Abstract
An Akita Inu, living in Belgium, was presented with unusual clinical manifestations of acute babesiosis that included neurological signs and pancytopenia. Diagnosis was made by identifying Babesia canis in the blood smear. Neurological signs resolved after treatment with imidocarb diproprionate. Normalization of hematological abnormalities was gradual over 5 months.
Résumé
Forme inhabituelle de babésiase canine. Un Akita Inu demeurant en Belgique a été présenté avec des manifestations cliniques inhabituelles de babésiase aiguë comprenant des signes neurologiques et de la pancytopénie. Le diagnostic a été établi par identification de Babesia canis dans les frottis sanguins. Les signes neurologiques se sont résorbés après traitement au diproprionate d’imidocarb. Une normalisation graduelle des anomalies sanguines s’est échelonnée sur 5 mois.
(Traduit par Docteur André Blouin)
A 10-year old, male Akita Inu was referred to the Faculty of Veterinary Medicine with a 3-day history of seizures. These were ‘grand mal’ seizures; they occurred after exercise and lasted several minutes. Between seizures, the dog remained weak. No neurological signs were reported before this episode. Anorexia, appearing a day before presentation, was reported. Results from an electrocardiography and echocardiography performed by the referring veterinarian were within reference limits. Vaccinations were up-to-date and no exposure to drugs or history of travel was reported. However, the owner did report that the dog had been bitten by a tick 10 d before presentation.
Case description
On physical examination, the dog was weak and had an elevated temperature (39.8°C). Results from cardiac and respiratory auscultation were normal (heart rate 90 beats/min, respiratory rate 30 breaths/min). Mucous membranes were pale pink and capillary refill time was < 2 s. Dehydration was estimated clinically at 5%. Results from a neurological examination were normal, except for dullness. An enlarged spleen was palpated. Findings on a rectal examination were unremarkable.
Blood was collected for a complete blood (cell) count (CBC) and serum biochemical panel. A voided urine sample was submitted for urinalysis. Hematological abnormalities included a microcytic nonregenerative anemia (hematocrit 0.32 L/L; reference range, 0.43 to 0.59 L/L); mean corpuscular volume (MCV) (60 fL; reference range, 63 to 77 fL); reticulocyte-index (0.2%; reference range < 2%); leukopenia [total white blood cells (WBC) 4.9 × 109/L; reference range, 6.0 to 16.0 × 109/L]; and thrombocytopenia (platelet count 39 × 109/L; reference range, 160 to 510 × 109/L). Cytological evaluation of a peripheral blood smear showed low numbers of spherocytes. No abnormalities were noted on the biochemical profile, glycemia was within the reference ranges (4.33 mmol/L; reference range, 3.05 to 4.99 mmol/L). Urinalysis abnormalities included pyuria (WBC 50/μL, reference range, <25/μL), hemoglobinuria, and bilirubinuria (3+). Urine specific gravity was high (1.050), compatible with clinical dehydration. Results from aerobic urine culture were negative. Therefore, the pyuria most likely resulted from genital or urinary contamination, since urinalysis had been performed on a free catch sample and there had been no previous antibiotic treatment. Hemoglobinuria and bilirubinuria were attributed to intra- and extravascular hemolysis.
Results of aerobic and anaerobic blood culture and an indirect fluorescent antibody (IFA) test for Ehrlichia canis were negative.
Because of the pancytopenia, bone marrow was aspirated from the wing of the ileum and submitted for cytological examination. Results showed erythroid hyperplasia (myeloid:erythroid <1); the myeloid series was complete in number with orderly maturation; adequate numbers of megakaryocytes were present. Abdominal radiographs showed splenomegaly; thoracic radiographic images were unremarkable. Abdominal ultrasonographs confirmed a homogeneously enlarged spleen with normal echogenicity. Fine needle aspirates of the spleen were not taken because of the thrombocytopenia.
