60 PDs) of 2BS cells were also analyzed as controls. Figure 1A showed that 2BS with enforced expression of wild type SIRT1 displayed lower frequency of SA-ß-gal staining than cells transfected with mock vector and H363YSIRT1 constructs. But few significant differences in SA-ß-gal activity were observed between mock vector-transfected cells and H363YSIRT1-transfected cells.
Figure 1. Changes of senescence-associated features in 2BS cells were induced by enforced expression of SIRT1.
(A) Stable transfected 2BS cells expressing empty Vector, SIRT1 and H363YSIRT1 were passaged until one of them underwent senescence, then stained for SA-β-gal staining, a classical biomarker for senescence. Early passage (23 PDs) 2BS cells were showed as negative control. Late passage (60 PDs) 2BS cells were stained as positive control. (B) Senescence-associated heterochromatic foci (SAHF), another classical marker of senescence, was showed in 2BS cell lines DNA foci accumulate was visualized by DAPI staining in 2BS cells. Scale bars were equal to 20 µm. (C) Enlarged images of DAPI staining are shown in the lower panels. Scale bars were equal to 8 µm.
The accumulation of Senescence-associated heterochromatin foci (SAHF) is another specific biomarker of senescent cells [16]. As shown in Figure 1B and C , senescence induced by extensive passage culture typically displayed punctuated DNA foci which were visualized by DAPI staining. In contrast, exponentially growing human fibroblasts usually displayed several small nucleoli and a more uniform DAPI staining pattern. We observed that 2BS cells expressing SIRT1 did not develop pronounced nucleoli or DNA foci. However mock-transfected and H363YSIRT1-transfected cells appeared prominent DNA foci which are nearly indistinguishable from those in senescent 2BS cells. The appearance of DNA foci in different transformations coincided precisely with SA-ß-gal activity and the onset of senescence.
Consist with the SIRT1-induced reduction of senescence-associated biomarker, SIRT1-tansfected 2BS displayed an increased lifespan while H363YSIRT1-transfected 2BS showed a little decreased life span, as compared with mock-transfected cells and untransfected cells, shown in Table 1 .
Table 1. Cumulative population doublings of SIRT1, H363YSIRT1, Mock-transfected cells and untransfected cells.
| Cells | Cumulative population doublings(PDs) | Number of test samples |
| untransfected cells | 54–59 | 6 |
| Mock vector | 51–55 | 5 |
| SIRT1 | 63–70 | 6 |
| H363SIRT1 | 46–53 | 5 |
SIRT1 promoted cell proliferation in 2BS cells
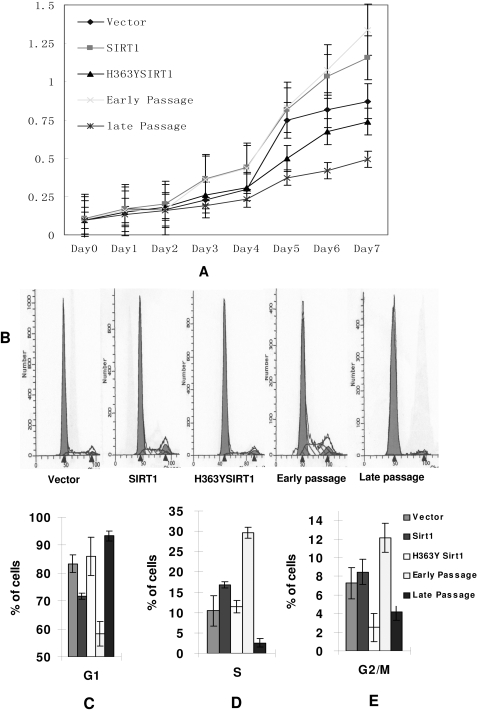
As cells undergo senescence, their proliferation also decline significantly. To observe the variations in proliferation, the growth rates of different transformations and the control cells were measured by MTT method ( Figure 2A ). The growth curves of 2BS cells expressing SIRT1 advanced quickly even at 50 PDs, displaying a strong proliferation potential similar to young cells (23 PDs), whereas the curves of cells containing mock vector and H363YSIRT1 constructs were approaching to those of senescent cells (58 PDs).
Figure 2. Induced expression of SIRT1 promoted cell proliferation in 2BS cells.
(A) Growth curves of transfected 2BS cells (all at PD50) with expression of Vector, SIRT1, and H353YSIRT1. Early passage (23PDs) and late passage cells (58PDs) of 2BS were shown for comparison. Cell proliferation was determined by the MTT assay. Each experiment was performed on 6 independent wells at each time indicated. (B) Flow cytometric analysis of 2BS cells with expression of empty vector, SIRT1 and H363YSIRT1. Early passage (23 PDs) and late passage 2BS cells (58 PDs) were also shown for comparison. Experiments were repeated 3∼4 times with similar results. (C, D, E) Typical cell cycle histograms representing percentage of cells at G1, S, G2/M phases were recorded from 3 replicate experiments. The mean percentage of cells at different phases was showed below.
