Abstract
The aim of this study was to investigate the effect of bovine viral diarrhea virus (BVDV) infections (unapparent acute infections and persistent infections) on the overall health and performance of feedlot cattle. Calves from 25 pens (7132 calves) were enrolled in the study. Overall and infectious disease mortality rates were significantly higher (P < 0.05) in pens categorized at arrival as positive for type I BVDV and lower in pens that were positive for type II BVDV than in negative pens. Mortality attributed to BVDV infection or enteritis was significantly more common (P < 0.05) in the pens containing persistently infected (PI) calves than in pens not containing PI calves (non-PI pens). There were no statistically detectable (P ≥ 0.05) differences in morbidity, overall mortality, average daily gain, or the dry matter intake to gain ratio between PI and non-PI pens. Although type-I BVDV infections in feedlots appear to contribute to higher mortality rates, the presence of PI calves alone does not appear to have a strong impact on pen-level animal health and feedlot performance.
Résumé
Impact des infections au virus de la diarrhée virale bovine sur la santé et la performance des bovins en parcs d’engraissement. Le but de cette étude était d’examiner les effets de l’infection au virus de la diarrhée virale bovine (VDVB) (infections silencieuses aigües et infections persistantes) sur la santé générale et la performance des bovins en parcs d’engraissement. Des veaux provenant de 25 différents enclos (7132 animaux) ont été inclus dans cette étude. Les taux généraux de mortalité et ceux reliés aux maladies infectieuses étaient significativement plus élevés (P < 0,05) dans les enclos classés positifs au type 1 de VDVB à l’arrivée et plus bas dans les enclos classés positifs au type II de VDVB que dans les enclos classés négatifs. La mortalité attribuée aux infections au VDVB ou aux entérites était significativement plus fréquente (P < 0,05) dans les enclos occupés par des veaux infectés de façon persistante (IP) que dans les enclos occupés par des veaux non IP (enclos non IP). Il n’y avait pas de différence significatives (P ≥ 0,05) dans la morbidité, la mortalité globale, le gain corporel moyen quotidien ou la prise de matière sèche par rapport a l’indice de gain entre les enclos IP et non IP. Bien que les infections de type 1 au VDVB dans les parcs d’engraissement semblent contribuer aux taux plus élevés de mortalité, la présence seule de veaux IP ne semble par avoir d’impact important sur le niveau de santé dans les enclos et sur la performance des parcs d’engraissement.
(Traduit par Docteur André Blouin)
Introduction
Bovine viral diarrhea virus (BVDV) can cause different diseases that include subclinical benign infection, fatal mucosal disease, peracute fatal diarrhea, thrombocytopenia and hemorrhagic disease, reproductive failure, and congenital abnormalities (1).
In a survey of 256 beef herds in the USA, over 90% of herds had been exposed to BVDV through either natural exposure or vaccination (2). In western Canada, the prevalence of BVDV infection in a population of feedlot calves was estimated to be 27%, using an ELISA test, and the seroconversion risk was 40%, according to a virus neutralization test (3). In the same study, the prevalence of persistently infected (PI) calves was < 0.1%, which was deemed to be low (3). In a 1-year study of a beef herd, the prevalence of PI calves was estimated to range from 9.1% to 12.7% and the PI calves had poor survivability and were “poor doers” to 1 y of age compared with BVDV-negative herd mates (4).
Bovine viral diarrhea virus infection continues to play an important role in commercial feedlot production, even though BVDV vaccination programs are in use. One of the most important infectious diseases in commercial feedlot production is the undifferentiated fever (UF)/bovine respiratory disease (BRD) complex and, in spite of preventive and control strategies, there appears to be evidence that BVDV infection continues to play an important role in UF/BRD in commercial feedlot production (5–9). Current management practices have focused on successfully managing this disease complex through the use of prophylactic and therapeutic antimicrobial strategies, as well as vaccination programs targeting the common viral and bacterial etiologic agents of feedlot UF/BRD described in the veterinary literature (6–9).
Serologic studies have demonstrated that feedlot animals with higher antibody titers, seroconversion to BVDV, or both, are at a lower risk of developing UF/BRD (10–13). In addition, results from a prospective cohort study of new cases of UF/BRD occurring after day 70 of the feeding period (cases) and “healthy” pen mates (controls) demonstrate that BVDV was 4.55 times more likely to be isolated from the serum of case animals than control animals (P < 0.05) (14). Immunohistochemical (IHC) staining of postmortem specimens from selected feedlot cattle populations has demonstrated an association between the presence of BVDV and the occurrence of fatal BRD (15–17). In addition, it appears that BVDV was associated with UF/BRD in large-scale field trials where vaccinating calves at arrival with multivalent viral vaccines containing BVDV antigens significantly (P < 0.05) reduced UF/BRD morbidity, overall chronicity, overall wastage, and/or overall mortality, as compared with calves that were not vaccinated against BVDV (7,18).
There are no data that describe BVDV transmission in commercial feedlot production and how this transmission affects the development of UF/BRD, because, until recently, assays for BVDV infection were not suitable for cost-effective, rapid identification and differentiation between PI and acutely infected (AI) animals. Polymerase chain reaction (PCR) for BVDV detection is now widely used and may detect both PI and AI animals; the ability to pool individual samples reduces the cost and increases the efficiency of the assay. Immunohistochemical staining has been used on formalin-fixed tissues for BVDV detection for some years and the finding that the assay is applicable to skin biopsy specimens and can be used to differentiate PI from AI animals has expanded its use to the confirmation of persistent infections in the living animal (19).