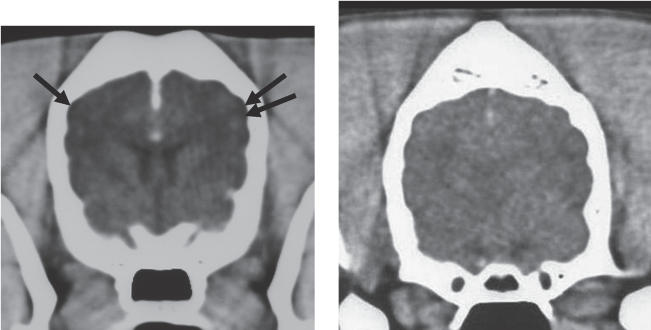
Computer tomography (CT) of the brain suggested the presence of cortical atrophy (Figure 1). Symmetry of the brain hemispheres and the ventricle size were normal. Some diffuse hypodense areas were seen in the cerebrum. Intravenous injection of contrast medium (Optiray 350; Codali, Brussels, Belgium) 2 mL/kg bodyweight (BW) revealed normal CT enhancement of the brain parenchyma without any enhancement in the hypodense areas. A cervical cerebral spinal fluid (CSF) puncture was performed and results from the CSF analysis were within normal limits [no cells, protein content 18 g/L (reference range, <27.5 g/L)].
Figure 1.
A — Transverse CT-slice of the brain after the administration of intravenous contrast medium. Normal ventricle size, normal symmetry, and enhancement of the brain parenchyma are visible. Cortical atrophy (arrows) is present. B — Transverse CT-slice of the brain without significant abnormalities for comparison.
Treatment of the clinical signs was instituted with NaCl 0.9%, 70 mL/h, IV, cefazoline (Kefzol; Eli Lilly, Brussels, Belgium), 20 mg/kg BW, IV, q8h, and phenobarbital (Gardenal; Aventis, Diegem, Belgium), 5 mg/kg BW, PO, q24h.
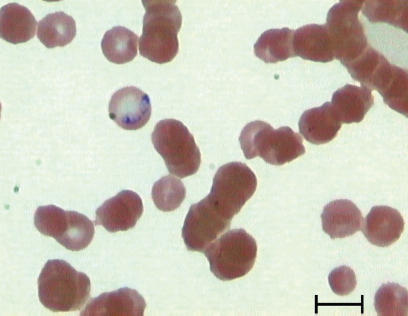
Peripheral blood smears were examined cytologically daily. On day 3, intraerythrocytic organisms, compatible with B. canis, were detected (Figure 2). Imidocarb diproprionate (Carbesia; Shering-Plough, Levallois-Perret, France), 6 mg/kg BW, SC, was administered and this dose was repeated 2 wk later. Within 48 h, the dog’s mentation returned to normal and the anorexia resolved. The leucocyte count had returned to normal (15.6 × 109/L) within 3 d, and the thrombocytopenia resolved within 6 d (platelet count 41.5 × 109/L) following the imidocarb injection. The anemia improved (day 6: hematocrit 0.36 L/L). No seizures were observed for 5 d and the phenobarbital was discontinued after 6 d. The administration of cefazoline was discontinued after 7 d.
Figure 2.
Large (2.4 μm to 5.0 μm), piriform-shaped organisms, compatible with Babesia canis occuring paired within an erythrocyte of a dog. Wright-Giemsa stain. Bar = 5 μm.
Five months later, the hematocrit was within the reference range (0.45 L/L); the MCV was 62 fL, and a urinalysis did not reveal any abnormalities.
Discussion
Canine babesiosis is an emerging disease in nonendemic regions in Europe (1). The expansion of the geographic range of babesiosis in the United States was also documented recently (2). The risk of spreading parasite species to nonendemic regions, including Canada and the northern part of Europe, is enhanced by the international transport of animals (1).
The clinical signs of canine babesiosis have been well described (3,4). The severity of the disease depends on species pathogenicity and the host’s immune response (5). Because of the history of a recent tick bite, the acute clinical signs, the hematological abnormalities, and the response to imidocarb treatment, this dog most likely had acute, atypical babesiosis. The incubation period for acute babesiosis ranges from 10 to 21 d (4), suggesting that the dog became infected in Belgium.