To clarify the mechanisms of SIRT1-drived cell proliferation described above, the cell cycle profiles of transfected and untransfected 2BS cells were analyzed by flow cytometry. Each experiment was performed on 6 independent wells and representative data were shown in Figure 2B, C, D and E . Consisted with growth profiles observed in Figure 2A , 2BS cells transfected with SIRT1 markedly postponed G1 phase arrest while mock-transfected cells and H363YSIRT1-transfected cells reached G1 phase arrest after confluent culture. In addition, SIRT1 seemed to have less effect on S and G2 phase. Thus, we concluded that enforced SIRT1 expression led to cell proliferation by suppressing G1 arrest and initiated the G1/S transition.
SIRT1 suppressed cellular senescence through p16INK4A/Rb pathway
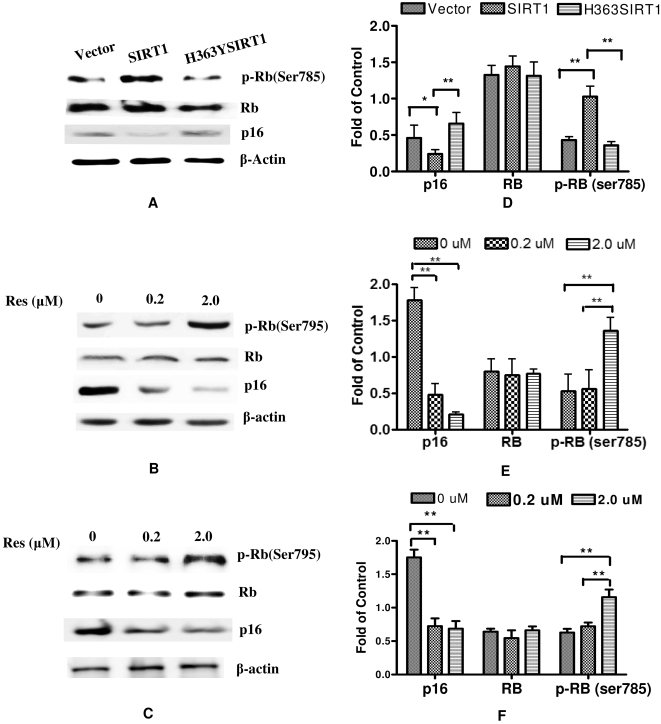
In 2BS, cellular senescence is largely associated with the inactivation of p16INK4A/Rb signaling pathway [23]. Increased p16INK4A prevents activation of the cyclin D/CDK4/CDK6 and subsequently promotes the growth inhibitory functions of RB. As well as the accumulation of p16INK4A in vitro, p16INK4A also displays a robust age-dependent increase in vivo [24]. To explore the mechanisms by which SIRT1 delays cellular senescence in human fibroblasts, p16INK4A/Rb signaling pathway was detected subsequently among different transformations. The total cellular proteins were prepared and analyzed by western blot for detecting the expression of p16INK4A, Rb, and phosphorylation of Rb at Ser795. Figure 3A, 3D indicated that, compared with mock vector and H363YSIRT1 transfected 2BS cells, p16INK4A displayed a two-fold decrease in SIRT1-transfected cells, accordingly phospho-Rb (Ser795) was increased for more than two times whereas the levels of β-actin and Rb remained unchanged in different batches of transfected cells. Resveratrol (Res) is regarded as an activator of SIRT1, promoting the survival of human cells in vitro [25]. To further investigate the role of SIRT1 affecting p16INK4A/Rb signaling pathway, resveratrol was used to treat WI38 cells and 2BS cells with different concentrations for 72 hr. As shown in Figure 3B, 3C , resveratrol decreased the expression of p16INK4A and increased phosphorylation of Rb (Ser795) in a dose-dependent manner, whereas the levels of β-actin and Rb were invariable in either presence or absence of resveratrol. Accordingly, the quantitative protein expression variations were summarized by bar graph in Figure 3D, 3E .
Figure 3. SIRT1-dependend suppression of cellular senescence was through p16INK4A/Rb pathway.
(A) Transfected 2BS cells containing pcDNA3.1, pcDNA-SIRT1 and pcDNA-H363YSIRT1 were lysated and prepared for western blot analysis by using specific antibodies against phospho-retinoblastoma (Ser795), retinoblastoma, p16INK4A and β-actin. (B, C) 2BS cells and WI38 cells at 40 PDs were treated with solvent alone or 0.2 or 2 µM of resveratrol (Res) for 72 h. Cell lysates were prepared for Western blot with the same antibodies. (D, E, F) The quantitative protein expression was summarized in bar graphs (left) presented by mean values (±SEM) of 3 independent experiments. Error bars represent the S.D. Significant difference compared between controls, *, p<0.05; **, p<0.01.