The objectives of the present study were to describe the frequency and character (type I and II) of BVDV infections (PI and AI) at feedlot arrival; to describe the frequency of BVDV identification in postmortem tissue samples, using IHC tests; to investigate the effect of BVDV infections on subsequent animal health outcomes; and to investigate the effects of PI animals on pen-level animal health and feedlot performance.
Materials and methods
Study overview
A prospective, longitudinal study was conducted to investigate associations between BVDV-infection status and measures of morbidity, mortality, and production in cattle populations housed at commercial feedlots. Infection status, determined by using a combination of PCR, IHC, or both, was investigated for all enrolled cattle at the time of initial processing, at the time that sick cattle were examined, and at the time of postmortem examination in study animals that died. At each of 3 study sites, feeder cattle were purchased from auction markets throughout western Canada, as per the standard procurement procedures for each feedlot. Animals were managed as per a standard set of animal husbandry procedures for feedlot calves, which included the collection of individual animal event information on a chute-side computer system. Pens of animals were convenience-selected for enrollment, based on study site and laboratory time constraints. Sixteen pens (10 pens at site A and 6 pens at site B) were enrolled in the fall of 2003 and 9 pens (5 pens at site B and 4 pens at site C) were enrolled in the fall of 2004. Study animals were followed from feedlot arrival until feedlot exit (shipment for harvest or death).
Study facilities
Three feedlots in Alberta (1 feedlot from each of the Mossleigh, Strathmore, and Brant areas) were selected for feedlot calf enrollment. The capacity of these feedlots ranges from 14 000 to 48 000 animals and, collectively, more than 45 000 beef calves are fed annually. The basic design of each feedlot is representative of the standard design used in western Canada. The animals were housed in open-air, dirt-floor pens, arranged side-by-side, with central feed alleys and 20% porosity wood-fence windbreaks. Each pen holds approximately 250 to 350 animals. Hospital and cattle handling facilities are located in each feedlot. Each cattle handling facility has a hydraulic chute, an electronic scale, a chute-side computer for animal health data collection [Feedlot Health Animal Record Management (FHARM); Feedlot Health Management Services (FHMS), Okotoks, Alberta], and separation alleys to facilitate the return of animals to designated pens.
Study animals
The animals enrolled in this study (7132 calves; 25 pens) were crossbred beef steer and bull calves purchased from auction markets throughout western Canada, using the standard procurement procedures employed by each feedlot. Animals were transported by truck to the feedlots after assembly at the auction markets. Upon arrival at the feedlot, the animals were moved through a hydraulic chute for processing. At processing, animals received the following: unique individual animal identification tag; a modified-live infectious bovine rhinotracheitis virus (IBRV) and BVDV (both type I and type II viruses) vaccine; a multivalent clostridial bacterin/toxoid; a Mannheimia haemolytica-Pasteurella multocida bacterin/toxoid; a Histophilus somni bacterin; a prophylactic, parenteral, long-acting antimicrobial; an anabolic growth implant; and a topical external and internal parasite control product. In addition, all bull calves were castrated. One to 10 wk postarrival, all animals received a modified-live IBRV, BVDV (type I virus), parainfluenza-3 virus, and bovine respiratory syncytial virus booster vaccine.
Experimental design
In addition to the standard processing procedures described previously, a citrated whole blood sample (collected from the jugular vein) and a skin biopsy (obtained from the outer edge of the ear) were collected from all study animals at processing (population-based BVDV testing survey to identify BVDV PI and AI at the time of feedlot arrival). Subsequently, a 2nd citrated whole blood sample was collected from the study animals at the time of initial diagnosis of UF or no fever (NF) during the first 30 d of the feeding period (morbidity-based BVDV testing survey to identify BVDV AI at the time of initial morbidity diagnosis). A diagnosis of UF was made when animals showed evidence of depression, as characterized by lack of response to visual stimulation, reluctance to move, and/or abnormal posture/carriage of the head; a lack of abnormal clinical signs referable to body systems other than the respiratory system; a rectal temperature > 40.5°C; and no previous treatment history for UF/BRD. A diagnosis of NF was made when animals showed similar clinical symptoms to UF, but rectal temperature was ≤ 40.5°C.
At the time of gross postmortem examination, skin (from the outer edge of the ear), lung, heart, ileum, and synovial membrane samples were collected from each animal by an FHMS veterinarian (mortality-based BVDV testing survey to identify BVDV in tissues of dead animals). On a daily basis, FHMS personnel transported all samples from the study sites to the FHMS office.
Finally, confirmation samples (a citrated whole blood sample and a skin biopsy) were collected from all PI animals (positive IHC test on arrival skin biopsy — refer to next section for more information) that survived to harvest.
Sample processing
Citrated whole blood samples collected from the study animals for both the population-based and morbidity-based surveys were transported to the Western College of Veterinary Medicine (WCVM), University of Saskatchewan, 2–3 times per wk for PCR testing. An RNA extraction method was used to isolate RNA from the blood cells of each sample. The extracts of 5 animals were pooled and RT-PCR was used to identify positive pools (and subsequently to identify the infected individual animal(s) within each positive pool). The method used distinguished between BVDV genotypes (type I or II) (20,21).