Regenerative anemia and thrombocytopenia are frequent features of canine babesiosis (4); however, pancytopenia is an infrequent finding (3). The leukocyte count can be extremely variable, ranging from leukopenia to a leukemoid reaction. Pancytopenia is also described in association with other infectious disorders such as ehrlichiosis, parvovirus infection, canine hepatitis virus infection, histoplasmosis, septicemia, and endotoxemia. During chronic ehrlichiosis, pancytopenia is due to bone marrow hypoplasia (3). However, in this case, an IFA test for E. canis was negative. False negative serologic results have been reported in early infections and with low serum antibody levels (6). However, the negative serologic result and the hyperplastic bone marrow did not support chronic ehrlichiosis. Septicemia was unlikely, because clinical and hematological signs did not improve after antibiotic therapy but resolved after imidocarb diproprionate injection. History and diagnostic tests results did not support toxic or drug-related causes, or neoplasia, myelofibrosis, and myelophthisis as possible causes of the pancytopenia. In humans, babesiosis has been associated with a hemophagocytic syndrome, resulting in pancytopenia (7). In this case, results from the bone marrow cytologic examination did not support this syndrome as a possible cause of pancytopenia.
Additionally, a nonregenerative microcytic anemia was encountered. Bone marrow aspiration was hypercellular with a decreased myeloid to erythroid ratio, indicating early or ineffective erythropoiesis due to hemolysis. Microcytic red blood cells have been described in the Japanese Akita (8). Other causes of microcytosis, such as iron deficiency or hepatic vascular anomalies, seemed unlikely, as no signs of chronic gastrointestinal blood loss were present and levels of liver enzymes and bile acids were within reference ranges. Also, the low MCV range persisted after resolution of the anemia and clinical signs. Therefore, the low erythrocyte MCV is likely a limitation of the reference range used (inappropriate for this breed) rather than a true alteration.
Cerebral babesiosis (CB) refers to the occurrence of nervous symptoms associated with parasitized erythrocytes. Only a small number of affected animals (1% to 10%) develop CB (4). Clinical signs such as seizures and altered consciousness, similar to those described for this dog, have been reported (3). Other neurological signs of CB can include ataxia, paresis, muscle tremor, anisocoria, and vestibular signs (3,4). The pathogenesis of CB is related to parasitized erythrocytes that become sequestrated in the central nervous system microvasculature and to the release of inflammatory mediators and tissue hypoxia, which can lead to neurological signs (3,4,9). Cerebral babesiosis is usually associated with high mortality (3,4). In this case, the clinical signs improved markedly 2 d following imidocarb diproprionate administration. Early diagnosis and treatment may have contributed to a favorable outcome. Indeed, clinical improvement is usually seen within 24 h after initiation of antibabesial compounds (4). Additionally, B. canis canis, the most common strain of B. canis in Europe, has an intermediate pathogenicity (4). Large (2.4 μm to 5.0μ), piriform-shaped organisms, occuring paired within erythrocytes, typical for B. canis, were found on blood smear cytologic examination (4). Polymerase chain reaction testing is capable of differentiating between B. canis strains; however, this was not available to us. Therefore, although organisms typical for B. canis were detected on cytologic examination, infection with B. canis canis was not confirmed.
In humans, neuroimaging is routinely performed in patients with infectious diseases such as cerebral malaria, neuroborreliosis, and Rocky Mountain spotted fever (9–12). Abnormalities seen on CT images, although mostly nonspecific, correlate well with illness severity and clinical outcome (9–12). The most frequent patterns encountered are no abnormalities, cerebral edema, thalamic and cerebellar hypoattentuation, and cerebral atrophy. According to the author’s knowledge, brain CT findings for canine cerebral babesiosis have not been reported. In this case, general cortical atrophy and diffuse hypodense areas could relate to abnormalities encountered in other human infectious diseases with cerebral involvement (11). However, considering the age of the dog and the atypical abnormalities found on the CT images, cortical atrophy related to aging cannot be ruled out as a possible cause of these abnormalities. Because of complete resolution of clinical signs and because of financial restraints, a follow-up CT scan was not performed.
