Abstract
Hepatitis C virus (HCV) chronic infection is characterized by low or undetectable cellular immune responses against HCV antigens. Some studies have suggested that HCV proteins manipulate the immune system by suppressing the specific antiviral T-cell immunity. We have previously reported that the expression of HCV core and E1 proteins (CE1) in dendritic cells (DC) impairs their ability to prime T cells in vitro. We show here that immunization of mice with immature DC transduced with an adenovirus encoding HCV core and E1 antigens (AdCE1) induced lower CD4+- and CD8+-T-cell responses than immunization with DC transduced with an adenovirus encoding NS3 (AdNS3). However, no differences in the strength of the immune response were detected when animals were immunized with mature DC subsequently transduced with AdCE1 or AdNS3. According to these findings, we observed that the expression of CE1 in DC inhibited the maturation caused by tumor necrosis factor alpha or CD40L but not that induced by lipopolysaccharide. Blockade of DC maturation by CE1 was manifested by a lower expression of maturation surface markers and was associated with a reduced ability of AdCE1-transduced DC to activate CD4+- and CD8+-T-cell responses in vivo. Our results suggest that HCV CE1 proteins modulate T-cell responses by decreasing the stimulatory ability of DC in vivo via inhibition of their physiological maturation pathways. These findings are relevant for the design of therapeutic vaccination strategies in HCV-infected patients.
Hepatitis C virus (HCV) is an enveloped, single-stranded RNA virus belonging to the family Flaviviridae that is responsible for the majority of non-A, non-B hepatitis (29), which affects an estimated 170 million people worldwide. Infection by HCV is characterized by a high tendency to evolve to chronicity and by the ability to cause chronic hepatitis that may progress to liver cirrhosis and eventually to hepatocellular carcinoma (10). In acute HCV infection, strong T-cell responses against viral antigens are associated with viral clearance, mediated by both CD4+ and CD8+ T cells (11, 14, 30, 46). However, chronically infected patients show very weak or undetectable antiviral T-cell reactivity (6, 21, 34, 37), while maintaining immune competence against other antigens. These findings suggest that HCV may have developed strategies to specifically inhibit the induction of responses toward its constituents. The great variability of HCV, as evidenced by the existence of quasispecies in the same infected individual (26), may allow the emergence of escape mutants, which cannot be efficiently recognized by the immune system. Indeed, several escape mutants have been described that not only affect antibody recognition but also T-cell recognition (7, 41, 47). Although sequence variability is one of the most important mechanisms used by HCV to evade immune response, there are other viral mechanisms of evasion. HCV not only infects hepatocytes but can also infect hematopoietic cells. Viral replication has been described in different subsets of cells of the immune system, and this may favor viral persistence (3, 23, 32) through interactions between viral proteins, mainly HCV core, with promoters and signaling proteins that are relevant for viral clearance (reviewed in reference 45). Regarding the immunomodulatory activity of core protein, contradictory effects have been reported. It has been shown that immunization with a recombinant vaccinia virus containing HCV core resulted in immunosuppression against vaccinia antigens, an effect that was not observed when immunization was performed with vaccinia virus containing HCV nonstructural genes (19). In accordance with these findings it has been reported that transgenic mice expressing HCV core in T cells manifested inhibition of T-lymphocyte responsiveness (42). In contrast, other authors have found that immunization with adenovirus expressing HCV core (24) or immunization of transgenic mice producing HCV structural proteins in the liver (43) did not reveal any significant change of immune reactivity. We have recently described that dendritic cells (DC) expressing HCV core and E1 proteins (DC-CE1) have an impaired ability to induce in vitro primary and secondary CD4+-T-cell responses (38). Similarly, monocyte-derived DC obtained from HCV-infected patients have been shown to exhibit an impaired in vitro stimulatory ability (3, 18). These findings prompted us to study the in vivo immunomodulatory role of HCV structural proteins core and E1. We show here that in vivo immunization with DC expressing HCV CE1 induces lower CD4+- and CD8+-T-cell responses than immunization with DC expressing HCV nonstructural protein 3 (NS3). Also, we show that the lower immunostimulatory capability of DC expressing CE1 is dependent on a maturation defect caused by the expression of HCV structural proteins in the antigen-presenting cell (APC).
MATERIALS AND METHODS
Recombinant adenoviruses.
Recombinant adenoviruses AdCE1 (coding for HCV core and E1 proteins), AdNS3 (coding for HCV NS3 protein), and AdLacZ (expressing β-galactosidase) have been described elsewhere (1, 5). Viruses were propagated on 293 cells, purified in a CsCl isopicnic banding step, and kept in aliquots at −80°C.
Antigens.
Peptides were synthesized manually in a multiple peptide synthesizer by using Fmoc (9-fluorenylmethoxy carbonyl) chemistry. The Kaiser ninhydrin test was used to monitor every step. At the end of the synthesis the peptides were cleaved and deprotected with trifluoroacetic acid and then washed with diethyl ether. Purity of the peptides was always above 90%. HCV core protein (genotype 1b) expressed in baculovirus was kindly provided by B. Rodgers (Murex, England). HCV NS3 was kindly provided by G. Paranhos-Baccala (Centre Nationale de la Recherche Scintifique, Lyon, France). Adenoviral particles used for in vitro stimulation of splenocytes were heat inactivated at 100°C for 30 min.
Mice.
Six- to eight-week-old BALB/c, C57BL/6, and A/J mice were obtained from Harlan (Barcelona, Spain). The animals were maintained in pathogen-free conditions and treated according to the guidelines of our institution. OT-I T-cell-receptor-transgenic mice recognizing peptide OVA(257-264) presented by H-2 Kb were originally generated by F. Carbone (Walter and Eliza Hall Institute of Medical Research, Victoria, Australia) (16), and a founder was received with permission from B. Alarcón (Centro de Biologíia Molecular, Madrid, Spain). That founder was crossed to C57BL/6 females, and the resulting colony was inbred to obtain a homozygous trait of inheritance of the transgenes.
Cell lines.
P815 mastocytoma cells (H-2d) and EL-4 thymoma cells (H-2b) were used as target cells in chromium release assays with cytotoxic T lymphocytes (CTLs) from BALB/c or A/J and from C57BL/6 or OT-I mice, respectively. They were grown in complete medium (CM; RPMI 1640 containing 10% fetal calf serum (FCS), 100 U of penicillin/ml, 100 μg of streptomycin/ml, 2 mM glutamine, and 50 μM 2-mercaptoethanol). XS52 and XS106 DC lines (17, 48) were kindly provided by A. Takashima (University of Texas Southwestern Medical Center, Dallas) and were grown in CM containing 10% of NS47 fibroblast cell line supernatant plus 2 ng of murine granulocyte-macrophage colony-stimulating factor (mGM-CSF; R&D Systems; Minneapolis, Minn.)/ml and in CM containing 5% NS47 supernatant plus 0.5 ng of mGM-CSF/ml, respectively. These cell lines have been described as having immature and mature phenotypes, respectively. The NS47 fibroblast cell line was also obtained from A. Takashima and was grown in CM. The cell lines 3T3-CD40L and 3T3-SAMEN (used as control) were gifts from P. Hwu (National Cancer Institute, Bethesda, Md.) and were derived from NIH 3T3 by stable transduction with murine CD40L or empty vector.
DC generation.