The skin biopsies collected at the time of feedlot arrival were placed in a cryoprotectant media (60% ethylene glycol in phosphate buffered saline) and stored at −18°C. Biopsy samples from animals that tested positive by PCR analysis of the citrated whole blood samples were retrieved and shipped to the WCVM for BVDV IHC staining to differentiate between PI and AI animals (19). Tissues collected from animals that died during the study were placed in formalin and sent to the WCVM for BVDV IHC staining. At the WCVM, formalinized samples were trimmed and processed into paraffin-embedded tissue blocks. Serial sections of each tissue block were immunohistochemically stained for the gp48 protein of BVDV, using monoclonal antibody 15C5 and an avidin-biotin complex immunoperoxidase method (19). Stained tissues were examined under light microscopy and scored on a scale of negative to 3+, relative to the staining in tissues of a known PI positive control animal tested concurrently with each group of tissues from test animals.
Calves were considered to be acutely infected with BVDV when virus was detected by PCR but was not present at detectable levels by IHC staining of the skin biopsy. Animals were designated as PI when BVDV was detected by PCR and IHC staining on samples obtained at entry to the feedlot and subsequently on samples obtained at postmortem, at the confirmation sampling prior to harvest, or both. The sensitivity and reproducibility of the PCR method was established by using samples from 5 animals confirmed as PI with BVDV by multiple virus isolations. Samples from these animals were pooled with variable numbers of samples from known negative cattle and the limits of sensitivity for detection of the virus in pooled samples established, based on the ability of the assay to detect each and all of the known PI animals on multiple testing.
Data collection and management
The results of the BVDV testing surveys were entered into an electronic spreadsheet (Microsoft Office Excel 2003; Microsoft Corporation, Redmond, Washington, USA) and verified. Animal health information for the entire feeding period describing initial treatment of animals diagnosed with UF and with NF, as well as the gross postmortem diagnoses, were extracted from the chute-side animal health data collection system (FHARM), collated, and verified (Table 1). Feedlot performance data from each site-specific feedlot administrative software package were entered into an electronic spreadsheet (Microsoft Office Excel 2003, Microsoft Corporation) and verified. Finally, the BVDV testing survey, animal health, and feedlot performance data were merged to form complete pen-level and individual animal-level data sets.
Table 1.
Morbidity and mortality data summary by pen persistent infection status in a study to investigate the effect of bovine viral diarrhea virus (BVDV) infections on the overall health and performance of feedlot cattle
| Pen persistent infection status
|
|||||
|---|---|---|---|---|---|
| Variable | PI pensf | Non-PI pensg | Relative riskh | 95% CIi | P-value |
| Number of pens | 9 | 16 | |||
| Number of animals | 2504 | 4628 | |||
| Initial UF treatmenta,j (%) | 11.64 | 10.99 | 1.06 | 0.75–1.49 | 0.742 |
| Initial NF treatmenta,j (%) | 7.03 | 9.07 | 0.77 | 0.52–1.15 | 0.201 |
| Overall mortalityj (%) | 2.22 | 2.83 | 0.79 | 0.55–1.12 | 0.179 |
| Infectious mortalityj (%) | 1.28 | 1.98 | 0.65 | 0.40–1.06 | 0.082 |
| BRD mortalityb,j (%) | 0.64 | 1.07 | 0.60 | 0.31–1.14 | 0.115 |
| HS mortalityc,j (%) | 0.54 | 0.76 | 0.72 | 0.39–1.33 | 0.288 |
| AR mortalityd,j (%) | 0.07 | 0.11 | 0.62 | 0.15–2.59 | 0.517 |
| BVDV/Enteritis mortalitye,j (%) | 0.24 | 0.03 | 7.92 | 1.01–62.43 | 0.049 |
| Metabolic mortalityj (%) | 0.30 | 0.28 | 1.07 | 0.54–2.10 | 0.854 |
| Miscellaneous mortalityj (%) | 0.44 | 0.50 | 0.89 | 0.49–1.62 | 0.699 |
UF — undifferentiated fever; NF — no fever
BRD — mortality caused by bovine respiratory disease
HS — mortality caused by Histophilus somni disease
AR — mortality caused by arthritis
BVDV/Enteritis — mortality caused by bovine viral diarrhea virus and/or other infections of the gastrointestinal tract
PI Pens includes pens that had at least 1 animal with a BVDV persistent infection (PI)
Non-PI Pens includes pens that did not have an animal with a BVDV PI
Relative Risk is the ratio of the rate of disease in the PI Pens divided by the rate of the disease in the Non-PI Pens
95% CI is the 95% confidence interval calculated for each relative risk, corrected for feedlot and initial weight effects and intra-pen clustering of animal health events using generalized linear modeling techniques. The partially maximized likelihood function was used to calculate the confidence intervals. When convergence of the confidence interval could not be attained using the maximized likelihood function, asymptotic normality was used to calculate the confidence intervals
Values for morbidity and mortality variables are least squares means, expressed as percents, controlling for the effects of feedlot, initial weight, and intra-pen clustering of animal health events
In the pen-level data set, pens were categorized by PI status as follows: PI pens consisted of pens that had at least 1 PI animal and non-PI pens consisted pens that did not have any PI animals. In addition, pens were categorized as having evidence of type I or II BVDV infection, based on the PCR results from the arrival samples. At the individual animal level, the arrival and morbidity PCR results were used to categorize individual animals as having evidence of type I or II BVDV infection.