SIRT1 promoted phosphorylations of kinases associated with cell proliferation
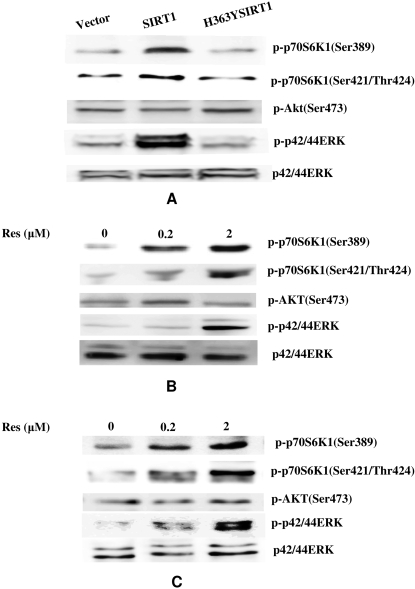
Our results ( Figure 2 ) showed that SIRT1 promotes cell proliferation in 2BS. And activities of kinase signaling usually contribute to cell viability in human fibroblasts [26]–[29]. To confirm our hypothesis that SIRT1 promotes cell proliferation by activating some kinase signaling, we examined the activation of ERK, AKT and p70 S6K1 in different batches of transformations. As shown in Figure 4A , phosphorylations of ERK (Thr202/Tyr204) and S6K1(Thr389, Thr421/Ser424) were enhanced in SIRT1-tansfected 2BS cells, whereas that of AKT (Ser473) was unchanged by SIRT1 overexpression. Compared to mock-transfected cells, 2BS with enforced expression of H363YSIRT1 did not significantly influence the phosphorylations of ERK, AKT and p70 S6K1. Figure 4A also showed that total ERK protein levels were not altered in different batches of transformations. To confirm such SIRT1-associated activation of Kinases, resveratrol was used to treat WI38 cells and 2BS cells with different concentrations. As shown in Figure 4B , resveratrol increased the phosphorylations of ERK (Thr202/Tyr204) and S6K1(Thr389, Thr421/Ser424)in a dose-dependent manner while that of AKT was not altered in either treated or untreated cells. These data were consistent with the results obtained above from SIRT1-transfected 2BS, suggesting that SIRT1 was associated with the promoted phosphorylations of ERK and S6K1.
Figure 4. SIRT1-dependend phosphorylation of kinase was associated with the cell proliferation.
(A) Transfected 2BS cell containing pcDNA3.1, pcDNA-SIRT1 and pcDNA-H363YSIRT1 were lysated and subjected to western blot analysis by using specific antibodies against Phospho-p44/42 ERK (Thr202/Tyr204), Phospho-AKT (Ser473), Phospho-p70 S6K1(Thr389), Phospho-p70 S6K1 (Thr421/Ser424)and p44/42 ERK. (B, C) 2BS cells and WI38 cells at 40 PDs were treated with solvent alone or 0.2 or 2 µM of resveratrol (Res) for 72 h. Cell lysates analyzed by western blot, as described in Figure 4A.
Inhibition of S6K1 activity led to increased p16INK4A/Rb pathway
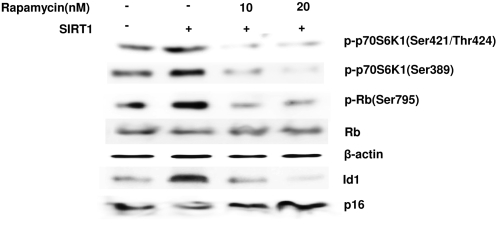
Since SIRT1 regulated both p16INK4A/Rb pathway and ERK/S6K1 signaling, it will be interesting to investigate whether SIRT1-stimulated S6K1 phosphorylation is associated with the regulation of p16INK4A/Rb pathway. SIRT1-transfected 2BS cells were treated with increased concentrations of Rapamycin for inhibiting SIRT1-induced S6K1 phosphorylation. The signaling, e.g. Id1, p16INK4A and Rb, which were observed to lead to 2BS senescence [23], [30], were subsequently detected by western blot. As shown in Figure 5 , treating SIRT1-transfected cells with Rapamycin resulted in a decreased phosphorylation of S6K1 and Id1 expression, subsequently led to an increase in the expression of p16INK4A and a decreased phosphorylation of Rb at 795 whereas the levels of Rb and β-actin remained unaltered.
Figure 5. Inhibition of S6K1 activity led to increased p16/Rb pathway with Id1 suppression.