DC were grown from bone marrow cells. After the erythrocytes were lysed, the cells were washed and subsequently depleted of lymphocytes and granulocytes by incubation with a mixture of antibodies to CD4 (GK1.5; American Type Culture Collection [ATCC], Manassas, Va.), CD8 (53.6.72; ATCC), Ly-6G/Gr1 (BD Pharmingen; San Diego, Calif.), and CD45R/B220 (BD Pharmingen), followed by rabbit complement. Remaining cells were grown at 106 cells/ml in 12-well plates in CM supplemented with 20 ng of mGM-CSF and 20 ng of murine interleukin-4 (IL-4; both from Peprotech, London, United Kingdom)/ml. Every 2 days, two-thirds of the medium was replaced with fresh medium containing cytokines. Nonadherent DC were harvested at day 7 and transfected with adenovirus. In some experiments, DC maturation was induced by treating the cells with 1 μg of lipopolysaccharide (LPS; Sigma)/ml or 200 ng of tumor necrosis factor alpha (TNF-α; Peprotech)/ml or by culturing them on a monolayer of 3T3-CD40L cells for 48 h.
Transfection of DC with adenovirus.
DC harvested at day 7 of culture were transfected by incubation at 107 cells/ml in RPMI 1640 with recombinant defective adenoviruses at a multiplicity of infection of 3,000. After 1 h, CM was added to dilute the DC to a final concentration of 1 × 106 to 2 × 106 cells/ml. Cells were harvested 24 h later, extensively washed in order to discard any carryover of adenoviral particles, and used for immunization. In some cases, cells were pulsed with 1 μM CTL epitope I10 from human immunodeficiency virus type 1 envelope protein (44) or 1 μM peptide OVA(257-264) during the last 24 h of incubation.
Immunization.
Groups of three mice were immunized intraperitoneally with 109 PFU of adenovirus resuspended in 0.5 ml of phosphate-buffered saline (PBS) or with 2 × 105 DC transfected with adenovirus. Ten to fourteen days later, animals were sacrificed, and spleens were removed, homogenized, and pooled for immunological analysis. In some experiments, mice were bled from the retro-orbital plexus to collect serum before removal of their spleens.
Stimulation of spleen cells for cytokine induction.
Spleen cells were resuspended in CM and plated at 8 × 105 cells/well in 0.2 ml in U-bottom 96-well plates in the presence of the following antigens: recombinant HCV core protein (1 μg/ml), recombinant HCV NS3 protein (1 μg/ml), or heat-inactivated adenoviral particles from AdLacZ. Cells cultured in the absence of antigen were used as a negative control. In antibody blocking experiments, cells were incubated in the presence of 50 μg of anti-CD4 or anti-CD8 antibodies/ml. Two days later, supernatants were harvested and gamma interferon (IFN-γ) and IL-4 were measured by enzyme-linked immunosorbent assay (BD Pharmingen) according to the manufacturer's instructions.
In the case of cells from OT-I mice, CD8+ cells were purified from the spleens of naive animals by using CD8 Microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer's instructions. These cells (2 × 105/well) were stimulated with graded numbers of adenovirus-transfected DC pulsed or not pulsed with 1 μM peptide OVA(257-264). Two days later, supernatants were harvested, and IFN-γ levels were measured as described above.
Measurement of CD69 expression.
Immune spleen cells were stimulated in 96-well plates as for the measurement of cytokine production. One day later, cells were washed at 4°C with PBS containing 2% FCS and stained with fluorescein isothiocyanate-labeled anti-CD4 antibodies in combination with phycoerythrin-labeled anti-CD69 antibodies (all from BD Pharmingen). After 30 min, the cells were washed, and the number of double-positive CD4+ CD69+ cells was analyzed by using a FACSCalibur flow cytometer (Becton Dickinson).
Detection of antiadenoviral antibodies.
Antiadenoviral antibodies were quantified by enzyme-linked immunosorbent assay as described previously (20). Briefly, microtiter wells (Nunc Maxisorp, Nunc, Denmark) were coated overnight with 1.5 × 108 PFU/well of AdLacZ in carbonate buffer (pH 10). The next day, the wells were washed with PBS containing 0.1% Tween 20 and blocked for 1 h at room temperature with PBS containing 0.1% Tween 20 and 1% nonfat milk. After a blocking step, different serum dilutions were added, followed by incubation for 1 h at 37°C. Antibodies were detected by incubation with biotinylated goat anti-mouse immunoglobulin G at a 1/500 dilution (Amersham Biosciences, Buckinghamshire, England), followed by incubation with horseradish peroxidase at a 1/500 dilution (Amersham). Finally, color was developed by incubation with a TMB solution (Substrate Reagent Set; BD Pharmingen) and stopped by addition of 2N H2SO4.
Measurement of CTL responses.
Splenocytes from animals immunized with DC were incubated with peptides I10 (1 μM), OVA(257-264) (10 μM), E1(121-135) (10 μM), or NS3(1215-1224) (10 μM) for 2 h at 37°C, washed twice, and cultured in 24-well plates at 7.5 × 106 cells/well. Two days later, IL-2 (2.5 U/ml) was added to the wells, and on days 6 to 7 the cells were harvested for chromium release assays. Lytic activity was measured by incubating different numbers of effector cells with 3,000 P815 or EL-4 target cells previously loaded with 51Cr and with or without CTL peptide for 4 h. The percentage of specific lysis was calculated according to the following formula: [(counts per minute [cpm] experimental − cpm spontaneous)/(cpm maximum − cpm spontaneous)] × 100, where spontaneous lysis corresponds to target cells incubated in the absence of effector cells, and maximum lysis is obtained by incubating target cells with 5% Triton X-100.
In the case of splenocytes from OT-I mice, 2 × 106 CD8+ purified naive cells were stimulated in 24-well plates with 2 × 105 adenovirus-transfected DC obtained from C57BL/6 mice and pulsed with OVA(257-264) peptide. Four days later, cells were harvested and CTL activity was measured against EL-4 target cells pulsed or unpulsed with 1 μM OVA(257-264) peptide.
Flow cytometric analysis of DC.
Analysis of the expression of DC surface molecules was done at different times after infection with adenovirus. Cells (105/well) were stained at 4°C in PBS containing 2% FCS with the following fluorescein isothiocyanate-labeled antibodies: anti-CD11c, anti-CD80, anti-CD86, anti-I-Ad, anti-H-2Kd, and isotype control (all from BD Pharmingen). After 30 min, cells were washed and surface expression of the different molecules was analyzed.
RESULTS
HCV structural proteins can act as efficient immunogens.