Statistical analysis
The data were analyzed using a software program (SAS for Windows, Release 9.1; SAS Institute, Cary, North Carolina, USA). In the pen-level data, animal health variables were compared between PI pens and non-PI pens using log linear modeling techniques, controlling for clustering of disease and lack of independence created by grouped management of cattle, using generalized estimating questions, as previously described (22,23). The feedlot performance variables were compared between PI pens and non-PI pens, using least squares analysis of variance for site and pen PI status effects (24).
In both the pen-level and individual animal-level data, frequency distributions and descriptive statistics were calculated, and cross-tabulations were used to evaluate simple associations between the arrival and morbidity BVDV infection variables and the subsequent animal health outcome variables (24,25). Generalized linear modeling techniques were used to evaluate the complex associations between the arrival and morbidity BVDV infection variables and the subsequent animal health outcome variables, controlling for feedlot and initial weight effects and intrapen clustering of animal health events (22,23).
Results
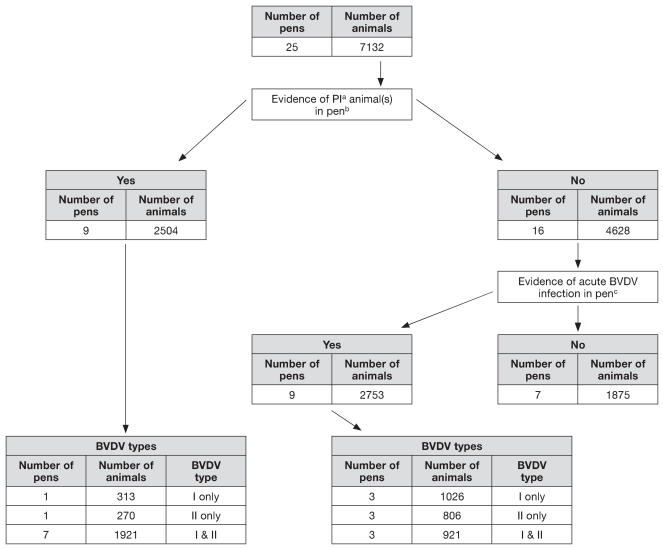
Results of the BVDV testing that was performed on samples collected on arrival of animals at the feedlot is presented in Figure 1. Nine of the 16 non-PI pens had evidence of BVDV infection at feedlot arrival, even though there were no PI animals in those pens. During the 1st mo of the feeding period, only 4 of the 7 non-PI pens with no evidence of BVDV infection at arrival also had no evidence of BVDV infection at the time of initial UF/NF diagnosis. Therefore, 3 of the pens with no evidence of BVDV infection at arrival had BVDV circulating in the pen during the 1st mo on feed. Conversely, 3 of the 9 PI pens had no evidence of BVDV infection at the time of initial UF/NF diagnosis, which suggests that BVDV was not actively circulating in the pen during the 1st mo on feed.
Figure 1.
Results of bovine viral diarrhea virus (BVDV) testing performed on samples collected on arrival of animals at the feedlot in a study to investigate the effect of BVDV infections on the overall health and performance of feedlot cattle.
a PI — persistently infected with BVDV
b Evidence for a PI infection with BVDV was established when BVDV was detected by polymerase chain reaction (PCR) and immunohistochemical (IHC) staining on samples obtained at entry to the feedlot
c Evidence for an acute infection with BVDV was established when BVDV was detected by PCR testing but was not present at detectable levels by IHC staining of the skin biopsy
Thirteen PI animals (0.18%) were detected in 9 of 25 pens enrolled in the study. Eleven of the PI animals had a type I strain of BVDV, 1 PI animal had a type II strain, and 1 PI animal had an undetermined strain of BVDV that was lost during laboratory follow-up. Death occurred prior to harvest in 8/13 (61.5%) PI animals: 3 were diagnosed with mucosal disease, 1 was diagnosed with peritonitis, and 4 were diagnosed with BRD.
Animal health and feedlot performance data, at the pen level, for PI pens and non-PI pens are summarized in Tables 1 and 2, respectively. The BVDV/enteritis mortality rate was significantly higher in PI pens than in non-PI pens (P < 0.05). However, there were no significant (P ≥ 0.05) differences in any of the other morbidity or mortality variables that were studied. The animal health outcome was numerically better in PI pens than in non-PI pens (Table 1). There were no significant (P ≥ 0.05) differences in average daily gain (ADG) or the dry matter intake to gain ratio (DM:G) between PI pens and non-PI pens. Although it was not statistically significant, there were trends of 1% to 2% improvements in ADG and DM:G in the non-PI pens when compared with the PI pens (Table 2).
Table 2.