SIRT1-transfected 2BS were treated for one day with increasing concentrations of Rapamycin (10nM and 20nM). Following treatment, the cells were lysed and subjected to western analysis for determining phosphorylation of S6k1 and signaling associated with the cell cycle G1 arrest (Id1, p16INK4A, Rb and phosphorylated Rb). Transfected 2BS containing SIRT1 and Mock vector were also analyzed as a positive and a negative control. Levels of β-actin were used as a loading control.
The levels of SIRT1 protein and phosphorylations of ERK and S6K1 were declined in senescent 2BS cells
Having demonstrated that SIRT1-induced ERK/S6K1 signaling was associated with SIRT1-dependent interference of cellular senescence and cell proliferation, it was reasonable to determinate the alterations of SIRT1/ERK/S6k1 signaling in cell aging. As shown in Figure 6 , the levels of SIRT1 protein were declined in late passage of 2BS (63PDs), and the phosphorylations of ERK and S6K1, but not that of AKT, were also decreased in senescent cells. Our results suggested that the decline of SIRT1/ERK/S6K1 signaling in senescent cells may be for the cell aging in 2BS.
Figure 6. The alterations of SIRT1 expression and phosphorylation of ERK, AKT and S6K1 were determined in senescent 2BS Cells.
The total cellular lysates from early passage (23 PDs) and late passage of 2BS (63 PDs) were prepared and subjected to western blot analysis by using specific antibodies against SIRT1,β-actin, Phospho-p44/42 ERK(Thr202/Tyr204), p44/42 ERK, Phospho-p70 S6K1 (Thr389), Phospho-p70 S6K1(Thr421/Ser424)and Phospho-AKT (Ser473).
Discussion
It is well known that overexpression of Sir2 orthologs extends life span in a wide range of lower eukaryotes [3]–[6]. These findings inspired the assumptions that SIRT1 has a role in regulating life span in mammals [29] and as a potential pharmacological target to treat the major diseases of aging [31]. Studies by Michishita et al indicated that overexpression of SIRT1 had no effects on replicative life span of foreskin-derived NHF cells and prostate epithelial cells [10]. But he further documented that SIRT1 was down-regulated in human senescent cells, suggesting that SIRT1 may be required to extend replicative life span. Chua et al. revealed that SIRT1-deficient mouse embryonic fibroblasts (MEFs) had extended replicative life span under sub-lethal oxidative stress, but showed p19 induction and cell cycle arrest upon acute DNA damage [32]. Our results showed that enforced SIRT1 expression in 2BS promoted cell life span and prevented cellular senescence with the features of delayed SA-β-gal staining, reduced SAHF formation and G1 arrest, increased cell growth rate and extended replicate life span. However, H363YSIRT1 might not show any pronounced cellular senescence features but manifested small but significantly decreased life span compared to controls. Studies by Sasak et al [14] documented that the level of SIRT1 was declined significantly with serial cell culture passage in both human and murine cells, and showed a positive correlation with cell proliferation and a negative correlation with SA-β-gal staining. In similar ways, a high SA-β-gal staining was observed in WI38, IMR90 and human endothelial cells induced by sirtinol or splitomicin, SIRT1 inhibitors, by Ota H et al [15], [33]. The inconsistencies on the function of SIRT1 in the process of cellular senescence may depend on cell-type-specific context and different molecular mechanisms involved.
Ota H et al observed that WI38 and IMR90 could be induced into senescence by SIRT1 inhibitor [15]. But detail molecular mechanisms were unknown. Like WI38 and IMR90, the senescence of 2BS was largely associated with the accumulation of p16INK4A at late passage [23], [30]. And anti-sense p16INK4A expression could largely delay the senescence of 2BS [23]. It will enforce us to assume that the SIRT1 activation may prevent 2BS senescence. Furthermore it was documented that age-associated p16INK4 accumulation, in some rodent tissues, could be attenuated by CR and SIRT1 acts as a key regulator orchestrating the response to CR in mammals, it promoted the assumption that SIRT1 could partly regulate the expression of p16INK4A. These assumptions were consequently confirmed by SIRT1 overexpresion in 2BS and treating 2BS and WI38 cells with dose-dependent resveratrol, a SIRT1 activator. A latest research indicated that p16INK4A/Rb pathway was required for SAHF formation and cellular senescence [34]. Consistent with their conclusions, our results indicated that the SIRT1-associated prevention of SAHF formation may be dependent on suppressing p16INK4A/Rb pathway. Although there is no data previously reported that SIRT1 could suppressed the expression of p16INK4A, there were studies documented that histone deacetylase inhibitors (HDACIs) sodium dibutyrate (SDB) and trichostatin A (TSA) induced senescence in human cells by regulating p16INK4A/Rb pathway [35], indicating that SIRT1 may suppressed the expression of p16INK4A in a similar way. Since the p16INK4A expression was reported as a robust in vivo biomarker in human and rodent with advancing age [21], [24], the suppression of p16INK4A by SIRT1 may benefit to attenuate age-related diseases.