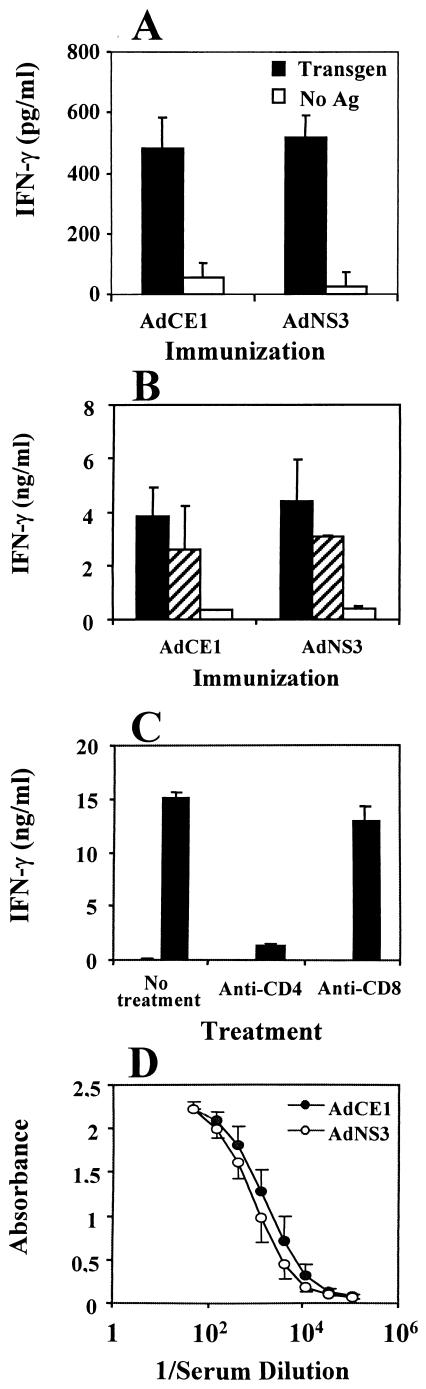
In order to determine the ability of HCV core and E1 proteins to elicit T-cell immunity, we immunized BALB/c mice with AdCE1. We used as control mice injected with AdNS3 since it has been shown that NS3 is a good immunogen and that immunization with vaccinia virus encoding HCV nonstructural antigens did not have any immunosuppressive effect (19). We found that intraperitoneal immunization of BALB/c mice with 109 PFU of AdCE1 or AdNS3 resulted in the induction of similar T-cell immune responses against the transgenes contained in the adenoviral vectors (Fig. 1A). Likewise, the splenocytes of animals immunized with AdCE1 or AdNS3 produced similar levels of IFN-γ upon stimulation with heat-inactivated adenoviral particles (Fig. 1B). As shown in Fig. 1C, the production of IFN-γ by spleen cells in the presence of antigen was mainly mediated by CD4+ T cells. With regard the production of antiadenoviral antibodies, a CD4+-T-helper-dependent phenomenon (9, 49), a similar humoral response was elicited by AdCE1 and AdNS3 (Fig. 1D). Thus, immunization with an adenovirus encoding HCV core and E1 proteins does not cause a blunted immune response either against the transgene or against the integral adenoviral antigens.
FIG. 1.
Injection of recombinant defective adenovirus expressing HCV CE1 proteins does not suppress the CD4+-T-cell response compared to adenovirus expressing HCV NS3. Groups of three BALB/c mice were immunized intraperitoneally with 109 PFU of AdCE1 or AdNS3. Ten days later the animals were sacrificed and spleen cells were stimulated with different antigens to induce IFN-γ production. (A) IFN-γ production after stimulation with 1 μg of transgene protein HCV core or NS3/ml. (B) IFN-γ production after incubation with 6 × 109 PFU of AdLacZ heat-inactivated adenoviral particles/ml (▪), with 1.2 × 109 PFU/ml (▨), or with no antigen (□). (C) Spleen cells from AdCE1-immunized mice were stimulated with 6 × 109 PFU of heat-inactivated adenoviral particles/ml in the presence of different antibodies, and IFN-γ production was measured. (D) Before sacrificing the animals, serum was obtained and antiadenoviral antibodies were measured in both groups.
Immunization with DC expressing HCV core and E1 proteins impairs CD4+-T-cell responses.
After the injection of an adenoviral vector, many different cell types (including APC, hepatocytes, and other epithelial and nonepithelial cells) can be infected, and all of them will release the adenoviral and transgenic proteins to the circulation. These antigens will be taken up by normal (noninfected) DC, which will initiate an immune response. An immunization procedure different from the injection of adenoviral vectors would be immunization with DC which had been transduced ex vivo with AdCE1 or with AdNS3. In this case both the adenoviral and transgenic antigens will be presented to T cells by infected DC. In a previous study we showed that expression of HCV core and E1 proteins in DC impairs their stimulatory ability in vitro, leading to abnormal priming of CD4+ T cells (38). Thus, we sought to determine whether synthesis of HCV proteins inside DC might impair their ability to induce T-cell immunity in vivo. For this purpose, bone marrow-derived DC differentiated for 7 days with GM-CSF and IL-4 (immature DC) were transduced with AdCE1 (DC-CE1), AdNS3 (DC-NS3), or AdLacZ (DC-LacZ) or left uninfected. Phenotypic analysis of these cells showed that DC-CE1 expressed lower levels of CD86 (as reported previously [15]), CD80, and I-Ad class II molecules (Table 1) than DC-NS3 or DC-LacZ, and levels similar to those expressed by uninfected DC. The percentages of positive cells were the same in all groups: 86 and 88% for CD80, 64 and 67% for CD86, and 84 and 87% for I-Ad for DC-CE1 and DC-NS3 cells, respectively. Thus, differences in fluorescence values are not due to a lower number of cells expressing CD80, CD86, or I-Ad in DC-CE1 but to a lower level of expression of these molecules on a per-cell basis. No differences were observed on CD11c and H-2Kd class I molecules between DC-CE1 and DC-NS3 or DC-LacZ.
TABLE 1.
Phenotypic analysis of day 7 DC transfected with AdCE1 or AdNS3
| DC group | Mean fluorescence ± SD with surface markera:
|
|||||
|---|---|---|---|---|---|---|
| Isotype control | CD11c | CD80 | CD86 | IAd | Kd | |
| Uninfected DC | 6 ± 0.7 | 32 ± 6 | 72 ± 3 | 33 ± 4 | 423 ± 48 | 142 ± 9 |
| DC-CE1 | 5 ± 0.2 | 56 ± 8 | 76 ± 11 | 46 ± 8 | 476 ± 24 | 170 ± 16 |
| DC-NS3 | 5 ± 0.6 | 61 ± 2 | 102 ± 4 | 78 ± 5 | 570 ± 17 | 177 ± 12 |
| DC-LacZ | 6 ± 0.7 | 70 ± 10 | 120 ± 3 | 76 ± 4 | 550 ± 34 | 144 ± 6 |
Bone marrow-derived DC were grown for 7 days with GM-CSF and IL-4 and then infected with AdCE1, AdNS3, or AdLacZ or left untreated. One day later, surface marker expression was analyzed by flow cytometry. The numbers indicate the mean fluorescence values for each marker and are representative of three different experiments.
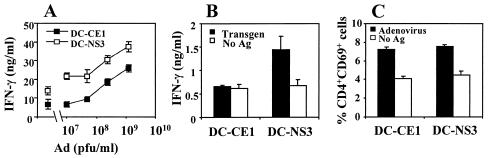
BALB/c mice were then immunized with DC-CE1 or DC-NS3 as a control group, and 2 weeks later the CD4+-T-cell responses to adenoviral antigens were measured. Stimulation of splenocytes from these animals with inactivated adenoviral particles showed that cells from DC-CE1-immunized mice produced lower levels of IFN-γ than cells from animals immunized with DC-NS3 (Fig. 2A). Interestingly, IFN-γ production in the absence of adenoviral particles was also lower in the group of DC-CE1-immunized mice, possibly reflecting a lower stimulation of T cells specific for bovine antigens present in FCS. Analysis of the responses to HCV antigens showed that DC-CE1 was unable to induce a response to core protein, whereas DC-NS3 induced the production of IFN-γ by T cells specific for NS3. In order to confirm that the decrease in IFN-γ production was due to reduced T-cell activation and not to a shift in the cytokine profile, IL-4 was measured in both supernatants. We found that both DC-CE1 and DC-NS3 failed to induce detectable levels of IL-4 (data not shown), ruling out the possibility that a Th2 pattern of response could be mechanism responsible for the lower IFN-γ production observed in DC-CE1-immunized mice.
FIG. 2.