Diagnosis of babesiosis was made by identifying organisms on blood smears. Babesia organisms can be easily missed with low parasitemia (3,4). Chronic carriers rarely have evident organisms present on cytologic examination and are usually without clinical signs (4). In the present case, we suspected that low parasitemia was present (babesia organisms seen on day 3), which was most likely related to early disease. An IFA test can also be used to support diagnosis. Seropositive results can occur in clinically normal dogs and serologic testing alone cannot be used to make a definitive diagnosis (3). For this reason and also because the initial suspicion of babesiosis was low, IFA testing for B. canis was not performed here.
This case illustrates the presence of canine babesiosis in Belgium, which is a nonendemic region. In addition, the manifestation of the disease was atypical and included neurological signs and pancytopenia.
Acknowledgments
The authors acknowledge Dr. H. Lefebure for the referral of the dog, and Dr. L. Van Ham for the neurological examination. CVJ
Footnotes
Reprints will not be available from the authors.
References
- 1.Daugschies A. Import of parasites by tourism and animal trading. Dtsch Tierarztl Wochenschr. 2001;108:348–352. [PubMed] [Google Scholar]
- 2.Birkenheuer AJ, Correa MT, Levy MG, Breitschwerdt EB. Geographic distribution of babesiosis among dogs in the United States and association with dog bites: 150 cases (2000–2003) J Am Vet Med Assoc. 2005;227:942–947. doi: 10.2460/javma.2005.227.942. [DOI] [PubMed] [Google Scholar]
- 3.Boozer AL, Macintire DK. Canine babesiosis. Vet Clin North Am Small Anim Pract. 2003;33:885–904. doi: 10.1016/s0195-5616(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 4.Taboada J. Babesiosis. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 2. Philadelphia: WB Saunders; 1998. pp. 473–481. [Google Scholar]
- 5.Neer TM. Ehrlichiosis. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 2. Philadelphia: WB Saunders; 1998. pp. 473–481. [Google Scholar]
- 6.Brandão LP, Hagiwara MK, Myiashiro SI. Humoral immunity and reinfection resistance in dogs experimentally inoculated with B. canis and either treated or untreated with imidocarb dipropionate. Vet Parasitol. 2003;114:253–265. doi: 10.1016/s0304-4017(03)00130-4. [DOI] [PubMed] [Google Scholar]
- 7.Gupta P, Hurley RW, Helseth PH, Goodman JL, Hammerschmidt DE. Pancytopenia due to hemophagocytic syndrome as the presenting manifestation of babesiosis. Am J Hematol. 1995;50:60–62. doi: 10.1002/ajh.2830500113. [DOI] [PubMed] [Google Scholar]
- 8.Degen M. Pseudohyperkalemia in Akitas. J Am Vet Med Assoc. 1987;190:541–543. [PubMed] [Google Scholar]
- 9.Schetters TP, Eling WM. Can Babesia infections be used as a model for cerebral malaria? Parasitol Today. 1999;15:492–497. doi: 10.1016/s0169-4758(99)01566-5. [DOI] [PubMed] [Google Scholar]
- 10.Bonawitz C, Castillo M, Mukherji K. Comparison of CT and MR features with clinical outcome in patients with rocky mountain spotted fever. Am J Neuroradiol. 1997;18:459–464. [PMC free article] [PubMed] [Google Scholar]
- 11.Tarasów E, Ustymowicz A, Zajkowska J, Hermanowska-Szpakowicz T. Neuroborreliosis: CT and MRI findings in 14 cases. Preliminary communication. Neurolog Neurochir Pol. 2001;35:803–813. [PubMed] [Google Scholar]
- 12.Patankar TF, Karnad DR, Shetty PG, Desai AP, Prasad SR. Adult cerebral malaria: Prognostic importance of imaging findings and correlation with postmortem findings. Radiology. 2002;224:811–816. doi: 10.1148/radiol.2243010588. [DOI] [PubMed] [Google Scholar]