Activation of ERK, AKT and p70 S6K is required for cell proliferation. The inhibition of such kinases could induce cell arrest in fibroblasts [26]–[28]. Our data showed that both SIRT1 transfection and Resveratrol treatment promoted phosphorylations of ERK (Thr202/Tyr204) and S6K1 (Thr389, Thr421/Ser424), whereas that of AKT (Ser473) was unchanged. Consistent with our results, SIRT1 was reported to induce the phosphorylation of ERK in HEK293 cells [36]. Though no works illuminated the mechanisms of SIRT1-induced phoshorylation of ERK, Kobayashi et al documented that HDAC inhibitor FK228 suppressed the MAP kinase signaling pathway by upregulating Rap1 transcription [37], suggesting that SIRT1 might recruit a similar way to provoke ERK signaling.
S6k1 was required for cell growth and G1 cell cycle progression and involved in translational control of 5′ oligopyrimidine tract mRNAs via promoting the phosphorylation of S6 protein of the 40S ribosomal subunit [38]. These indicated that SIRT1 may be functionally for the mRNA translation by activating S6K1 phosphorylation. Recent works issued that the tumor suppressor protein tuberous sclerosis complex 2 (TSC 2), an upstream regulator of mTOR, could be phosphorylated by ERK signaling [39], [40], indicating that the phosphorylation of S6K1 may be mediated by ERK signaling.
Since the activation of S6K1 was obligatory for cell cycle progression and the inhibition of such enzyme led to cell cycle arrest [41], [42], it was presumed that S6K1 may be associated with SIRT1-dependent p16INK4A decline in 2BS. Latest data revealed that mTOR, a direct upstream signaling for the phosphorylation of S6k1, was for the induction of Id1 expression in mammary epithelial cells. Moreover Id1 was responsible for the down regulation of p16INK4A in human diploid fibroblasts [30], [43]. These researches suggested that there will be a potential association between S6K1 and p16INK4A. Our data showed that, after treating SIRT1-transfected 2BS with dose-dependent Rapamycin, phosphorylation of S6K1 and Id1 expression was suppressed, subsequently p16INK4A/Rb pathway was provoked. These results implied that SIRT1-induced phosphorylation of S6K1 may be responsible to the accumulation of p16INK4A via the introduction of Id1. But further witnesses should be investigated by S6K1 RNA interfere approach or transfecting dominant negative S6K1 mutaition.
It was worthy of note that SIRT1 expression and the phosphorylations of ERK and S6K1 were decreased in senescent 2BS. The decline of SIRT1/ERK/S6K1 in the late of passage may be responsible for the senescence of 2BS. The elimination of S6K1 phosphorylation in senescent fibroblasts was previously reported by Zhang et al [44]. The variations of ERK signaling in senescent cells still remained disruptions [43], [45]. The inconsistencies may result from the use of different cell lines or different culture conditions used.
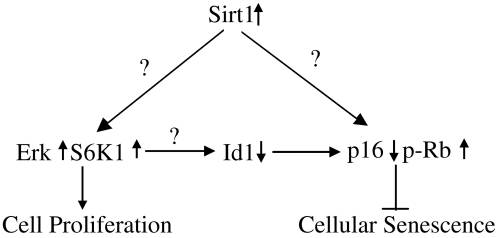
In summary, our data suggested that, in the presence of stress, overexpression of SIRT1 contributed to cell proliferation and prevention of cellular senescence in human diploid fibroblasts. The delay of senescence by SIRT1 was associated with down-regulation of p16INK4A/Rb pathway and the activation of ERK/S6K1 signaling. The decline of SIRT1-dependent ERK/S6K1 signaling in senescent cell may be contribute to cell progression loss and cellular senescence at late passage of human diploid fibroblasts. Based on above evidence we have put forward a possible model of how SIRT1 antagonize cellular senescence via promoted ERK/S6K1 singling, shown in Figure 7 .
Figure 7. Schematic model for a mechanism of Sirt1 activating ERK/S6K1 signaling pathway involved in cell senescence.
The activation of SIRT1 in human lung fibroblasts suppressed p16INK4A/Rb pathway and provoked ERK/S6K1 signaling, which, in turn, may inhibit p16INK4A/Rb signaling via Id1 induction.
Materials and Methods
Cell Culture and Synchronization
2BS cell line was isolated from female fetal lung fibroblast tissue in the National Institute of Biological Products, Beijing, China. The characters of 2BS have been fully characterized since its isolation in 1962 [26]–[28]. The maximal life span of 2BS is about 60 population doublings (PDs). 2BS cells are considered to be young below 30 PDs and go into senescence at 55 PDs or above. Cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum, 100 units/ml of penicillin and 100 µg/ml streptomycin at 37°C in 5% CO2. WI38 cell line was cultured in the same way.