Performance data summary by pen persistant infection status in a study to investigate the effect of bovine viral diarrhea virus (BVDV) infections on the overall health and performance of feedlot cattle
| Pen persistent infection status
|
||||
|---|---|---|---|---|
| Variable | PI pensa | Non-PI pensb | Standard error of mean (Sχ̄) | P-value |
| Average daily gainc,e (lb/animal/day) | 3.47 | 3.51 | Sχ̄ = 0.04 | 0.468 |
| Dry matter intake to gain ratiod,e | 5.76 | 5.66 | Sχ̄ = 0.05 | 0.092 |
PI pens includes pens that had at least 1 animal with a BVDV persistent infection (PI)
Non-PI pens includes pens that did not have an animal with a BVDV PI
Average Daily Gain (ADG) is the average number of pounds gained per day during the feeding period. The effect of animals that died has been removed from the ADG values
Dry Matter Intake to Gain Ratio (DM:G) is a ratio of the pounds of feed (expressed on a 100% dry matter basis) necessary for 1 lb of gain. The effect of animals that died has been removed from the DM:G values
Values for ADG and DM:G are least squares means, controlling for the effects of feedlot and initial weight
The occurrence of BVDV infection, based on results of the whole blood PCR testing, was low at feedlot arrival (0.41% and 0.27% of animals were positive for type I and II BVDV infection, respectively). There was a significant (P < 0.05) association between type-specific BVDV infection and pen-level morbidity and mortality outcomes (Table 3). Overall mortality and infectious disease mortality rates were significantly (P < 0.05) higher in pens categorized as positive for type I BVDV at arrival than in negative pens. In addition, initial UF treatment and initial NF treatment frequencies were numerically higher in pens that were categorized as positive for type I BVDV than in negative pens. However, overall mortality rates were significantly (P < 0.05) lower and infectious mortality and initial UF and NF treatment frequencies were numerically lower in pens that were positive for type II BVDV infection at arrival than in negative pens.
Table 3.
Effect of arrival bovine viral diarrhea virus (BVDV) infection on subsequent pen-level morbidity and mortality in a study to investigate the effect of BVDV infections on the overall health and performance of feedlot cattle
| Variable | Relative riska | 95% CIc | P-value |
|---|---|---|---|
| Initial UF treatmentb | |||
| Evidence of type I BVDV — Yes vs. No | 1.40 | 0.84–2.35 | 0.195 |
| Evidence of type II BVDV — Yes vs. No | 0.82 | 0.56–1.20 | 0.314 |
| Initial NF treatmentb | |||
| Evidence of type I BVDV — Yes vs. No | 1.51 | 1.14–2.00 | 0.004 |
| Evidence of type II BVDV — Yes vs. No | 0.69 | 0.49–0.98 | 0.036 |
| Overall mortality | |||
| Evidence of type I BVDV — Yes vs. No | 2.01 | 1.26–3.21 | 0.003 |
| Evidence of type II BVDV — Yes vs. No | 0.58 | 0.35–0.96 | 0.034 |
| Infectious mortality | |||
| Evidence of type I BVDV — Yes vs. No | 2.83 | 1.64–4.88 | < 0.001 |
| Evidence of type II BVDV — Yes vs. No | 0.52 | 0.26–1.03 | 0.061 |
Relative risk — the ratio of the rate of disease in pens with evidence of type-specific BVDV infection on arrival divided by the rate of disease in pens with no evidence of type-specific BVDV viraemia on arrival
UF — undifferentiated fever and NF is no fever
95% CI — the 95% confidence interval calculated for each relative risk, corrected for feedlot and initial weight effects and intra-pen clustering of animal health events using generalized linear modeling techniques. The partially maximized likelihood function was used to calculate the confidence intervals. When convergence of the confidence interval could not be attained using the maximized likelihood function, asymptotic normality was used to calculate the confidence intervals
At the individual animal level, acute BVDV infections were associated with a significantly (P < 0.05) increased risk of overall mortality and infectious disease mortality (Tables 4 and 5). However, these associations were observed more consistently with type I BVDV infection than with type II BVDV infection. Type I BVDV infection was detected at the time of initial diagnosis in 4.00% of UF cases and in 2.55% of NF cases, and type II BVDV infection was detected at the time of initial diagnosis in 2.5% of UF cases and in 1.7% of NF cases.
Table 4.
Effect of arrival bovine viral diarrhea virus (BVDV) infection on subsequent individual animal morbidity and mortality in a study to investigate the effect of BVDV infections on the overall health and performance of feedlot cattle
| Variable | Relative riska | 95% CIc | P-value |
|---|---|---|---|
| Initial UF Treatmentb | |||
| Evidence of type I or II BVDV — Yes vs. No | 0.30 | 0.04–2.23 | 0.240 |
| Evidence of type I BVDV — Yes vs. No | N/A | N/A | N/A |
| Evidence of type II BVDV — Yes vs. No | 0.57 | 0.08–4.04 | 0.570 |
| Initial NF Treatmentb | |||
| Evidence of type I or II BVDV — Yes vs. No | 1.34 | 0.49–3.64 | 0.568 |
| Evidence of type I BVDV — Yes vs. No | 2.77 | 1.02–7.51 | 0.046 |
| Evidence of type II BVDV — Yes vs. No | N/A | N/A | N/A |
| Overall Mortality | |||
| Evidence of type I or II BVDV — Yes vs. No | 5.00 | 1.82–13.71 | 0.002 |
| Evidence of type I BVDV — Yes vs. No | 4.38 | 1.52–12.65 | 0.006 |
| Evidence of type II BVDV — Yes vs. No | 2.76 | 0.6–12.64 | 0.192 |
| Infectious Mortality | |||
| Evidence of type I or II BVDV — Yes vs. No | 6.03 | 1.81–20.06 | 0.003 |
| Evidence of type I BVDV — Yes vs. No | 3.83 | 0.91–16.10 | 0.067 |
| Evidence of type II BVDV — Yes vs. No | 4.37 | 0.96–19.95 | 0.057 |
Relative risk — the ratio of the rate of disease in non-persistently infected animals with evidence of type-specific BVDV infection on arrival divided by the rate of disease in animals with no evidence of type-specific BVDV viraemia on arrival
UF — undifferentiated fever and NF is no fever
95% CI — the 95% confidence interval calculated for each relative risk, corrected for feedlot effects and intra-pen clustering of animal health events using generalized linear modeling techniques. The partially maximized likelihood function was used to calculate the confidence intervals. When convergence of the confidence interval could not be attained using the maximized likelihood function, asymptotic normality was used to calculate the confidence intervals. Values for unstable statistical models that would not solve are recorded as N/A
Table 5.