Injection of DC transduced with a recombinant adenovirus expressing HCV CE1 proteins impairs CD4+-T-cell responses. Groups of three BALB/c mice were immunized intraperitoneally with 2 × 105 DC transduced with AdCE1 (DC-CE1) or AdNS3 (DC-NS3). Ten to fourteen days later the animals were sacrificed, and spleen cells were pooled and stimulated with different antigens. (A) IFN-γ production by splenocytes stimulated with heat-inactivated adenoviral particles. The symbols on the left side of the graph correspond to IFN-γ production in the absence of adenoviral particles. The results are representative of three different experiments. (B) IFN-γ production after stimulation with 1 μg of transgene protein HCV core or NS3/ml. (C) CD69 expression on CD4+ T cells after stimulation with adenoviral antigens.
In a previous study (38) we had reported that in vitro stimulation of CD4+ T cells with CE1-expressing DC led to an abnormal priming of T cells (e.g., inhibition of T-cell proliferation and IL-2 production but with normal upregulation of early activation markers such as CD25 or CD69). Thus, we wanted to study whether in vivo immunization with DC-CE1 induced CD4+ T cells with a similar phenotype. Analysis of CD69 expression on CD4+ T cells showed that DC-CE1 was as potent as DC-NS3 in the induction of this early activation marker (Fig. 2C). Thus, CD4+ T cells induced after immunization with DC-CE1 show a phenotype of incomplete activation, with upregulation of CD69 but without full IFN-γ production.
Immunization with DC expressing HCV core and E1 proteins impairs CTL responses.
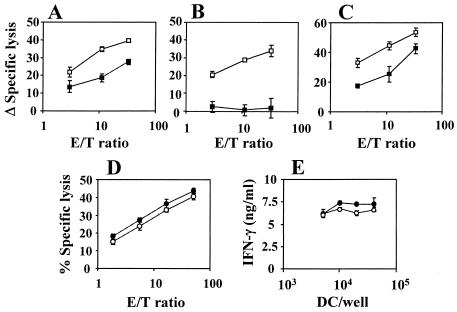
CD4+ T cells are important for the induction of CTL responses, since they produce many cytokines required for CTL priming. Moreover, activated CD4+ T cells license DC through the CD40-CD40L pathway for the activation of naive CD8+ T cells (4, 35, 39). Since we found that expression of HCV CE1 proteins had an immunosuppressive effect on the stimulation of CD4+-T-cell responses induced by DC, we next analyzed whether CTL responses were also affected by expression of CE1 in DC. Thus, DC were infected with AdCE1 or AdNS3 and used to immunize BALB/c mice as in previous experiments. In order to measure the CTL responses, both groups of DC were pulsed before injection with the CTL epitope I10, from human immunodeficiency virus type 1 gp120 envelope protein. Two weeks later, splenocytes were stimulated in vitro with I10, and CTL activity against this epitope was measured in chromium release assays. Lower CTL responses against I10 were induced by immunization with DC-CE1 compared to those generated by immunization with DC-NS3 (Fig. 3A). We also studied the CTL responses to HCV antigens by using peptides E1(121-135) and NS3(1215-1224), which were recognized after immunization with AdCE1 or AdNS3, respectively (1, 20). As shown in Fig. 3B, DC-CE1 did not induce any CTL response against E1(121-135), whereas DC-NS3 induced a clear CTL response against NS3(1215-1224). These results indicate that the expression of CE1 in DC not only reduces the ability of DC to activate CD4+-T-cell responses but also impairs CTL induction.
FIG. 3.
Expression of HCV CE1 proteins in DC impairs their in vivo ability to induce CTL responses. (A) Groups of three BALB/c mice were immunized intraperitoneally with 2 × 105 DC transduced with AdCE1 (DC-CE1) or AdNS3 (DC-NS3) previously pulsed with CTL epitope I10. Two weeks later, animals were sacrificed and their spleen cells were pooled and stimulated with I10. Six days later, CTL activity against I10 was measured in chromium release assays with peptide-pulsed or unpulsed P815 cells. The data represent the change in specific lysis (Δ specific lysis), which was calculated by subtracting the percent lysis with unpulsed target cells from the percent lysis with peptide-pulsed cells. (B) Splenocytes from BALB/c mice immunized with DC-CE1 or DC-NS3 were stimulated with peptide E1(121-135) or NS3(1215-1224), respectively. Six days later, the CTL activity against the corresponding peptide was measured in chromium release assays. The results are expressed as in panel A. (C) C57BL/6 mice were immunized intraperitoneally with 2 × 105 syngeneic DC transduced with AdCE1 (DC-CE1) or AdNS3 (DC-NS3) previously pulsed with CTL epitope OVA(257-264). Two weeks later, animals were sacrificed and CTL activity was measured as in panel A. (D) Purified CD8+ T cells from OT-I mice were stimulated in vitro with C57BL/6 DC transfected with AdCE1 or AdNS3 and pulsed with peptide OVA(257-264). Five days later, lytic activity was measured against EL-4 peptide pulsed or unpulsed target cells. The percentage of lysis against unpulsed target cells was always <2%. (E) IFN-γ production by CD8+ T cells from OT-I mice stimulated with DC-CE1 or DC-NS3 pulsed with peptide OVA(257-264) as in panel D was measured in 48-h culture supernatants. The levels of IFN-γ production stimulated by unpulsed DC were always <0.1 ng/ml (not shown). In all figures, the solid symbols correspond to results obtained with DC-CE1, whereas the open symbols correspond to results with DC-NS3.
The decreased ability of DC-CE1 to stimulate CD8+-T-cell responses might be due to impaired activation of CD4+ T cells or to a direct effect on CTL priming. Thus, we studied the ability of DC-CE1 to prime CD8+-T-cell responses in a CD4-independent system. To this aim, DC-CE1 or DC-NS3 were used to stimulate in vitro purified naive CD8+ T cells from OT-I transgenic mice (C57BL/6 background), which recognize peptide 257-264 from OVA. As shown in Fig. 3D and E, OT-I CD8+ T cells primed with either DC-CE1 or DC-NS3 presenting the peptide OVA(257-264) showed a similar degree of activation, measured as CTL activity (Fig. 3D) or IFN-γ production (Fig. 3E). As a control of response in a CD4-dependent system, normal C57BL/6 mice were immunized with adenovirus-infected DC pulsed with OVA(257-264). In this case (Fig. 3C) and similar to results found in Fig. 3A, DC-CE1 was not as efficient as DC-NS3 for the induction of CTL responses. This suggests that the expression of CE1 in DC does not directly affect CTL priming but reduces CTL activation through decreased stimulation of CD4+ T cells.
HCV core and E1 protein expression does not affect the immunostimulatory function of mature DC.