For synchronization, human fibroblasts were rendered quiescent by serum deprivation for 48 hr and then stimulated to reenter the cell cycle by the addition of serum to a final concentration of 10%. G1 phase cells were harvested at 8 hr after serum stimulation.
Antibodies and Reagents
Antibodies against p44/42 ERK (#9102), Phosphorylated p44/42 ERK (Thr202/Tyr204) (#9101), Phosphorylated Rb (Ser795) (#9301), Rb (4H1) mouse Antibody (#9309), Phosphorylated S6K1 (Thr389, Thr421/Ser424) (#9430) were purchased from Cell Signaling Technology (Beverly, MA). p-Akt123 (Ser473) Antibody (SC7985-R), SIRT1 (H300) Antibody (SC15404), β-actin (I-19) Antibody (sc-1616) and Id1 (C-20) Antibody (sc-488) were purchased from Santa Cruz Biotech., Inc. p16INK4A (Ab-1) Antibody (#MS-218-P1) was purchased from NeoMarkers Corp. Resveratrol (R5010) was purchased from Sigma-Aldrich Cop. Rapamycin (#553210) was from Calbiochem. Other chemicals and reagents were of the purest grade available.
Stable Transfection of Human Diploid Fibroblasts
pcDNA SIRT1, pcDNA H363YSIRT1 and pcDNA 3.1B were transfected into young 2BS cells at 20 PDs with Lipofectamine 2000, performed according to standard method. After 48 hr, the cells were selected by G418 (100 µg/ml; Life Technologies). Colonies of stable transformations were isolated 3∼4 weeks later and propagated accordingly in complete medium containing 50 µg/mL G418. The resulting transformations were termed as SIRT1, H363YSIRT1 and Vector respectively.
Senescence-associated beta-Galactosidase Staining
Transfected 2BS and untransfected early passage and late passage 2BS cells were washed twice in PBS, fixed to plates using 3% formaldehyde for 3∼5 min, and washed with PBS again. Then these cells were incubated overnight at 37°C without CO2 in a freshly prepared staining buffer (1 mg/ml X-gal, 40 mM citric acid/sodium phosphate, pH 6.0, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM NaCl, 2 mM MgCl2) [40].
Senescence-associated beta-heterochromatic foci (SAFH) analysis
To determine SAFH formation, cells were cultured directly on glass cover slips and then fixed with 4% Para formaldehyde. After washing with PBS, Cells were permeabilized with 0.2% Triton X-100/PBS for 10 min. DNA was visualized by DAPI (1 µg/ml) for 1 min, and then washed with PBS for twice. Cover slips were mounted in a 90% glycerol PBS solution. Cover slips were examined under Leica DMIRB inverted microscope.
MTT Growth Curve
2BS cells were detached and seeded into 96-well plates 2500 cells per well. At the indicated times, cells were stained with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (10 µg/ml) in PBS for 3 hr, then dissolved with 50% N,N dimethylformamide and 10% SDS for 3hr at 37°C. The optical density at 570 nm was determined. Each point was determined in sextuplet.
Cell Cycle Analysis
When cells reached 70–80% confluence, they were washed with PBS, detached with 0.25% trypsin, and fixed with 75% ethanol overnight. Samples were resuspended in 0.5 ml PBS and stained with propidium iodide in the dark for 30 min, and the DNA contents were measured by fluorescence-activated cell sorting (FACS) on a Becton-Dickinson FACScan flow cytometry system. The data were analyzed using a CellFIT software.
Examination of Replicative Lifespan
All transfected 2BS cells and untransfected 2BS cells were subcultured at a split ratio of 1:2. The number of cells was counted at each passage, and the number of population doublings (PDs) achieved between passages was determined by log2 (number of cells obtained/number of cells inoculated). When the cells stopped dividing and remained impossible to be further passaged for 2 weeks, they were regarded as growth-arrested. Conclusions were confirmed by two independent transduction tests
Western Blot
Cells were lysed in radioimmune precipitation assay buffer (1×PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 100 µg/ml phenylmethylsulfonyl fluoride, 50 kallikrein-inactivating units/ml aprotinin, and 1 mM sodium orthovanadate). Protein concentration of each sample was determined by BCA Protein Assay Reagent (Pierce). 100 µg of proteins was loaded on 15% SDS-polyacrylamide gel and transferred to polyvinylidene difluoride filters (Millipore). The filter was blocked and then incubated with the primary antibody in 5% nonfat dry milk in TBST (10 mM Tris-Cl, pH 7.5, 150 mM NaCl, 0.05% Tween 20) overnight at 4°C. After washing, the blots were incubated with secondary antibody (1 µg/ml) conjugated to horseradish peroxidase at 1:2,500 in TBST for 1 hr at room temperature. Proteins were visualized with Chemiluminescent Substrate (Pierce) according to the manufacturer's instruction.