Effect of bovine viral diarrhea virus (BVDV) infection at the time of undifferentiated fever (UF) or no fever (NF) diagnosis on subsequent individual animal mortality in a study to investigate the effect of BVDV infections on the overall health and performance of feedlot cattle
| Variable | Relative riska | 95% CIb | P-value |
|---|---|---|---|
| Overall mortality | |||
| Evidence of type I or II BVDV — Yes vs. No | 2.16 | 1.14–4.09 | 0.018 |
| Evidence of type I BVDV — Yes vs. No | 2.73 | 1.67–4.46 | < 0.001 |
| Evidence of type II BVDV — Yes vs. No | 1.31 | 0.35–4.82 | 0.688 |
| Infectious mortality | |||
| Evidence of type I or II BVDV — Yes vs. No | 2.24 | 1.21–4.16 | 0.011 |
| Evidence of type I BVDV — Yes vs. No | 3.42 | 1.96–5.96 | < 0.001 |
| Evidence of type II BVDV — Yes vs. No | 0.96 | 0.26–3.56 | 0.950 |
Relative risk — the ratio of the rate of disease in non-persistently infected animals with evidence of type-specific BVDV infection at the time of initial UF or NF diagnosis divided by the rate of disease in animals with no evidence of type-specific BVDV infection at the time of initial UF or NF diagnosis
95% CI — the 95% confidence interval calculated for each relative risk, corrected for feedlot and initial diagnosis effects and intra-pen clustering of animal health events using generalized linear modeling techniques. The partially maximized likelihood function was used to calculate the confidence intervals. When convergence of the confidence interval could not be attained using the maximized likelihood function, asymptotic normality was used to calculate the confidence intervals
In the study animals that died during the feeding period (mortality-based BVD testing), evidence of BVDV infection was found, using IHC staining in 5.56% of non-PI animals (number of positive animals/total number of animals) and in 1.89% of postmortem samples from non-PI animals (number of positive tissue samples/total number of tissues tested). Evidence of BVDV infection, using IHC staining, was found in 100% of PI animals and in 96.30% of postmortem samples from those animals (number of positive tissue samples/total number of tissue samples).
Discussion
This study demonstrates that the presence of PI animals alone does not have a large negative impact on pen-level animal health and feedlot performance outcomes in feedlot animals. Most PI animals were determined to be infected with BVDV type I. In addition, presence of a PI animal in a pen appeared to be protective to the overall health outcomes, since PI pens had numerically fewer overall mortalities than in non-PI pens. This is consistent with what was reported by O’Connor et al (26), but contrary to what was reported by Loneragan et al (27). O’Connor et al reported that the presence of PI animals was associated with a significant (P < 0.05) reduction in the pen-level risk of respiratory disease. However, Loneragan et al reported that the incidence of respiratory disease morbidity was significantly higher in pens containing PI animals or in pens adjacent to pens containing PI animals than in pens with no exposure to PI animals, but the baseline level of respiratory disease was lower than in the current study. In both the O’Connor et al and the Loneragan et al studies, the prevalence of PI animals was similar. The level of acute BVDV infection in the population was not measured or characterized in either of the previously mentioned studies. However, in our study, the occurrence of acute BVDV infection and BVDV type identification were reported.
The effect of PI animals on the animal health of adjacent pens has been studied previously (19); this was not evaluated in the current study because of cost-related issues. As a result, the fact that the PI status of pens adjacent to the study pens was unknown must be taken into consideration when interpreting the results of the current study. The presence of PI animals in pens adjacent to the study pens may have had an effect on the outcome parameters that were measured.
Immunohistochemical staining results from postmortem specimens of non-PI animals demonstrated that BVDV affected a small proportion of feedlot animals that die during the feeding period. These findings were not consistent with those of other studies from animals with chronic disease, animals with multi-systemic disease that have variable BVDV vaccination histories, or both (15–17; Campbell, WCVM, unpublished observations). However, the difference in the frequency of BVDV detection in postmortem tissues between the current study and other studies is likely due to 2 factors: 1) in the current study, all causes of mortality in the target population were investigated, and 2) in the current study, animals received a comprehensive program of type I and II BVDV vaccination.