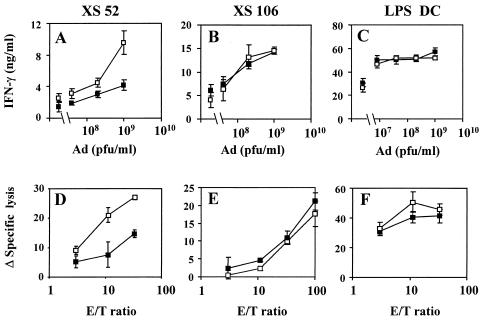
Since in the experiments described above we used immature DC, we decided to analyze whether expression of CE1 might also affect the immunostimulatory function of mature DC. Several signaling pathways are activated in the maturation of DC, which differ according to the maturation stimuli provided. Thus, in a first set of experiments we used two long-term DC lines (XS52 and XS106) that have the immature and mature phenotypes, respectively (17, 48). Immunization of BALB/c mice with immature XS52 cells showed that expression of CE1 induced weaker CD4+-T-cell responses to adenoviral antigens than immunization with the same cells transduced with NS3 (Fig. 4A). However, when immunization was done with mature XS106 cells, expression of CE1 proteins did not affect the generation of CD4+-T-cell responses compared to cells transduced with NS3 (Fig. 4B). Similarly, when we tested CTL responses to peptide I10 we found that expression of CE1 on XS52 immature DC caused an impairment in their CTL stimulation ability (Fig. 4D), whereas when mature XS106 DC were used the expression of CE1 in these cells did not modify the intensity of CTL response compared to that obtained with cells transduced with NS3 (Fig. 4E). In order to confirm these results with bone marrow-derived DC, DC were treated with LPS (LPS-DC) and infected with adenovirus 2 days later, once they had reached a mature phenotype. These cells were pulsed with I10 and used for immunization experiments. LPS-DC behaved as XS106, and no differences between cells expressing CE1 or NS3 were found in CD4+ (Fig. 4C) and CTL responses (Fig. 4F). Thus, mature DC are not susceptible to the inhibitory effects caused by the expression of CE1 in the APCs.
FIG. 4.
Expression of HCV core and E1 proteins in mature DC does not impair their stimulatory ability. BALB/c (A, C, D, and F) or A/J (B and E) mice were immunized with XS52 (A and D)-, XS106 (B and E)-, or LPS (C and F)-stimulated bone marrow DC transfected with AdCE1 (▪) or AdNS3 (□) and then pulsed with peptide I10. Two weeks later, the animals were sacrificed and the spleens were removed. Cells were cultured in 96-well plates, and CD4+-T-cell responses to heat-inactivated adenoviral particles were measured as IFN-γ production (A to C). The symbols on the left side of the graph correspond to IFN-γ production in the absence of adenoviral particles. Splenocytes were also cultured for 5 days in 24-well plates with peptide I10, and the CTL activity was measured in chromium release assays (D to F). The data represent the change in specific lysis (Δ specific lysis), which was calculated by subtracting the percent lysis with unpulsed target cells from the percent lysis with peptide-pulsed cells.
Expression of HCV CE1 in DC impairs the TNF-α- and CD40L-dependent maturation process.
According to data reported above, we hypothesized that expression of HCV CE1 might impair maturation of DC, and as a result, their ability to stimulate T-cell responses. Thus, we decided to analyze whether expression of CE1 in DC might modify the response to a maturation stimulus. To investigate this point, we infected day 7 immature DC with AdCE1 or with AdNS3 (as a control), and 24 h later, both groups of cells were treated with different stimuli, namely, LPS, TNF-α, or CD40L. Two days after DC stimulation, maturation surface markers were analyzed by flow cytometry. Table 2 shows that LPS treatment induced maturation of both DC-CE1 and DC-NS3 with similar upregulation of maturation surface markers in both cases. In clear contrast, stimulation with TNF-α or CD40L induced a higher expression of maturation markers on DC-NS3 compared to DC-CE1. These results indicate that HCV CE1 expression does not affect the LPS-induced DC maturation but inhibits both TNF-α- and CD40L-dependent maturation pathways.
TABLE 2.
Effect of HCV CE1 proteins expression on DC maturation pathways
| Treatment | Transgene | Mean fluorescence with surface markera:
|
|||
|---|---|---|---|---|---|
| Isotype control | CD80 | CD86 | IAd | ||
| None | CE1 | 7 | 108 | 93 | 462 |
| NS3 | 7 | 139 | 129 | 552 | |
| LPS | CE1 | 6 | 220 | 136 | 846 |
| NS3 | 7 | 173 | 126 | 815 | |
| TNF-α | CE1 | 6 | 105 | 92 | 738 |
| NS3 | 8 | 186 | 169 | 1100 | |
| 3T3-SAMEN | CE1 | 7 | 86 | 79 | 519 |
| NS3 | 6 | 120 | 117 | 669 | |
| 3T3-CD40L | CE1 | 6 | 120 | 109 | 572 |
| NS3 | 5 | 163 | 152 | 942 | |
Bone marrow-derived DC were grown for 7 days with GM-CSF and IL-4 and then infected with AdCE1 or AdNS3. One day after infection, DC were incubated with LPS (1 μg/ml), TNF-α (200 ng/ml), or 3T3-CD40L fibroblasts to induce maturation. As a control, cells were left untreated or cultured in the presence of 3T3-SAMEN fibroblasts. After two additional days, surface marker expression was analyzed by flow cytometry. The numbers indicate the mean fluorescence values for each marker and are representative of three different experiments.
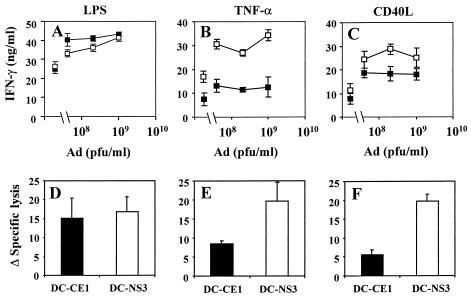
In order to corroborate in vivo the effect of CE1 on DC maturation observed in vitro, mice were immunized with DC infected with AdCE1 or AdNS3 and stimulated with different maturation reagents. As in previous experiments, CD4+- and CD8+-T-cell responses induced by these DC were evaluated 2 weeks after immunization. As shown in Fig. 5 A and D, similar CD4+- and CD8+-T-cell responses were obtained after immunization with DC-CE1 or DC-NS3 when the DC were treated with LPS after adenoviral infection. In contrast, when DC were treated with TNF-α or CD40L after adenoviral infection, the expression of CE1 caused a clear reduction of both CD4+- and CD8+-T-cell responses (Fig. 5B, C, E, and F), confirming that viral structural proteins are interfering with the maturation pathways of DC and consequently with the induction of T-cell immunity.
FIG. 5.
Expression of HCV CE1 in DC inhibits maturation induced by treatment with TNF-α or CD40L but not with LPS. Day 7 bone marrow-derived DC were transduced with AdCE1 (▪) or AdNS3 (□). One day after infection, DC were treated with 1 μg of LPS/ml (A and D), 200 ng of TNF-α/ml (B and E), or 3T3-CD40L fibroblasts (C and F) for two additional days. Cells were pulsed with peptide I10 for the last 24 h of culture, washed, and injected intraperitoneally (2 × 105 DC/mouse). Two weeks later, spleens were removed and pooled, and splenocytes were stimulated with antigens. CD4+-T-cell responses were measured as IFN-γ production against heat-inactivated adenoviral particles (A to C). The symbols on the left side of each graph correspond to IFN-γ production in the absence of adenoviral particles. CTL responses were measured as lytic activity on cells cultured for 5 days in the presence of CTL peptide I10 (D to F). I10-stimulated splenocytes were assayed at an effector/target ratio of 100.
DISCUSSION
The lack of an efficient antiviral cellular immune response is a feature of chronic HCV infection. Interaction of viral proteins within infected cells with components of the cell machinery may constitute an important mechanism that would allow HCV to escape from the immune response. Immunomodulatory activities have been reported for several HCV proteins (45). In the case of core, contradictory results have been reported, which may be related to the experimental system used. We show here that immunization with either AdCE1 or control AdNS3 induces good CD4+-T-cell responses against both adenoviral and transgenic proteins, indicating that core and NS3 are equally good immunogens. These results are in agreement with our previous findings (1, 20) and with data from Liu et al. (24), who did not observe any immunosuppression induced by core after immunization with an adenovirus expressing this protein.