Acknowledgments
We would like to express our appreciation to Dr Fuyuki Ishikawa (University of Cambridge) and Dr. Eric Lam (Imperial College School of Medicine at Hammersmith Hospital) for providing us SIRT1 cDNA expression constructs (pcDNA SIRT1 and pcDNA H363YSIRT1). We also express our gratitude to Dr. Wengong Wang and Dr. Xiaowei Zhang for helpful discussions and Dr. Yousin Suh (Albert Einstein College of Medicine) for critical reading this manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants from the National Basic Research Programs of China (No. 2007CB507400), the National Natural Science Foundation of China (No.3050082 and No.30671064), and the National Post doctoral Foundation of China.
References
- 1.Lorenzini A, Tresini M, Austad SN, Cristofalo VJ. Cellular replicative capacity correlates primarily with species body mass not longevity. Mech Ageing Dev. 2005;126:1130–1133. doi: 10.1016/j.mad.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 4.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 6.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 8.Langley E, Pearson M, Faretta M, Bauer UM, Frye RA, et al. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J. 2002;21:2383–2396. doi: 10.1093/emboj/21.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C, Chen L, Hou X, Li Z, Kabra N, et al. Interactions between E2F1 and SirT1 regulate apoptotic response to DNA damage. Nat Cell Biol. 2006;8:1025–1031. doi: 10.1038/ncb1468. [DOI] [PubMed] [Google Scholar]
- 10.Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16:4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ford J, Jiang M, Milner J. Cancer-specific functions of SIRT1 enable human epithelial cancer cell growth and survival. Cancer Res. 2005;65:10457–10463. doi: 10.1158/0008-5472.CAN-05-1923. [DOI] [PubMed] [Google Scholar]
- 12.Kamel C, Abrol M, Jardine K, He X, McBurney MW. SirT1 fails to affect p53-mediated biological functions. Aging Cell. 2006;5:81–88. doi: 10.1111/j.1474-9726.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- 13.Solomon JM, Pasupuleti R, Xu L, McDonagh T, Curtis R, et al. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol Cell Biol. 2006;26:28–38. doi: 10.1128/MCB.26.1.28-38.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasaki T, Maier B, Bartke A, Scrable H. Progressive loss of SIRT1 with cell cycle withdrawal. Aging Cell. 2006;5:413–422. doi: 10.1111/j.1474-9726.2006.00235.x. [DOI] [PubMed] [Google Scholar]
- 15.Ota H, Tokunaga E, Chang K, Hikasa M, Iijima K, et al. Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene. 2006;25:176–185. doi: 10.1038/sj.onc.1209049. [DOI] [PubMed] [Google Scholar]
- 16.Shay JW, Wright WE. Ageing and cancer: the telomere and telomerase connection. Novartis Found Symp. 2001;235:116–125. [PubMed] [Google Scholar]
- 17.Forsyth NR, Evans AP, Shay JW, Wright WE. Developmental differences in the immortalization of lung fibroblasts by telomerase. Aging Cell. 2003;2:235–243. doi: 10.1046/j.1474-9728.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 18.Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27–S31. doi: 10.1016/s0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- 19.Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 20.Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al Regaiey K, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- 23.Duan J, Zhang Z, Tong T. Senescence delay of human diploid fibroblast induced by anti-sense p16INK4a expression. J Biol Chem. 2001;276:48325–48331. doi: 10.1074/jbc.M104814200. [DOI] [PubMed] [Google Scholar]
- 24.Ressler S, Bartkova J, Niederegger H, Bartek J, Scharffetter-Kochanek K, et al. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell. 2006;5:379–389. doi: 10.1111/j.1474-9726.2006.00231.x. [DOI] [PubMed] [Google Scholar]
- 25.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 26.Raffetto JD, Vasquez R, Goodwin DG, Menzoian JO. Mitogen-activated protein kinase pathway regulates cell proliferation in venous ulcer fibroblasts. Vasc Endovascular Surg. 2006;40:59–66. doi: 10.1177/153857440604000108. [DOI] [PubMed] [Google Scholar]
- 27.Thomas G. The S6 kinase signaling pathway in the control of development and growth. Biol Res. 2002;35:305–313. doi: 10.4067/s0716-97602002000200022. [DOI] [PubMed] [Google Scholar]