Interestingly, this study indicates that there may be differences in the effect of type I and II BVDV infection on pen-level and individual animal-level health outcomes. Animals that were in pens that contained animals with type I BVDV infections had significantly more initial UF and NF treatments, and overall mortalities, than animals in pens that did not have animals infected with type I BVDV infection. In addition, individual animals with BVDV type I infection were about 4 times more likely to die of all causes than to animals without BVDV type I infection. Moreover, at the pen level, BVDV type II infections appeared to convey a protective effect when compared with pens without BVDV type II infections. The exact cause for the increased risk of morbidity and mortality that is associated with BVDV type I infection is unknown. Previous studies have documented the virulence and immunsuppressive properties of both BVDV type I and BVDV type II isolates (28–29). Perhaps the particular BVDV type I isolates infecting cattle in this study population had more immunosuppressive properties than the BVDV II isolates. This could have rendered the calves infected with the BVDV type I isolates more susceptible to other infections and resultant diseases. Direct comparative studies of inter-isolate virulence are lacking. Few cohort studies have been conducted in a manner to allow for BVDV typing of PI animals and virtually none have focused on BVDV typing of acute infections, making it difficult to directly compare the relative virulence among specific BVDV isolates. Additional studies to further investigate the different effects of type I and II BVDV infections on pen-level and individual animal-level health outcomes are warranted.
The results of this study are in general agreement with previous seroepidemiologic work that has demonstrated highly variable correlations between pen-level evidence of BVDV infection and animal health outcome. However, the differences observed between types I and II BVDV infection on pen-level morbidity and mortality and the effect of acute BVDV infections on the risk of individual animal mortality have not been previously described. In this study, BVDV infection in non- PI animals occurred less frequently than in previously described studies, which may have been a result of the BVDV vaccination program used.
Acknowledgments
We thank the management and staff of the participating feedlots (Western Feedlots — Mossleigh site, Mossleigh, Alberta; Strangmuir Holdings, Strathmore, Alberta; and Ballco Feeders, Brant, Alberta) for their cooperation in conducting this study. In addition, we thank the research and laboratory personnel at the University of Saskatchewan and Feedlot Health Management Services for their dedicated efforts during the study. Finally, we acknowledge the contributions of Dr. Keith West from the University of Saskatchewan during the initial phases of laboratory project development. CVJ
Footnotes
This project was funded by the Alberta Livestock Industry Development Fund, Boehringer Ingelheim (Canada), Western Feedlots, Strangmuir Holdings, Ballco Feeders, and Feedlot Health Management Services. In addition, in-kind support was provided by The Saskatchewan Agriculture Development Fund.
References
- 1.Radostits OM, Gay CC, Blood DC, Hinchcliff KW. Veterinary Medicine: A Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses. 9. London: WB Saunders; 2000. pp. 1085–1105. [Google Scholar]
- 2.Paisley LG, Wells S, Schmitt BJ. Prevalence of bovine viral diarrhea antibodies in 256 US cow-calf operations: A survey. Theriogenology. 1996;46:1313–1323. [Google Scholar]
- 3.Taylor LF, Van Donkersgoed J, Dubovi EJ, Harland RJ, van den Hurk JV, Ribble CS, Janzen ED. The prevalence of bovine viral diarrhea virus infection in a population of feedlot calves in western Canada. Can J Vet Res. 1995;59:87–93. [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor LF, Janzen ED, Ellis JA, van den Hurk JV, Ward P. Performance, survival, necropsy, and virological findings from calves persistently infected with the bovine viral diarrhea virus originating from a single Saskatchewan beef herd. Can Vet J. 1997;38:29–37. [PMC free article] [PubMed] [Google Scholar]
- 5.National Animal Health Monitoring Service. Highlights of NAHMS Feedlot ‘99 Part III. Fort Collins, Colorado: Centers for Epidemiology and Animal Health, Veterinary Services, Animal and Plant Health Inspection Service, United States Department of Agriculture 2000; [Google Scholar]
- 6.Schunicht OC, Guichon PT, Booker CW, et al. A comparison of prophylactic efficacy of tilmicosin and a new formulation of oxytetracycline in feedlot calves. Can Vet J. 2002;43:355–362. [PMC free article] [PubMed] [Google Scholar]
- 7.Schunicht OC, Booker CW, Jim GK, Guichon PT, Wildman BK, Hill BW. Comparison of a multivalent viral vaccine program versus a univalent viral vaccine program on animal health, feedlot performance, and carcass characteristics of feedlot calves. Can Vet J. 2003;44:43–50. [PMC free article] [PubMed] [Google Scholar]