Injection of an adenoviral vector leads to infection (and transduction) of many different cell types, including APC and non-APC cells (such as hepatocytes and other epithelia). All transduced cells synthesize both adenoviral and transgenic proteins that will be released to the circulation and taken up and processed by noninfected DC that in turn activate an immune response. A different way to immunize animals is based on the injection of DC that had been transduced ex vivo with the vector. In this case the immune response against the adenoviral and transgenic proteins is induced only by transduced DC, that is, by DC that are endogenously synthesizing the antigenic proteins. HCV has been shown to infect not only liver cells but also extrahepatic tissues, including monocytes and DC (3, 13, 23, 32). In HCV infection it seems probable that the presentation of HCV antigens to T cells is done by DC that are infected with the virus. In a previous study we showed that DC that were transduced with an adenoviral vector to produce endogenously HCV core and E1 had lower stimulatory ability in vitro (38). Thus, we hypothesized that the immunomodulatory effects of HCV core and E1 might be found in vivo if immunization was carried out with DC transduced with AdCE1. We found that infection of immature DC with AdCE1 resulted in a lower expression of the costimulatory molecules CD80 and CD86 and I-Ad class II molecules. Moreover, when these immature DC were used for immunization 24 h after adenoviral infection, DC-CE1 induced lower CD4+- and CD8+-T-cell immune responses than did control DC. The fact that HCV CE1 cause immunosuppression only when expressed inside DC is also in agreement with the lack of immune defects observed in transgenic mice expressing core and envelope proteins selectively in hepatocytes (43), pointing to the concept that it is the expression of CE1 within the APC which interferes with their immunostimulatory activity. This reveals a new mechanism by which HCV might escape to T-cell immunity.
Interestingly, CD4+ T cells stimulated by DC-CE1 immunization were incompletely activated since they expressed early activation marker CD69 but produced IFN-γ deficiently, in contrast to CD4+ T cells stimulated by DC-NS3. These results reproduce in vivo our previous findings in an in vitro system (38), in which we found the same pattern of incomplete activation of CD4+ T cells stimulated by DC-CE1. It is also intriguing that CD4+ T cells from HCV-infected patients respond to core antigen with normal upregulation of early activation markers but markedly deficient production of IL-2 (38). These data, together with the present findings, suggest that HCV-specific CD4+ T cells from HCV-infected patients might be abnormally primed by HCV-infected DC.
As mentioned above, the expression of CE1 in DC has an immunosuppressive effect on the induction of both CD4+- and CD8+-T-cell responses. This effect of HCV structural proteins on the immunostimulatory function of DC may be the cause of the low CD4+ and CD8+ responses to HCV antigens observed in patients with chronic hepatitis C (6, 14, 21, 30, 34, 37, 46). According to our findings, the defective T-cell response against HCV antigens may indicate a predominant presentation of HCV antigens to T cells by HCV-infected DC, in which the expression of HCV structural antigens may impair their immunostimulatory ability. However, in patients with chronic HCV infection, viral replication might occur in only a proportion of DC, allowing the noninfected DC to present normally non-HCV antigens to T cells. This would explain how patients with chronic hepatitis C exhibit a selective deficit of anti-HCV immunity with preservation of normal immune response against unrelated antigens.
The importance of CD4+ T cells in CTL activity has been recently highlighted by studies in mice with chronic lymphocytic choriomeningitis virus infection, where it has been shown that viral persistence was facilitated by the presence of unresponsive CD8+ T cells because of the absence of an efficient CD4+ response (50). In our experiments with CD8+-T-cell priming carried out in a CD4+-independent system we observed that DC-CE1 could correctly stimulate CTL responses (Fig. 3D and E), indicating that the lower CD8+-T-cell response induced by DC-CE1 in normal animals is dependent on reduced activation of CD4+ T cells.
A common escape mechanism for viruses that infect DC is the interference with their maturation process. This effect, which has been observed in herpes simplex virus, vaccinia virus, measles virus, and human cytomegalovirus infections (12, 31, 36, 40), results in impaired development of efficient antiviral T-cell immunity. Our findings indicate that the blunted T-cell immune responses against antigens presented by DC-CE1 in the animals depends on a blockade of DC maturation by HCV structural antigens. In fact, when DC are treated with LPS to induce maturation before transduction with adenoviruses encoding CE1 or NS3, the immunostimulatory functions of DC-CE1 or DC-NS3 are comparable. Similarly, transduction with AdCE1 of a long-term DC line that does not require a maturation stimulus does not affect its ability to induce T-cell immune responses. Interestingly, we found that CE1 expression in the DC inhibited maturation induced by TNF-α or CD40L but not by LPS. The effect of CE1 on TNF-α-dependent maturation is in accordance with studies showing that DC from patients with chronic HCV infection, but not DC from subjects who cleared the virus, had a defect in their TNF-α-dependent maturation process, possibly due to the expression of viral proteins inside these cells (2). Although we cannot rule out a role for other HCV proteins in natural infection, our results indicate that CE1 may play a critical role in the inhibition of DC maturation mediated by TNF-α and CD40L in patients with chronic hepatitis C. Inhibition of the CD40-CD40L maturation pathway is another relevant finding of the present study. This pathway is mediated mainly by interaction of DC with CD40L-expressing CD4+ T cells. This interaction leads to upregulation of major histocompatibility complex class I, class II, and costimulatory molecules on DC and to the production of cytokines, necessary for full activation and differentiation of naive CD4+ T cells. Moreover, licensing of DC through this pathway enables them to activate CD8+-T-cell responses (4, 35, 39). Thus, interference with the maturation induced by CD40-CD40L leads to a lower costimulation via CD80 and/or CD86 and CD28, which might result in anergy instead of activation of T cells after T-cell-receptor triggering. Since CD4+ T cells are responsible for this maturation process, deficient CD4+-T-cell activation may result in decreased CD8+-T-cell responses. This may explain why the effect on CD8+ T cells, found with immunization with immature DC-CE1, is not observed when DC-CE1 are used in an in vitro CD4-independent system. Since CD40-CD40L and proinflammatory cytokines such as TNF-α are probably the main mechanisms responsible for DC maturation after immunization with immature DC, interference with these pathways may be the reason for the attenuation of CD4+ and CD8+-T-cell responses observed when animals are immunized with immature DC-CE1.
Interestingly, with the different maturation stimuli tested, TNF-α and CD40L, but not LPS, were affected by CE1 expression. CD40 is a member of the TNF receptor superfamily that includes TNF-α receptor 1, lymphotoxin-β receptor, and Fas (25). It should be noted that HCV core has been reported to bind to these receptors, thus modulating the signaling pathway activated by the corresponding ligands (8, 22, 27, 33, 51, 52). Future studies are needed to elucidate the molecular mechanism used by HCV CE1 to interfere with the intracellular signaling activated by TNF-α and CD40L in DC.
Besides pathophysiological implications, our findings are relevant with regard to the use of DC as a method to induce anti-HCV T-cell responses. HCV core is a very attractive protein for immunization due to its high degree of sequence conservation among different isolates, and synthetic peptides or protein fragments pulsed onto DC may be used to induce immune responses. However, if intracellular expression of the proteins is preferred in order to induce CTL responses, maturation of the DC should be carried out before transfection of the cells with the vector encoding HCV core. Recently, efficient anti-core CTL responses were obtained with DC engineered to produce HCV core, and in that study DC were matured with LPS before transduction (28).
To conclude, we found that HCV proteins can interfere with the maturation of DC. Impairment of the immunostimulatory function of DC as result of deficient maturation leads to blunted T-cell responses that may favor viral persistence. Thus, our data have revealed a new HCV strategy for evading T-cell immunity.