- 28.Gerasimovskaya EV, Tucker DA, Weiser-Evans M, Wenzlau JM, Klemm DJ, et al. Extracellular ATP-induced proliferation of adventitial fibroblasts requires phosphoinositide 3-kinase, Akt, mammalian target of rapamycin, and p70 S6 kinase signaling pathways. J Biol Chem. 2005;280:1838–1848. doi: 10.1074/jbc.M409466200. [DOI] [PubMed] [Google Scholar]
- 29.Guarente L, Picard F. Calorie restriction–the SIR2 connection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 30.Zheng W, Wang H, Xue L, Zhang Z, Tong T. Regulation of cellular senescence and p16(INK4a) expression by Id1 and E47 proteins in human diploid fibroblast. J Biol Chem. 2004;279:31524–31532. doi: 10.1074/jbc.M400365200. [DOI] [PubMed] [Google Scholar]
- 31.Haigis MC, Guarente LP. Mammalian sirtuins–emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 32.Chua KF, Mostoslavsky R, Lombard DB, Pang WW, Saito S, et al. Mammalian SIRT1 limits replicative life span in response to chronic genotoxic stress. Cell Metab. 2005;2:67–76. doi: 10.1016/j.cmet.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Ota H, Akishita M, Eto M, Iijima K, Kaneki M, et al. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol. 2007;43:571–579. doi: 10.1016/j.yjmcc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Narita M, Nunez S, Heard E, Narita M, Lin AW, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 35.Munro J, Barr NI, Ireland H, Morrison V, Parkinson EK. Histone deacetylase inhibitors induce a senescence-like state in human cells by a p16-dependent mechanism that is independent of a mitotic clock. Exp Cell Res. 2004;295:525–538. doi: 10.1016/j.yexcr.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Gan L, Han Y, Bastianetto S, Dumont Y, Unterman TG, et al. FoxO-dependent and -independent mechanisms mediate SirT1 effects on IGFBP-1 gene expression. Biochem Biophys Res Commun. 2005;337:1092–1096. doi: 10.1016/j.bbrc.2005.09.169. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi Y, Ohtsuki M, Murakami T, Kobayashi T, Sutheesophon K, et al. Histone deacetylase inhibitor FK228 suppresses the Ras-MAP kinase signaling pathway by upregulating Rap1 and induces apoptosis in malignant melanoma. Oncogene. 2006;25:512–524. doi: 10.1038/sj.onc.1209072. [DOI] [PubMed] [Google Scholar]
- 38.Pullen N, Thomas G. The modular phosphorylation and activation of p70s6k. FEBS Lett. 1997;410:78–82. doi: 10.1016/s0014-5793(97)00323-2. [DOI] [PubMed] [Google Scholar]
- 39.Arvisais EW, Romanelli A, Hou X, Davis JS. AKT-independent phosphorylation of TSC2 and activation of mTOR and ribosomal protein S6 kinase signaling by prostaglandin F2alpha. J Biol Chem. 2006;281:26904–26913. doi: 10.1074/jbc.M605371200. [DOI] [PubMed] [Google Scholar]
- 40.Tee AR, Anjum R, Blenis J. Inactivation of the tuberous sclerosis complex-1 and -2 gene products occurs by phosphoinositide 3-kinase/Akt-dependent and -independent phosphorylation of tuberin. J Biol Chem. 2003;278:37288–37296. doi: 10.1074/jbc.M303257200. [DOI] [PubMed] [Google Scholar]
- 41.Hidayat S, Yoshino Ki, Tokunaga C, Hara K, Matsuo M, et al. Inhibition of amino acid-mTOR signaling by a leucine derivative induces G1 arrest in Jurkat cells. Biochemical and Biophysical Research Communications. 2003;301:417–423. doi: 10.1016/s0006-291x(02)03052-8. [DOI] [PubMed] [Google Scholar]
- 42.Gao N, Flynn DC, Zhang Z, Zhong XS, Walker V, et al. G1 cell cycle progression and the expression of G1 cyclins are regulated by PI3K/AKT/mTOR/p70S6K1 signaling in human ovarian cancer cells. Am J Physiol Cell Physiol. 2004;287:C281–C291. doi: 10.1152/ajpcell.00422.2003. [DOI] [PubMed] [Google Scholar]
- 43.Ohtani N, Zebedee Z, Huot TJ, Stinson JA, Sugimoto M, et al. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature. 2001;409:1067–1070. doi: 10.1038/35059131. [DOI] [PubMed] [Google Scholar]
- 44.Zhang H, Hoff H, Marinucci T, Cristofalo VJ, Sell C. Mitogen-independent phosphorylation of S6K1 and decreased ribosomal S6 phosphorylation in senescent human fibroblasts. Exp Cell Res. 2000;259:284–292. doi: 10.1006/excr.2000.4965. [DOI] [PubMed] [Google Scholar]
- 45.Hutter E, Unterluggauer H, Uberall F, Schramek H, Jansen-Durr P. Replicative senescence of human fibroblasts: the role of Ras-dependent signaling and oxidative stress. Exp Gerontol. 2002;37:1165–1174. doi: 10.1016/s0531-5565(02)00136-5. [DOI] [PubMed] [Google Scholar]