- 8.Schunicht OC, Booker CW, Guichon PT, et al. An evaluation of the relative efficacy of a new formulation of oxytetracycline for the treatment of undifferentiated fever in feedlot calves in western Canada. Can Vet J. 2002;43:940–945. [PMC free article] [PubMed] [Google Scholar]
- 9.Jim GK, Booker CW, Guichon PT, et al. A comparison of florfenicol and tilmicosin for the treatment of undifferentiated fever in feedlot calves in western Canada. Can Vet J. 1999;40:179–184. [PMC free article] [PubMed] [Google Scholar]
- 10.Booker CW, Guichon PT, Jim GK, Schunicht OC, Harland RJ, Morley PS. Seroepidemiology of undifferentiated fever in feedlot calves in western Canada. Can Vet J. 1999;40:40–48. [PMC free article] [PubMed] [Google Scholar]
- 11.Martin SW, Bateman KG, Shewan PE, Rosendal S, Bohac JG, Thorburn M. A group level analysis of the associations between antibodies to seven putative pathogens and respiratory disease and weight gain in Ontario feedlot calves. Can J Vet Res. 1990;54:337–342. [PMC free article] [PubMed] [Google Scholar]
- 12.Martin SW, Bateman KG, Shewan PE, Rosendal S, Bohac JE. The frequency, distribution and effects of antibodies, to seven putative respiratory pathogens, on respiratory disease and weight gain in feedlot calves in Ontario. Can J Vet Res. 1989;53:355–362. [PMC free article] [PubMed] [Google Scholar]
- 13.Martin SW, Bohac JG. The association between serological titers in infectious bovine rhinotracheitis virus, bovine virus diarrhea virus, parain-fluenza-3 virus, respiratory syncytial virus and treatment for respiratory disease in Ontario feedlot calves. Can J Vet Res. 1986;50:351–358. [PMC free article] [PubMed] [Google Scholar]
- 14.Booker CW. Effect of undifferentiated fever in feedlot production. Proc 74th West Vet Conf; 2002. [Google Scholar]
- 15.Haines DM, Moline KM, Sargent RA, Campbell JR, Myers DJ, Doig PA. Immunohistochemical study of Hemophilus somnus, Mycoplasma bovis, Mannheimia hemolytica, and bovine viral diarrhea virus in death losses due to myocarditis in feedlot cattle. Can Vet J. 2004;45:231–234. [PMC free article] [PubMed] [Google Scholar]
- 16.Haines DM, Martin KM, Clark EG, Jim GK, Janzen ED. The immunohistochemical detection of Mycoplasma bovis and bovine viral diarrhea virus in tissues of feedlot cattle with chronic, unresponsive respiratory disease and/or arthritis. Can Vet J. 2001;42:857–860. [PMC free article] [PubMed] [Google Scholar]
- 17.Shahriar FM, Clark EG, Janzen ED, West K, Wobeser G. Coinfection with bovine viral diarrhea virus and Mycoplasma bovis in feedlot cattle with chronic pneumonia. Can Vet J. 2002;43:863–868. [PMC free article] [PubMed] [Google Scholar]
- 18.Booker CW. BVD virus and undifferentiated fever (bovine viral diarrhea). Proc 74th West Vet Conf; 2002. [Google Scholar]
- 19.Njaa BL, Clark EG, Janzen ED, Ellis JA, Haines DM. Diagnosis of cattle persistently infected with bovine viral diarrhea virus by immunohistochemical staining of formalin-fixed skin biopsies. J Vet Diagn Invest. 2000;12:393–399. doi: 10.1177/104063870001200501. [DOI] [PubMed] [Google Scholar]
- 20.Munoz-Zanzi CA, Johnson WO, Thurmond MC, Hietala SK. Pooled-sample testing as a herd-screening tool for detection of bovine viral diarrhea virus persistently infected cattle. J Vet Diagn Invest. 2000;12:195–203. doi: 10.1177/104063870001200301. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert SA, Burton KM, Prins SE, Deregt D. Typing of bovine viral diarrhea viruses directly from blood of persistently infected cattle by multiplex PCR. J Clin Microbiol. 1999;37:2020–2023. doi: 10.1128/jcm.37.6.2020-2023.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDermott JJ, Schukken YH. A review of methods used to adjust for cluster effects in explanatory epidemiological studies of animal populations. Prev Vet Med. 1994;18:155–173. [Google Scholar]
- 23.McDermott JJ, Schukken YH, Shoukri MM. Study design and analytic methods for data collected from clusters of animals. Prev Vet Med. 1994;18:175–191. [Google Scholar]
- 24.SAS Institute. SAS/STAT User’s Guide, Version 6. 4. Cary, North Carolina: SAS Institute; 1989. pp. 1pp. 2–1686. [Google Scholar]
- 25.Snedecor GW, Cochran WG. Statistical Methods. 7. Ames, Iowa: Iowa State Univ Pr; 1987. pp. 175–193. [Google Scholar]
- 26.O’Connor AM, Sorden SD, Apley MD. Association between the existence of calves persistently infected with bovine viral diarrhea virus and commingling on pen morbidity in feedlot cattle. Am J Vet Res. 2005;66:2130–2134. doi: 10.2460/ajvr.2005.66.2130. [DOI] [PubMed] [Google Scholar]
- 27.Loneragan GH, Thomson DU, Montgomery DL, Mason GL, Larson RL. Prevalence, outcome, and health consequences associated with persistent infection with bovine viral diarrhea virus in feedlot cattle. J Am Vet Med Assoc. 2005;226:595–601. doi: 10.2460/javma.2005.226.595. [DOI] [PubMed] [Google Scholar]
- 28.Carman S, van Dreumel T, Ridpath J, et al. Severe acute bovine viral diarrhea in Ontario, 1993–1995. J Vet Diagn Invest. 1998;10:27–35. doi: 10.1177/104063879801000106. [DOI] [PubMed] [Google Scholar]
- 29.Ridpath JF. Practical significance of heterogeneity among BVDV strains: Impact of biotype and genotype on U.S. control programs. Prev Vet Med. 2005;72:17–30. doi: 10.1016/j.prevetmed.2005.08.003. [DOI] [PubMed] [Google Scholar]