Acknowledgments
This study was supported by grants from Ministerio de Ciencia y Tecnología (SAF2001-1119 and SAF2002-023) to P. Sarobe and J. Prieto, respectively; from FIS (01/0733) to J. J. Lasarte; from CICYT (SAF2000-0059) to F. Borrás-Cuesta; and from the Instituto de Salud Carlos III C03/02 to all authors. A. Zabaleta is a recipient of a scholarship from Fundación Echebano, L. Arribillaga is a recipient of a scholarship from Gobierno de Navarra, and A. Arina is a recipient of a scholarship from FIS.
We thank B. Rodgers and G. Paranhos-Baccala for providing HCV core and NS3, respectively. We also thank A. Takashima and P. Hwu for providing cell lines.
REFERENCES
- 1.Arribillaga, L., A. L. de Cerio, P. Sarobe, N. Casares, M. Gorraiz, A. Vales, O. Bruna-Romero, F. Borras-Cuesta, G. Paranhos-Baccala, J. Prieto, J. Ruiz, and J. J. Lasarte. 2002. Vaccination with an adenoviral vector encoding hepatitis C virus (HCV) NS3 protein protects against infection with HCV-recombinant vaccinia virus. Vaccine 21:202-210. [DOI] [PubMed] [Google Scholar]
- 2.Auffermann-Gretzinger, S., E. B. Keeffe, and S. Levy. 2001. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood 97:3171-3176. [DOI] [PubMed] [Google Scholar]
- 3.Bain, C., A. Fatmi, F. Zoulim, J. P. Zarski, C. Trepo, and G. Inchauspe. 2001. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology 120:512-524. [DOI] [PubMed] [Google Scholar]
- 4.Bennett, S. R., F. R. Carbone, F. Karamalis, R. A. Flavell, J. F. Miller, and W. R. Heath. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393:478-480. [DOI] [PubMed] [Google Scholar]
- 5.Bruna-Romero, O., J. J. Lasarte, G. Wilkinson, K. Grace, B. Clarke, F. Borras-Cuesta, and J. Prieto. 1997. Induction of cytotoxic T-cell response against hepatitis C virus structural antigens using a defective recombinant adenovirus. Hepatology 25:470-477. [DOI] [PubMed] [Google Scholar]
- 6.Cerny, A., J. G. McHutchison, C. Pasquinelli, M. E. Brown, M. A. Brothers, B. Grabscheid, P. Fowler, M. Houghton, and F. V. Chisari. 1995. Cytotoxic T lymphocyte response to hepatitis C virus-derived peptides containing the HLA A2.1 binding motif. J. Clin. Investig. 95:521-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, K. M., B. Rehermann, J. G. McHutchison, C. Pasquinelli, S. Southwood, A. Sette, and F. V. Chisari. 1997. Immunological significance of cytotoxic T lymphocyte epitope variants in patients chronically infected by the hepatitis C virus. J. Clin. Investig. 100:2376-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, C. M., L. R. You, L. H. Hwang, and Y. H. Lee. 1997. Direct interaction of hepatitis C virus core protein with the cellular lymphotoxin-beta receptor modulates the signal pathway of the lymphotoxin-beta receptor. J. Virol. 71:9417-9426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coutelier, J. P., P. G. Coulie, P. Wauters, H. Heremans, and J. T. van der Logt. 1990. In vivo polyclonal B-lymphocyte activation elicited by murine viruses. J. Virol. 64:5383-5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dienstag, J. L. 1983. Non-A, non-B hepatitis. I. Recognition, epidemiology, and clinical features. Gastroenterology 85:439-462. [PubMed] [Google Scholar]
- 11.Diepolder, H. M., R. Zachoval, R. M. Hoffmann, E. A. Wierenga, T. Santantonio, M. C. Jung, D. Eichenlaub, and G. R. Pape. 1995. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet 346:1006-1007. [DOI] [PubMed] [Google Scholar]
- 12.Engelmayer, J., M. Larsson, M. Subklewe, A. Chahroudi, W. I. Cox, R. M. Steinman, and N. Bhardwaj. 1999. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J. Immunol. 163:6762-6768. [PubMed] [Google Scholar]
- 13.Goutagny, N., A. Fatmi, V. De Ledinghen, F. Penin, P. Couzigou, G. Inchauspe, and C. Bain. 2003. Evidence of viral replication in circulating dendritic cells during hepatitis C virus infection. J. Infect. Dis. 187:1951-1958. [DOI] [PubMed] [Google Scholar]
- 14.Gruener, N. H., T. J. Gerlach, M. C. Jung, H. M. Diepolder, C. A. Schirren, W. W. Schraut, R. Hoffmann, R. Zachoval, T. Santantonio, M. Cucchiarini, A. Cerny, and G. R. Pape. 2000. Association of hepatitis C virus-specific CD8+ T cells with viral clearance in acute hepatitis C. J. Infect. Dis. 181:1528-1536. [DOI] [PubMed] [Google Scholar]
- 15.Hiasa, Y., N. Horiike, S. M. Akbar, I. Saito, T. Miyamura, Y. Matsuura, and M. Onji. 1998. Low stimulatory capacity of lymphoid dendritic cells expressing hepatitis C virus genes. Biochem. Biophys. Res. Commun. 249:90-95. [DOI] [PubMed] [Google Scholar]
- 16.Hogquist, K. A., S. C. Jameson, W. R. Heath, J. L. Howard, M. J. Bevan, and F. R. Carbone. 1994. T-cell receptor antagonist peptides induce positive selection. Cell 76:17-27. [DOI] [PubMed] [Google Scholar]
- 17.Kitajima, T., K. Ariizumi, P. R. Bergstresser, and A. Takashima. 1995. T cell-dependent loss of proliferative responsiveness to colony-stimulating factor-1 by a murine epidermal-derived dendritic cell line, XS52. J. Immunol. 155:5190-5197. [PubMed] [Google Scholar]
- 18.Laporte, J., C. Bain, P. Maurel, G. Inchauspe, H. Agut, and A. Cahour. 2003. Differential distribution and internal translation efficiency of hepatitis C virus quasispecies present in dendritic and liver cells. Blood 101:52-57. [DOI] [PubMed] [Google Scholar]
- 19.Large, M. K., D. J. Kittlesen, and Y. S. Hahn. 1999. Suppression of host immune response by the core protein of hepatitis C virus: possible implications for hepatitis C virus persistence. J. Immunol. 162:931-938. [PubMed] [Google Scholar]
- 20.Lasarte, J. J., F. J. Corrales, N. Casares, A. Lopez-Diaz de Cerio, C. Qian, X. Xie, F. Borras-Cuesta, and J. Prieto. 1999. Different doses of adenoviral vector expressing IL-12 enhance or depress the immune response to a coadministered antigen: the role of nitric oxide. J. Immunol. 162:5270-5277. [PubMed] [Google Scholar]
- 21.Lasarte, J. J., M. Garcia Granero, A. Lopez, N. Casares, N. Garcia, M. P. Civeira, F. Borras Cuesta, and J. Prieto. 1998. Cellular immunity to hepatitis C virus core protein and the response to interferon in patients with chronic hepatitis C. Hepatology 28:815-822. [DOI] [PubMed] [Google Scholar]
- 22.Lasarte, J. J., P. Sarobe, P. Boya, N. Casares, L. Arribillaga, A. L. de Cerio, M. Gorraiz, F. Borras-Cuesta, and J. Prieto. 2003. A recombinant adenovirus encoding hepatitis C virus core and E1 proteins protects mice against cytokine-induced liver damage. Hepatology 37:461-470. [DOI] [PubMed] [Google Scholar]
- 23.Lerat, H., F. Berby, M. A. Trabaud, O. Vidalin, M. Major, C. Trepo, and G. Inchauspe. 1996. Specific detection of hepatitis C virus minus strand RNA in hematopoietic cells. J. Clin. Investig. 97:845-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, Z. X., H. Nishida, J. W. He, M. M. Lai, N. Feng, and G. Dennert. 2002. Hepatitis C virus genotype 1b core protein does not exert immunomodulatory effects on virus-induced cellular immunity. J. Virol. 76:990-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locksley, R. M., N. Killeen, and M. J. Lenardo. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487-501. [DOI] [PubMed] [Google Scholar]
- 26.Martell, M., J. I. Esteban, J. Quer, J. Genesca, A. Weiner, R. Esteban, J. Guardia, and J. Gomez. 1992. Hepatitis C virus (HCV) circulates as a population of different but closely related genomes: quasispecies nature of HCV genome distribution. J. Virol. 66:3225-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marusawa, H., M. Hijikata, T. Chiba, and K. Shimotohno. 1999. Hepatitis C virus core protein inhibits Fas- and tumor necrosis factor alpha-mediated apoptosis via NF-κB activation. J. Virol. 73:4713-4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsui, M., O. Moriya, N. Abdel-Aziz, Y. Matsuura, T. Miyamura, and T. Akatsuka. 2002. Induction of hepatitis C virus-specific cytotoxic T lymphocytes in mice by immunization with dendritic cells transduced with replication-defective recombinant adenovirus. Vaccine 21:211-220. [DOI] [PubMed] [Google Scholar]
- 29.Miller, R. H., and R. H. Purcell. 1990. Hepatitis C virus shares amino acid sequence similarity with pestiviruses and flaviviruses as well as members of two plant virus supergroups. Proc. Natl. Acad. Sci. USA 87:2057-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Missale, G., R. Bertoni, V. Lamonaca, A. Valli, M. Massari, C. Mori, M. G. Rumi, M. Houghton, F. Fiaccadori, and C. Ferrari. 1996. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J. Clin. Investig. 98:706-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moutaftsi, M., A. M. Mehl, L. K. Borysiewicz, and Z. Tabi. 2002. Hum. cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells. Blood 99:2913-2921. [DOI] [PubMed] [Google Scholar]
- 32.Muller, H. M., B. Kallinowski, C. Solbach, L. Theilmann, T. Goeser, and E. Pfaff. 1994. B-lymphocytes are predominantly involved in viral propagation of hepatitis C virus (HCV). Arch. Virol. 9(Suppl.):307-316. [DOI] [PubMed] [Google Scholar]
- 33.Ray, R. B., K. Meyer, R. Steele, A. Shrivastava, B. B. Aggarwal, and R. Ray. 1998. Inhibition of tumor necrosis factor (TNF-alpha)-mediated apoptosis by hepatitis C virus core protein. J. Biol. Chem. 273:2256-2259. [DOI] [PubMed] [Google Scholar]
- 34.Rehermann, B., K. M. Chang, J. G. McHutchison, R. Kokka, M. Houghton, and F. V. Chisari. 1996. Quantitative analysis of the peripheral blood cytotoxic T lymphocyte response in patients with chronic hepatitis C virus infection. J. Clin. Investig. 98:1432-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridge, J. P., F. Di Rosa, and P. Matzinger. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393:474-478. [DOI] [PubMed] [Google Scholar]
- 36.Salio, M., M. Cella, M. Suter, and A. Lanzavecchia. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur. J. Immunol. 29:3245-3253. [DOI] [PubMed] [Google Scholar]
- 37.Sarobe, P., J. I. Jauregui, J. J. Lasarte, N. Garcia, M. P. Civeira, F. Borras-Cuesta, and J. Prieto. 1996. Production of interleukin-2 in response to synthetic peptides from hepatitis C virus E1 protein in patients with chronic hepatitis C: relationship with the response to interferon treatment. J. Hepatol. 25:1-9. [DOI] [PubMed] [Google Scholar]
- 38.Sarobe, P., J. J. Lasarte, N. Casares, A. Lopez-Diaz de Cerio, E. Baixeras, P. Labarga, N. Garcia, F. Borras-Cuesta, and J. Prieto. 2002. Abnormal priming of CD4+ T cells by dendritic cells expressing hepatitis C virus core and E1 proteins. J. Virol. 76:5062-5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoenberger, S. P., R. E. Toes, E. I. van der Voort, R. Offringa, and C. J. Melief. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393:480-483. [DOI] [PubMed] [Google Scholar]
- 40.Servet-Delprat, C., P. O. Vidalain, H. Bausinger, S. Manie, F. Le Deist, O. Azocar, D. Hanau, A. Fischer, and C. Rabourdin-Combe. 2000. Measles virus induces abnormal differentiation of CD40 ligand-activated human dendritic cells. J. Immunol. 164:1753-1760. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu, Y. K., M. Hijikata, A. Iwamoto, H. J. Alter, R. H. Purcell, and H. Yoshikura. 1994. Neutralizing antibodies against hepatitis C virus and the emergence of neutralization escape mutant viruses. J. Virol. 68:1494-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soguero, C., M. Joo, K. A. Chianese-Bullock, D. T. Nguyen, K. Tung, and Y. S. Hahn. 2002. Hepatitis C virus core protein leads to immune suppression and liver damage in a transgenic murine model. J. Virol. 76:9345-9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun, J., F. Bodola, X. Fan, H. Irshad, L. Soong, S. M. Lemon, and T. S. Chan. 2001. Hepatitis C virus core and envelope proteins do not suppress the host's ability to clear a hepatic viral infection. J. Virol. 75:11992-11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi, H., Y. Nakagawa, K. Yokomuro, and J. A. Berzofsky. 1993. Induction of CD8+ cytotoxic T lymphocytes by immunization with syngeneic irradiated HIV-1 envelope derived peptide-pulsed dendritic cells. Int. Immunol. 5:849-857. [DOI] [PubMed] [Google Scholar]
- 45.Tellinghuisen, T. L., and C. M. Rice. 2002. Interaction between hepatitis C virus proteins and host cell factors. Curr. Opin. Microbiol. 5:419-427. [DOI] [PubMed] [Google Scholar]
- 46.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C. Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, H., and D. D. Eckels. 1999. Mutations in immunodominant T-cell epitopes derived from the nonstructural 3 protein of hepatitis C virus have the potential for generating escape variants that may have important consequences for T-cell recognition. J. Immunol. 162:4177-4183. [PubMed] [Google Scholar]
- 48.Xu, S., P. R. Bergstresser, and A. Takashima. 1995. Phenotypic and functional heterogeneity among murine epidermal-derived dendritic cell clones. J. Investig. Dermatol. 105:831-836. [DOI] [PubMed] [Google Scholar]
- 49.Yang, Y., Q. Li, H. C. Ertl, and J. M. Wilson. 1995. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J. Virol. 69:2004-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu, N., A. Khoshnan, R. Schneider, M. Matsumoto, G. Dennert, C. Ware, and M. M. Lai. 1998. Hepatitis C virus core protein binds to the cytoplasmic domain of tumor necrosis factor (TNF) receptor 1 and enhances TNF-induced apoptosis. J. Virol. 72:3691-3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu, N., C. F. Ware, and M. M. Lai. 2001. Hepatitis C virus core protein enhances FADD-mediated apoptosis and suppresses TRADD signaling of tumor necrosis factor receptor. Virology 283:178-187. [DOI] [PubMed] [Google Scholar]